Emerging Nanoparticle Strategies for Modulating Tumor-Associated Macrophage Polarization
Abstract
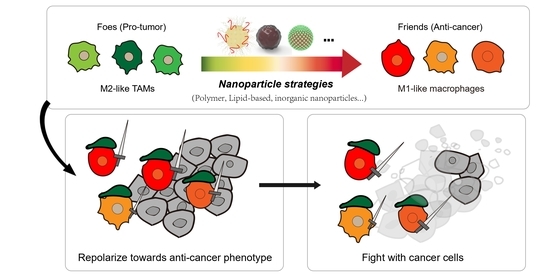
:1. Introduction
2. Polymeric Nanoparticles and Macrophage Repolarization
3. Lipid-Based Nanomaterials and Macrophage Repolarization
4. Inorganic Nanoparticles and Macrophage Repolarization
5. Other Nanomaterials
6. Conclusion and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Maude, S.L.; Teachey, D.T.; Porter, D.L.; Grupp, S.A. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood 2015, 125, 4017–4023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamanishi, J.; Mandai, M.; Ikeda, T.; Minami, M.; Kawaguchi, A.; Murayama, T.; Kanai, M.; Mori, Y.; Matsumoto, S.; Chikuma, S.; et al. Safety and Antitumor Activity of Anti-PD-1 Antibody, Nivolumab, in Patients With Platinum-Resistant Ovarian Cancer. J. Clin. Oncol. 2015, 33, 4015–4022. [Google Scholar] [CrossRef] [PubMed]
- Iwai, Y.; Hamanishi, J.; Chamoto, K.; Honjo, T. Cancer immunotherapies targeting the PD-1 signaling pathway. J. Biomed. Sci. 2017, 24, 26. [Google Scholar] [CrossRef] [Green Version]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.; Woodward, W.A.; Wang, X.; Reuben, J.M.; Ueno, N.T. Inflammatory breast cancer biology: The tumour microenvironment is key. Nat. Rev. Cancer 2018, 18, 485–499. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, Z.; Ren, Z.; Tang, F.; Li, Y. Obstacles and Coping Strategies of CAR-T Cell Immunotherapy in Solid Tumors. Front. Immunol. 2021, 12, 687822. [Google Scholar] [CrossRef] [PubMed]
- Koustas, E.; Sarantis, P.; Papavassiliou, A.G.; Karamouzis, M.V. The Resistance Mechanisms of Checkpoint Inhibitors in Solid Tumors. Biomolecules 2020, 10, 666. [Google Scholar] [CrossRef]
- Duan, Q.; Zhang, H.; Zheng, J.; Zhang, L. Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends Cancer 2020, 6, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, L.; Pollard, J.W. Targeting macrophages: Therapeutic approaches in cancer. Nat. Rev. Drug Discov. 2018, 17, 887–904. [Google Scholar] [CrossRef] [PubMed]
- Poh, A.R.; Ernst, M. Targeting Macrophages in Cancer: From Bench to Bedside. Front. Oncol. 2018, 8, 49. [Google Scholar] [CrossRef] [Green Version]
- Najafi, M.; Hashemi Goradel, N.; Farhood, B.; Salehi, E.; Nashtaei, M.S.; Khanlarkhani, N.; Khezri, Z.; Majidpoor, J.; Abouzaripour, M.; Habibi, M.; et al. Macrophage polarity in cancer: A review. J. Cell Biochem. 2019, 120, 2756–2765. [Google Scholar] [CrossRef] [PubMed]
- Van Dalen, F.J.; van Stevendaal, M.; Fennemann, F.L.; Verdoes, M.; Ilina, O. Molecular Repolarisation of Tumour-Associated Macrophages. Molecules 2018, 24, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Ginhoux, F.; Schultze, J.L.; Murray, P.J.; Ochando, J.; Biswas, S.K. New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat. Immunol. 2016, 17, 34–40. [Google Scholar] [CrossRef]
- Alberto Mantovani, S.S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Bercovici, N.; Guerin, M.V.; Trautmann, A.; Donnadieu, E. The Remarkable Plasticity of Macrophages: A Chance to Fight Cancer. Front. Immunol. 2019, 10, 1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Guo, J.; Huang, L. Tackling TAMs for Cancer Immunotherapy: It’s Nano Time. Trends Pharm. Sci. 2020, 41, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Noy, R.; Pollard, J.W. Tumor-associated macrophages: From mechanisms to therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeNardo, D.G.; Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Cully, M. Cancer: Re-educating tumour-associated macrophages with nanoparticles. Nat. Rev. Drug Discov. 2018, 17, 468. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xie, Y.; Xiong, Y.; Liu, S.; Qiu, C.; Zhu, Z.; Mao, H.; Yu, M.; Wang, X. TLR 7/8 agonist reverses oxaliplatin resistance in colorectal cancer via directing the myeloid-derived suppressor cells to tumoricidal M1-macrophages. Cancer Lett. 2020, 469, 173–185. [Google Scholar] [CrossRef]
- Rodell, C.B.; Arlauckas, S.P.; Cuccarese, M.F.; Garris, C.S.; Li, R.; Ahmed, M.S.; Kohler, R.H.; Pittet, M.J.; Weissleder, R. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat. Biomed. Eng. 2018, 2, 578–588. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, A.N.; Contractor, S.; Castaneda, I.; Cathcart, C.S.; Razdan, D.; Klyde, D.; Kisza, P.; Gonzales, S.F.; Salazar, A.M. A Phase I trial using local regional treatment, nonlethal irradiation, intratumoral and systemic polyinosinic-polycytidylic acid polylysine carboxymethylcellulose to treat liver cancer: In search of the abscopal effect. J. Hepatocell Carcinoma 2017, 4, 111–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Qu, S.; Chen, X.; Wu, Q.; Shi, M. Promising Targets for Cancer Immunotherapy: TLRs, RLRs, and STING-Mediated Innate Immune Pathways. Int. J. Mol. Sci. 2017, 18, 404. [Google Scholar] [CrossRef] [PubMed]
- Self-Fordham, J.B.; Naqvi, A.R.; Uttamani, J.R.; Kulkarni, V.; Nares, S. MicroRNA: Dynamic Regulators of Macrophage Polarization and Plasticity. Front. Immunol. 2017, 8, 1062. [Google Scholar] [CrossRef] [Green Version]
- Curtale, G.; Rubino, M.; Locati, M. MicroRNAs as Molecular Switches in Macrophage Activation. Front. Immunol. 2019, 10, 799. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Hagan, C.T.t.; Vincent, B.G.; Wang, A.Z. Emerging Nano-/Microapproaches for Cancer Immunotherapy. Adv. Sci. 2019, 6, 1801847. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.; Zaki, A.A.; Hui, J.Z.; Muzykantov, V.R.; Tsourkas, A. Multifunctional Nanoparticles: Cost Versus Benefit of Adding Targeting and Imaging Capabilities. Science 2012, 338, 903–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberg, M.S. Improving cancer immunotherapy through nanotechnology. Nat. Rev. Cancer 2019, 19, 587–602. [Google Scholar] [CrossRef]
- Beg, S.; Almalki, W.H.; Khatoon, F.; Alharbi, K.S.; Alghamdi, S.; Akhter, M.H.; Khalilullah, H.; Baothman, A.A.; Hafeez, A.; Rahman, M.; et al. Lipid/polymer-based nanocomplexes in nucleic acid delivery as cancer vaccines. Drug Discov. Today 2021, 26, 1891–1903. [Google Scholar] [CrossRef] [PubMed]
- Perciani, C.T.; Liu, L.Y.; Wood, L.; MacParland, S.A. Enhancing Immunity with Nanomedicine: Employing Nanoparticles to Harness the Immune System. ACS Nano 2020, 15, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef] [PubMed]
- MacParland, S.A.; Tsoi, K.M.; Ouyang, B.; Ma, X.Z.; Manuel, J.; Fawaz, A.; Ostrowski, M.A.; Alman, B.A.; Zilman, A.; Chan, W.C.; et al. Phenotype Determines Nanoparticle Uptake by Human Macrophages from Liver and Blood. ACS Nano 2017, 11, 2428–2443. [Google Scholar] [CrossRef]
- Haber, T.; Cornejo, Y.R.; Aramburo, S.; Flores, L.; Cao, P.; Liu, A.; Mooney, R.; Gilchrist, M.; Tirughana, R.; Nwokafor, U.; et al. Specific targeting of ovarian tumor-associated macrophages by large, anionic nanoparticles. Proc. Natl. Acad. Sci. USA 2020, 117, 19737–19745. [Google Scholar] [CrossRef]
- Yu, M.; Chen, Z.; Guo, W.; Wang, J.; Feng, Y.; Kong, X.; Hong, Z. Specifically targeted delivery of protein to phagocytic macrophages. Int. J. Nanomed. 2015, 10, 1743–1757. [Google Scholar] [CrossRef] [Green Version]
- Ngambenjawong, C.; Cieslewicz, M.; Schellinger, J.G.; Pun, S.H. Synthesis and evaluation of multivalent M2pep peptides for targeting alternatively activated M2 macrophages. J. Control. Release 2016, 224, 103–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conde, J.; Bao, C.; Tan, Y.; Cui, D.; Edelman, E.R.; Azevedo, H.S.; Byrne, H.J.; Artzi, N.; Tian, F. Dual targeted immunotherapy via in vivo delivery of biohybrid RNAi-peptide nanoparticles to tumour-associated macrophages and cancer cells. Adv. Funct. Mater. 2015, 25, 4183–4194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cieslewicz, M.; Tang, J.; Yu, J.L.; Cao, H.; Zavaljevski, M.; Motoyama, K.; Lieber, A.; Raines, E.W.; Pun, S.H. Targeted delivery of proapoptotic peptides to tumor-associated macrophages improves survival. Proc. Nati. Acad. Sci. USA 2013, 110, 15919–15924. [Google Scholar] [CrossRef] [Green Version]
- Sur, S.; Rathore, A.; Dave, V.; Reddy, K.R.; Chouhan, R.S.; Sadhu, V. Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano-Struct. Nano-Objects 2019, 20, 100397. [Google Scholar] [CrossRef]
- Banik, B.L.; Fattahi, P.; Brown, J.L. Polymeric nanoparticles: The future of nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 271–299. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, L.; Wei, X.; Zhou, S. Polymer-based drug delivery systems for cancer treatment. J. Polym. Sci. Pol. Chem. 2016, 54, 3525–3550. [Google Scholar] [CrossRef]
- Fuchs, A.K.; Syrovets, T.; Haas, K.A.; Loos, C.; Musyanovych, A.; Mailander, V.; Landfester, K.; Simmet, T. Carboxyl- and amino-functionalized polystyrene nanoparticles differentially affect the polarization profile of M1 and M2 macrophage subsets. Biomaterials 2016, 85, 78–87. [Google Scholar] [CrossRef]
- Huang, Y.J.; Hung, K.C.; Hung, H.S.; Hsu, S.H. Modulation of Macrophage Phenotype by Biodegradable Polyurethane Nanoparticles: Possible Relation between Macrophage Polarization and Immune Response of Nanoparticles. Appl. Mater Interfaces 2018, 10, 19436–19448. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Kroll, A.V.; Holay, M.; Fang, R.H.; Zhang, L. Biomimetic Nanotechnology toward Personalized Vaccines. Adv Mater 2019, e1901255. [Google Scholar] [CrossRef]
- Fang, R.H.; Jiang, Y.; Fang, J.C.; Zhang, L. Cell memb.brane-derived nanomaterials for biomedical applications. Biomaterials 2017, 128, 69–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, G.; Sun, Z.; Li, S.; Peng, X.; Li, W.; Zhou, L.; Ma, Y.; Gong, P.; Cai, L. Cell-Membrane Immunotherapy Based on Natural Killer Cell Membrane Coated Nanoparticles for the Effective Inhibition of Primary and Abscopal Tumor Growth. ACS Nano 2018, 12, 12096–12108. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, Y.; Guo, X.; Zhao, J.; Zhou, S. A Biomimetic Polymer Magnetic Nanocarrier Polarizing Tumor-Associated Macrophages for Potentiating Immunotherapy. Small 2020, e2003543. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.G.; Camps, M.G.M.; Li, T.; Chan, A.B.; Ossendorp, F.; Cruz, L.J. Co-delivery of immunomodulators in biodegradable nanoparticles improves therapeutic efficacy of cancer vaccines. Biomaterials 2019, 220, 119417. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.; Lepland, A.; Scodeller, P.; Fontana, F.; Torrieri, G.; Tiboni, M.; Shahbazi, M.A.; Casettari, L.; Kostiainen, M.A.; Hirvonen, J.; et al. Peptide-guided resiquimod-loaded lignin nanoparticles convert tumor-associated macrophages from M2 to M1 phenotype for enhanced chemotherapy. Acta Biomater. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Liu, L.; Li, X.; Wang, Y.; Guo, X.; Zhao, J.; Zhou, S. Selectively targeting tumor-associated macrophages and tumor cells with polymeric micelles for enhanced cancer chemo-immunotherapy. J. Control. Release 2019, 313, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Son, S.; Park, K.S.; Moon, J.J. Modularly Programmable Nanoparticle Vaccine Based on Polyethyleneimine for Personalized Cancer Immunotherapy. Adv. Sci. 2021, 8, 2002577. [Google Scholar] [CrossRef] [PubMed]
- Qiu, N.; Wang, G.; Wang, J.; Zhou, Q.; Guo, M.; Wang, Y.; Hu, X.; Zhou, H.; Bai, R.; You, M.; et al. Tumor-Associated Macrophage and Tumor-Cell Dually Transfecting Polyplexes for Efficient Interleukin-12 Cancer Gene Therapy. Adv. Mater. 2021, 33, e2006189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Parayath, N.N.; Ene, C.I.; Stephan, S.B.; Koehne, A.L.; Coon, M.E.; Holland, E.C.; Stephan, M.T. Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. Nat. Commun. 2019, 10, 3974. [Google Scholar] [CrossRef]
- Song, Y.; Tang, C.; Yin, C. Combination antitumor immunotherapy with VEGF and PIGF siRNA via systemic delivery of multi-functionalized nanoparticles to tumor-associated macrophages and breast cancer cells. Biomaterials 2018, 185, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Parayath, N.N.; Parikh, A.; Amiji, M.M. Repolarization of Tumor-Associated Macrophages in a Genetically Engineered Nonsmall Cell Lung Cancer Model by Intraperitoneal Administration of Hyaluronic Acid-Based Nanoparticles Encapsulating MicroRNA-125b. Nano Lett. 2018, 18, 3571–3579. [Google Scholar] [CrossRef] [PubMed]
- Zimel, M.N.; Horowitz, C.B.; Rajasekhar, V.K.; Christ, A.B.; Wei, X.; Wu, J.; Wojnarowicz, P.M.; Wang, D.; Goldring, S.R.; Purdue, P.E.; et al. HPMA-Copolymer Nanocarrier Targets Tumor-Associated Macrophages in Primary and Metastatic Breast Cancer. Mol. Cancer 2017, 16, 2701–2710. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; He, X.; Li, C.; He, Y.; Peng, Y.; Zhang, Y.; Lu, Y.; Chen, X.; Zhang, Y.; Chen, Q.; et al. Dandelion-Like Tailorable Nanoparticles for Tumor Microenvironment Modulation. Adv. Sci. 2019, 6, 1901430. [Google Scholar] [CrossRef]
- Wang, J.; Shen, S.; Li, J.; Cao, Z.; Yang, X. Precise Depletion of Tumor Seed and Growing Soil with Shrinkable Nanocarrier for Potentiated Cancer Chemoimmunotherapy. ACS Nano 2021, 15, 4636–4646. [Google Scholar] [CrossRef] [PubMed]
- Talluri, S.V.; Kuppusamy, G.; Karri, V.V.; Tummala, S.; Madhunapantula, S.V. Lipid-based nanocarriers for breast cancer treatment—Comprehensive review. Drug Deliv. 2016, 23, 1291–1305. [Google Scholar] [CrossRef] [PubMed]
- Vega-Vasquez, P.; Mosier, N.S.; Irudayaraj, J. Nanoscale Drug Delivery Systems: From Medicine to Agriculture. Front. Bioeng. Biotechnol. 2020, 8, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Du, C.; Guo, N.; Teng, Y.; Meng, X.; Sun, H.; Li, S.; Yu, P.; Galons, H. Composition design and medical application of liposomes. Eur. J. Med. Chem. 2019, 164, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Antimisiaris, S.G.; Mourtas, S.; Marazioti, A. Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery. Pharmaceutics 2018, 10, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuba, E. Liposome-based immunity-inducing systems for cancer immunotherapy. Mol. Immunol. 2018, 98, 8–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulikas, T. Clinical overview on Lipoplatin: A successful liposomal formulation of cisplatin. Expert Opin. Investig. Drugs 2009, 18, 1197–1218. [Google Scholar] [CrossRef] [PubMed]
- Damiati, S.; Kompella, U.B.; Damiati, S.A.; Kodzius, R. Microfluidic Devices for Drug Delivery Systems and Drug Screening. Genes 2018, 9, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa, S.; Auriola, S.; Monkkonen, J.; Maatta, J. Liposome encapsulated zoledronate favours M1-like behaviour in murine macrophages cultured with soluble factors from breast cancer cells. BMC Cancer 2015, 15, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajan, R.; Sabnani, M.K.; Mavinkurve, V.; Shmeeda, H.; Mansouri, H.; Bonkoungou, S.; Le, A.D.; Wood, L.M.; Gabizon, A.A.; La-Beck, N.M. Liposome-induced immunosuppression and tumor growth is mediated by macrophages and mitigated by liposome-encapsulated alendronate. J. Control. Release 2018, 271, 139–148. [Google Scholar] [CrossRef]
- Shobaki, N.; Sato, Y.; Suzuki, Y.; Okabe, N.; Harashima, H. Manipulating the function of tumor-associated macrophages by siRNA-loaded lipid nanoparticles for cancer immunotherapy. J. Control. Release 2020, 325, 235–248. [Google Scholar] [CrossRef]
- Li, H.; Somiya, M.; Kuroda, S.i. Enhancing antibody-dependent cellular phagocytosis by Re-education of tumor-associated macrophages with resiquimod-encapsulated liposomes. Biomaterials 2021, 268, 120601. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tiruthani, K.; Li, S.; Hu, M.; Zhong, G.; Tang, Y.; Roy, S.; Zhang, L.; Tan, J.; Liao, C.; et al. mRNA Delivery of a Bispecific Single-Domain Antibody to Polarize Tumor-Associated Macrophages and Synergize Immunotherapy against Liver Malignancies. Adv. Mater. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, A.; Kumar, S.; Nandi, D.; Kulkarni, A. CSF1R- and SHP2-Inhibitor-Loaded Nanoparticles Enhance Cytotoxic Activity and Phagocytosis in Tumor-Associated Macrophages. Adv. Mater. 2019, e1904364. [Google Scholar] [CrossRef]
- Ramesh, A.; Brouillard, A.; Kumar, S.; Nandi, D.; Kulkarni, A. Dual inhibition of CSF1R and MAPK pathways using supramolecular nanoparticles enhances macrophage immunotherapy. Biomaterials 2019, 227, 119559. [Google Scholar] [CrossRef] [PubMed]
- Tu, B.; He, Y.; Chen, B.; Wang, Y.; Gao, Y.; Shi, M.; Liu, T.; Asrorov, A.M.; Huang, Y. Deformable liposomal codelivery of vorinostat and simvastatin promotes antitumor responses through remodeling tumor microenvironment. Biomater. Sci. 2020, 8, 7166–7176. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Sui, D.; Liu, M.; Zhang, H.; Liu, M.; Wang, S.; Zhao, D.; Sun, W.; Liu, M.; Luo, X.; et al. Targeted delivery of zoledronic acid through the sialic acid—Siglec axis for killing and reversal of M2 phenotypic tumor-associated macrophages—A promising cancer immunotherapy. Int. J. Pharm. 2020, 590, 119929. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, S.; Kim, J.E.; Lee, S.N.; Shin, I.W.; Shin, H.S.; Jin, S.M.; Noh, Y.W.; Kang, Y.J.; Kim, Y.S.; et al. Lyophilizable and Multifaceted Toll-like Receptor 7/8 Agonist-Loaded Nanoemulsion for the Reprogramming of Tumor Microenvironments and Enhanced Cancer Immunotherapy. ACS Nano 2019, 13, 12671–12686. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; He, X.; Feng, X. Developing neobavaisoflavone nanoemulsion suppresses lung cancer progression by regulating tumor microenvironment. Biomed. Pharm. 2020, 129, 110369. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Ali, N. Nanovaccine: An emerging strategy. Expert Rev. Vaccines 2021, 20, 1273–1290. [Google Scholar] [CrossRef] [PubMed]
- Jiao, M.; Zhang, P.; Meng, J.; Li, Y.; Liu, C.; Luo, X.; Gao, M. Recent advancements in biocompatible inorganic nanoparticles towards biomedical applications. Biomater. Sci. 2018, 6, 726–745. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhong, X.; Li, J.; Liu, Z.; Cheng, L. Inorganic nanomaterials with rapid clearance for biomedical applications. Chem. Soc. Rev. 2021, 50, 8669–8742. [Google Scholar] [CrossRef]
- Shah, A.; Dobrovolskaia, M.A. Immunological effects of iron oxide nanoparticles and iron-based complex drug formulations: Therapeutic benefits, toxicity, mechanistic insights, and translational considerations. Nanomedicine 2018, 14, 977–990. [Google Scholar] [CrossRef]
- Zanganeh, S.; Hutter, G.; Spitler, R.; Lenkov, O.; Mahmoudi, M.; Shaw, A.; Pajarinen, J.S.; Nejadnik, H.; Goodman, S.; Moseley, M.; et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016, 11, 986–994. [Google Scholar] [CrossRef]
- Jin, R.; Liu, L.; Zhu, W.; Li, D.; Yang, L.; Duan, J.; Cai, Z.; Nie, Y.; Zhang, Y.; Gong, Q.; et al. Iron oxide nanoparticles promote macrophage autophagy and inflammatory response through activation of toll-like Receptor-4 signaling. Biomaterials 2019, 203, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Z.; Xue, Y.; Wang, G.; Cheng, Y.; Pan, Y.; Zhao, S.; Hou, Y. Anti-tumor macrophages activated by ferumoxytol combined or surface-functionalized with the TLR3 agonist poly (I: C) promote melanoma regression. Theranostics 2018, 8, 6307–6321. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.M.; Sanz-Ortega, L.; Mulens-Arias, V.; Gutierrez, L.; Perez-Yague, S.; Barber, D.F. Superparamagnetic iron oxide nanoparticle uptake alters M2 macrophage phenotype, iron metabolism, migration and invasion. Nanomedicine 2016, 12, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Kim, S.; Wong, D.S.H.; Jung, H.J.; Lin, S.; Zou, K.; Li, R.; Li, G.; Dravid, V.P.; Bian, L. Remote Manipulation of Ligand Nano-Oscillations Regulates Adhesion and Polarization of Macrophages in vivo. Nano Lett. 2017, 17, 6415–6427. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-C.; An, Q.; Guo, M.; Xiao, Y.; Li, B.; Gao, F.; Wang, Y.; Li, J.; Wang, Y.; Liu, Y.; et al. Oxygen-independent free radical generation mediated by core-shell magnetic nanocomposites synergizes with immune checkpoint blockade for effective primary and metastatic tumor treatment. Nano Today 2021, 36, 101024. [Google Scholar] [CrossRef]
- Yang, Y.; Tian, Q.; Wu, S.; Li, Y.; Yang, K.; Yan, Y.; Shang, L.; Li, A.; Zhang, L. Blue light-triggered Fe2+-release from monodispersed ferrihydrite nanoparticles for cancer iron therapy. Biomaterials 2021, 271. [Google Scholar] [CrossRef]
- Chen, L.; Ma, X.; Dang, M.; Dong, H.; Hu, H.; Su, X.; Liu, W.; Wang, Q.; Mou, Y.; Teng, Z. Simultaneous T Cell Activation and Macrophage Polarization to Promote Potent Tumor Suppression by Iron Oxide-Embedded Large-Pore Mesoporous Organosilica Core-Shell Nanospheres. Adv. Healthc. Mater. 2019, 8, e1900039. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Zhao, S.K.; Wen, C.; Tian, R.; Lin, L.; Cai, B.; Sun, Y.; Kang, F.; Yang, Z.; He, L.; et al. Activating Macrophage-Mediated Cancer Immunotherapy by Genetically Edited Nanoparticles. Adv. Mater. 2020, 32, e2004853. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cao, S.; Liang, S.; Tan, C.H.; Luo, B.; Xu, X.; Saw, P.E. Differently Charged Super-Paramagnetic Iron Oxide Nanoparticles Preferentially Induced M1-Like Phenotype of Macrophages. Front. Bioeng. Biotechnol. 2020, 8, 537. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Lu, L.; Xue, C.; Liu, J.; He, Y.; Zhou, J.; Xia, Z.; Dai, L.; Luo, Z.; Mao, Y.; et al. Polarization of tumor-associated macrophage phenotype via porous hollow iron nanoparticles for tumor immunotherapy in vivo. Nanoscale 2020, 12, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Dong, Z.; Fu, Y.; Gong, T.; Deng, L.; Zhang, Z. Hyaluronic acid modified doxorubicin loaded Fe3O4 nanoparticles effectively inhibit breast cancer metastasis. J. Mater. Chem. B 2019, 7, 5861–5872. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Zhang, Y.; Dong, X.; Zhang, L.; Liu, M.D.; Li, B.; Zhang, M.K.; Feng, J.; Zhang, X.Z. Artificially Reprogrammed Macrophages as Tumor-Tropic Immunosuppression-Resistant Biologics to Realize Therapeutics Production and Immune Activation. Adv. Mater. 2019, 31, e1807211. [Google Scholar] [CrossRef]
- Sun, W.; Yang, J.; Hou, M.; Xie, S.; Xiong, L.; Li, B.; Zhang, C. A Nano “Immune-Guide” Recruiting Lymphocytes and Modulating the Ratio of Macrophages from Different Origins to Enhance Cancer Immunotherapy. Adv. Funct. Mater. 2021. [Google Scholar] [CrossRef]
- Costa da Silva, M.; Breckwoldt, M.O.; Vinchi, F.; Correia, M.P.; Stojanovic, A.; Thielmann, C.M.; Meister, M.; Muley, T.; Warth, A.; Platten, M.; et al. Iron Induces Anti-tumor Activity in Tumor-Associated Macrophages. Front. Immunol. 2017, 8, 01479. [Google Scholar] [CrossRef] [Green Version]
- Pazar, B.; Ea, H.K.; Narayan, S.; Kolly, L.; Bagnoud, N.; Chobaz, V.; Roger, T.; Liote, F.; So, A.; Busso, N. Basic calcium phosphate crystals induce monocyte/macrophage IL-1beta secretion through the NLRP3 inflammasome in vitro. J. Immunol. 2011, 186, 2495–2502. [Google Scholar] [CrossRef] [Green Version]
- He, X.Y.; Liu, B.Y.; Xu, C.; Zhuo, R.X.; Cheng, S.X. A multi-functional macrophage and tumor targeting gene delivery system for the regulation of macrophage polarity and reversal of cancer immunoresistance. Nanoscale 2018, 10, 15578–15587. [Google Scholar] [CrossRef]
- Jiang, H.; Guo, Y.; Wei, C.; Hu, P.; Shi, J. Nanocatalytic Innate Immunity Activation by Mitochondrial DNA Oxidative Damage for Tumor-Specific Therapy. Adv. Mater. 2021, e2008065. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.; Hu, M.; Wang, Z.; Lyu, L.; Zhang, W.; Li, J.; Yang, H.; Lin, J.; Xing, B. Enhanced Cellular Ablation by Attenuating Hypoxia Status and Reprogramming Tumor-Associated Macrophages via NIR Light-Responsive Upconversion Nanocrystals. Bioconjug. Chem. 2018, 29, 928–938. [Google Scholar] [CrossRef]
- Kang, H.; Zhang, K.; Wong, D.S.H.; Han, F.; Li, B.; Bian, L. Near-infrared light-controlled regulation of intracellular calcium to modulate macrophage polarization. Biomaterials 2018, 178, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wen, Y.; Chen, X.; Zhu, X.; Yu, Q.; Gong, Y.; Yuan, G.; Liu, J.; Qin, X. Inflammation-responsive functional Ru nanoparticles combining a tumor-associated macrophage repolarization strategy with phototherapy for colorectal cancer therapy. J. Mater. Chem. B 2019, 7, 6210–6223. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Lin, C.; He, Y.; Lu, L.; Xu, K.; Tao, B.; Xia, Z.; Zeng, R.; Mao, Y.; Luo, Z.; et al. Engineering of Cascade-Responsive Nanoplatform to Inhibit Lactate Efflux for Enhanced Tumor Chemo-Immunotherapy. ACS Nano 2020, 14, 14164–14180. [Google Scholar] [CrossRef] [PubMed]
- Leonard, F.; Curtis, L.T.; Ware, M.J.; Nosrat, T.; Liu, X.; Yokoi, K.; Frieboes, H.B.; Godin, B. Macrophage Polarization Contributes to the Anti-Tumoral Efficacy of Mesoporous Nanovectors Loaded with Albumin-Bound Paclitaxel. Front. Immunol. 2017, 8, 693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mocan, T.; Matea, C.; Tabaran, F.; Iancu, C.; Orasan, R.; Mocan, L. In Vitro Administration of Gold Nanoparticles Functionalized with MUC-1 Protein Fragment Generates Anticancer Vaccine Response via Macrophage Activation and Polarization Mechanism. J. Cancer 2015, 6, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, M.; Xu, F.; Chen, Y.; Chen, B.; Chang, Y.; Zhong, H.; Jin, H.; Huang, Y. Biomimetic albumin-modified gold nanorods for photothermo-chemotherapy and macrophage polarization modulation. Acta Pharm. Sin. B 2018, 8, 74–84. [Google Scholar] [CrossRef]
- Chang, C.C.; Dinh, T.K.; Lee, Y.A.; Wang, F.N.; Sung, Y.C.; Yu, P.L.; Chiu, S.C.; Shih, Y.C.; Wu, C.Y.; Huang, Y.D.; et al. Nanoparticle Delivery of MnO2 and Antiangiogenic Therapy to Overcome Hypoxia-Driven Tumor Escape and Suppress Hepatocellular Carcinoma. ACS Appl. Mater. Interfaces 2020, 12, 44407–44419. [Google Scholar] [CrossRef]
- Song, M.; Liu, T.; Shi, C.; Zhang, X.; Chen, X. Bioconjugated Manganese Dioxide Nanoparticles Enhance Chemotherapy Response by Priming Tumor-Associated Macrophages toward M1-like Phenotype and Attenuating Tumor Hypoxia. ACS Nano 2016, 10, 633–647. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Tang, J.; Li, C.; Lu, Y.; Cheng, L.; Liu, J. A targeting black phosphorus nanoparticle based immune cells nano-regulator for photodynamic/photothermal and photo-immunotherapy. Bioact. Mater. 2021, 6, 472–489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, X.; Ren, Y.; Cao, F.; Hou, L.; Zhang, Z. An in situ microenvironmental nano-regulator to inhibit the proliferation and metastasis of 4T1 tumor. Theranostics 2019, 9, 3580–3594. [Google Scholar] [CrossRef]
- Yang, L.; Sun, J.; Liu, Q.; Zhu, R.; Yang, Q.; Hua, J.; Zheng, L.; Li, K.; Wang, S.; Li, A. Synergetic Functional Nanocomposites Enhance Immunotherapy in Solid Tumors by Remodeling the Immunoenvironment. Adv. Sci. 2019, 6, 1802012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Zhou, L.; Wang, C.; Han, Y.; Lu, Y.; Liu, J.; Hu, X.; Yao, T.; Lin, Y.; Liang, S.; et al. Tumor-Targeted Drug and CpG Delivery System for Phototherapy and Docetaxel-Enhanced Immunotherapy with Polarization toward M1-Type Macrophages on Triple Negative Breast Cancers. Adv. Mater. 2019, 31, e1904997. [Google Scholar] [CrossRef]
- Zhang, Y.; Sha, W.; Zhang, X.; Cheng, M.; Wu, Q.; Wang, W.; Yuan, Z. Zwitterionic chitooligosaccharide-modified ink-blue titanium dioxide nanoparticles with inherent immune activation for enhanced photothermal therapy. Biomater. Sci. 2019, 7, 5027–5034. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, D.; Harada, N.; Hayashi, T.; Tahara, Y.; Momose, F.; Sawada, S.-i.; Mukai, S.-a.; Akiyoshi, K.; Shiku, H. Nanogel-Based Immunologically Stealth Vaccine Targets Macrophages in the Medulla of Lymph Node and Induces Potent Antitumor Immunity. ACS Nano 2014, 8, 9209–9218. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, D.; Seo, N.; Hayashi, T.; Tahara, Y.; Fujii, K.; Tawara, I.; Miyahara, Y.; Okamori, K.; Yagita, H.; Imoto, S.; et al. Antigen delivery targeted to tumor-associated macrophages overcomes tumor immune resistance. J. Clin. Investig. 2019. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, C.; Zhang, X.; Chen, G.; Hu, Q.; Li, H.; Wang, J.; Wen, D.; Zhang, Y.; Lu, Y.; et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat. Nanotechnol. 2019, 14, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Wang, L.; Shang, W.; Chen, J.; Chen, Z.; Xiong, F.; Wang, Z.; Tong, Z.; Wang, K.; Yang, L.; et al. Intravesical In Situ Immunostimulatory Gel for Triple Therapy of Bladder Cancer. ACS Appl. Mater. Interfaces 2020, 12, 54367–54377. [Google Scholar] [CrossRef]
- Nie, W.; Wu, G.; Zhang, J.; Huang, L.L.; Ding, J.; Jiang, A.; Zhang, Y.; Liu, Y.; Li, J.; Pu, K.; et al. Responsive Exosome Nano-bioconjugates for Synergistic Cancer Therapy. Angew. Chem. 2020, 59, 2018–2022. [Google Scholar] [CrossRef] [PubMed]
- Gunassekaran, G.R.; Poongkavithai Vadevoo, S.M.; Baek, M.C.; Lee, B. M1 macrophage exosomes engineered to foster M1 polarization and target the IL-4 receptor inhibit tumor growth by reprogramming tumor-associated macrophages into M1-like macrophages. Biomaterials 2021, 278, 121137. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Sun, B.; Li, H.; Dong, J.; Zhang, L.; Wang, L.; Wang, P.; Zhao, Y.; Chen, C. Polyhydroxylated metallofullerenols stimulate IL-1beta secretion of macrophage through TLRs/MyD88/NF-kappaB pathway and NLRP(3) inflammasome activation. Small 2014, 10, 2362–2372. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Fu, Y.; Li, C.; Wu, Z.; Cao, W.; Hu, X.; Sun, X.; He, W.; Cao, X.; Ling, D.; et al. Metal-Organic Framework Nanoparticles for Ameliorating Breast Cancer-Associated Osteolysis. Nano Lett. 2020, 20, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, L.; Zhou, L.; Yu, S.; Lan, Y.; Liang, Q.; Liu, J.; Cao, A.; Liu, Y. Tumor Microenvironment-Triggered Charge Reversal Polymetformin-Based Nanosystem Co-Delivered Doxorubicin and IL-12 Cytokine Gene for Chemo-Gene Combination Therapy on Metastatic Breast Cancer. ACS Appl. Mater. Interfaces 2020, 12, 45873–45890. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Gao, M.; Hu, Z.; Xu, T.; Chen, J.; Ma, Y.; Li, S.; Gu, Y. Synergistic ferroptosis and macrophage re-polarization using engineering exosome-mimic M1 nanovesicles for cancer metastasis suppression. Chemi. Eng. J. 2021, 409, 128217. [Google Scholar] [CrossRef]
- Han, S.; Wang, W.; Wang, S.; Yang, T.; Zhang, G.; Wang, D.; Ju, R.; Lu, Y.; Wang, H.; Wang, L. Tumor microenvironment remodeling and tumor therapy based on M2-like tumor associated macrophage-targeting nano-complexes. Theranostics 2021, 11, 2892–2916. [Google Scholar] [CrossRef] [PubMed]
- Mao, K.; Cong, X.; Feng, L.; Chen, H.; Wang, J.; Wu, C.; Liu, K.; Xiao, C.; Yang, Y.G.; Sun, T. Intratumoral delivery of M-CSF by calcium crosslinked polymer micelles enhances cancer immunotherapy. Biomater. Sci. 2019, 7, 2769–2776. [Google Scholar] [CrossRef] [PubMed]
- Tu, K.; Deng, H.; Kong, L.; Wang, Y.; Yang, T.; Hu, Q.; Hu, M.; Yang, C.; Zhang, Z. Reshaping Tumor Immune Microenvironment through Acidity-Responsive Nanoparticles Featured with CRISPR/Cas9-Mediated Programmed Death-Ligand 1 Attenuation and Chemotherapeutics-Induced Immunogenic Cell Death. ACS Appl. Mater. Interfaces 2020, 12, 16018–16030. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, M.; Sun, M.; Wu, S.; Zhang, H.; Dai, Y.; Wang, D. Multifunctional Nanoparticles Boost Cancer Immunotherapy Based on Modulating the Immunosuppressive Tumor Microenvironment. ACS Appl. Mater. Interfaces 2020, 12, 50734–50747. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Zou, Z.Z.; Wang, Q.H.; Li, J.; Jin, H.; Yin, Q.X.; Xing, D. Targeting and Specific Activation of Antigen-Presenting Cells by Endogenous Antigen-Loaded Nanoparticles Elicits Tumor-Specific Immunity. Adv. Sci. 2020, 7, 1900069. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, W.; Zhou, X.; Liu, M.; Hou, X.; Cheng, Z.; Chen, D. Development of dual-targeted nano-dandelion based on an oligomeric hyaluronic acid polymer targeting tumor-associated macrophages for combination therapy of non-small cell lung cancer. Drug Deliv. 2019, 26, 1265–1279. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Zhang, J.; Hou, T.; Yin, X.; Zhang, N. Selective targeting of tumor cells and tumor associated macrophages separately by twin-like core-shell nanoparticles for enhanced tumor-localized chemoimmunotherapy. Nanoscale 2019, 11, 13934–13946. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.; Zhang, Y.; Li, W.S.; Su, Z.; Fadhil, J.I.; Zhang, C. Iron chelated melanin-like nanoparticles for tumor-associated macrophage repolarization and cancer therapy. Biomaterials 2019, 225, 119515. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhou, Y.; Chen, X.; Ning, T.; Chen, H.; Guo, Q.; Zhang, Y.; Liu, P.; Zhang, Y.; Li, C.; et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials 2021, 268, 120546. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lee, S.M.; Yi, C.; Li, C.W. Controllable synthesis of functional nanoparticles by microfluidic platforms for biomedical applications—A review. Lab. Chip 2017, 17, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Li, P.; Feng, J.; Lin, Z.; Chen, X.; Yang, N.; Wang, L.; Liu, D. Screening Therapeutic Agents Specific to Breast Cancer Stem Cells Using a Microfluidic Single-Cell Clone-Forming Inhibition Assay. Small 2019, e1901001. [Google Scholar] [CrossRef] [PubMed]
- Tavanti, F.; Menziani, M.C. Computational Insight on the Interaction of Common Blood Proteins with Gold Nanoparticles. Int. J. Mol. Sci. 2021, 22, 8722. [Google Scholar] [CrossRef] [PubMed]
- Reifarth, M.; Hoeppener, S.; Schubert, U.S. Uptake and Intracellular Fate of Engineered Nanoparticles in Mammalian Cells: Capabilities and Limitations of Transmission Electron Microscopy-Polymer-Based Nanoparticles. Adv. Mater. 2018, 30, 1703704. [Google Scholar] [CrossRef] [PubMed]
- Perez-Campana, C.; Gomez-Vallejo, V.; Martin, A.; San Sebastian, E.; Moya, S.E.; Reese, T.; Ziolo, R.F.; Llop, J. Tracing nanoparticles in vivo: A new general synthesis of positron emitting metal oxide nanoparticles by proton beam activation. Analyst 2012, 137, 4902–4906. [Google Scholar] [CrossRef] [PubMed]
- Shang, M. Microfluidic modelling of the tumor microenvironment for anti-cancer drug development. Lab. Chip 2019, 19, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Klichinsky, M.; Ruella, M.; Shestova, O.; Lu, X.M.; Best, A.; Zeeman, M.; Schmierer, M.; Gabrusiewicz, K.; Anderson, N.R.; Petty, N.E.; et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat. Biotechnol. 2020, 38, 947–953. [Google Scholar] [CrossRef] [PubMed]




| Main Matrix | Therapeutic Agent | Tumor Model | Reference |
|---|---|---|---|
| Polymeric nanoparticles | |||
| PLGA | Natural Killer cell membrane | 4T1 | [49] |
| PLGA | Iron oxide, M1 cell membrane | 4T1 | [50] |
| PLGA | CpG | B16F10 | [126] |
| PLGA | R848, Poly (I:C) | TC-1 | [51] |
| PGA | M-CSF | B16 | [127] |
| PEI | Hyaluronic acid, miR125b | Nonsmall cell lung tumor | [58] |
| β-cyclodextrin | R848 | MC38, B16F10 | [24] |
| Lignin | R848 | 4T1 | [52] |
| Poly (ε-caprolactone) (PCL), Sulfate protoporphyrin | R837 | 4T1 | [53] |
| PQDEA | IL-12 plasmid | KPC, BPD6, 4T1 | [55] |
| PEI | CpG | MC38, B16F10 | [54] |
| PEI | Paclitaxel, CRISPR/Cas9-Cdk5 | CT26, B16F10 | [128] |
| Trimethyl chitosan | siRNA blocking VEGF, PIGF | 4T1 | [57] |
| Poly-L-lysines | Zoledronic acid | 4T1 | [60] |
| Poly (ethylene glycol) -b-PHEP (PEG-b-PHEP) | BLZ-945 | 4T1 | [61] |
| PEI-PCL | Shikonin | CT26 | [129] |
| PCL-Hyd-PEG | CpG, anti-CD80 antibody | 4T1, B16F10 | [130] |
| Polymetformin | IL-12 plasmid, hyaluronic acid | 4T1 | [124] |
| Nanomicelles named as QHMF | Hyaluronic acid | A549 | [131] |
| Lipid-based nanoparticles | |||
| Lipid nanoparticles | siRNA blocking STAT3, HIF-1α | OS-RC-2 | [71] |
| Lipid nanoparticles | IMD-0354 | Hepa1-6 | [132] |
| Liposome | R848 | WiDr | [72] |
| Liposome | Bispecific antibody (binds CCL2, CCL5) | HCC, KPC liver tumor model | [73] |
| Liposome | BLZ945, anti-CD206 | 4T1, B16F10 | [74] |
| Liposome | Alendronate | TC-1 | [70] |
| Liposome | BLZ-945, Selumetinib | 4T1 | [75] |
| Liposome | Zoledronic acid | S180 | [77] |
| Lipid nanoemulsion | R848, R837 | B16F10-OVA, TC-1 | [78] |
| Lipid nanoemulsion | Neobavaisoflavone | A549 | [79] |
| Inorganic nanoparticles | |||
| Lanthanide-doped upconversion nanocrystals | Hyaluronic acid | / | [102] |
| Mesoporous silica with upconversion materials | Calcium ion | / | [103] |
| Iron oxide | Iron oxide, membrane blocking CD47-SIRPα | 4T1, B16F10 | [92] |
| Iron oxide | Iron oxide | / | [85] |
| Iron Oxide | Iron oxide | HT1080 | [93] |
| Iron Oxide | Iron Oxide, 3-MA | MDA-MB-231 | [94] |
| Iron Oxide | Iron oxide | 4T1 | [89] |
| Iron Oxide | Iron oxide | 4T1 | [90] |
| Iron Oxide | Iron oxide, hyaluronic acid | 4T1 | [96] |
| Iron Oxide | Iron oxide, hyaluronic acid | 4T1 | [97] |
| Iron Oxide | Iron oxide, poly (I:C) | B16F10 | [86] |
| Iron Oxide | Iron oxide and hyaluronic acid stimulated macrophages | 4T1 | [95] |
| Berlin blue | Hyaluronic acid | 4T1 | [112] |
| Iron Oxide | Iron Oxide | E.G7-OVA | [91] |
| Rubidium | BLZ-945 | CT26 | [104] |
| Iron chelated nanoparticles | Iron | CT26, 4T1 | [133] |
| Silica | Ferrous ion, rubidium ion | PANC-1 | [101] |
| Silica | siRNA blocking MCT-4 | 4T1, B16F10 | [105] |
| Calcium carbonate | Hyaluronic acid, IL-12 plasmid | / | [100] |
| Copper sulphide | CpG | 4T1 | [114] |
| Titanium dioxide | Chitooligosaccharide | H22 | [115] |
| Black phosphorus | Hyaluronic acid | 4T1 | [111] |
| Layered double hydroxides | miR155 | TC-1 | [113] |
| Other nanoparticles | |||
| Cholsterol pullulan nanogel | CpG | CMS5a | [117] |
| Fibrin gel (containing calcium carbonate) | Anti-CD47 antibody | B16F10 | [118] |
| Gel containing iron oxide and gold nanorod | Iron oxide | MB49 | [119] |
| Exosome | siRNA blocking galectin-9 | PANC-2 | [134] |
| Exosome | Exosome from M1 | 4T1 | [120] |
| Metal-Organic framework | CpG | MDA-MB-231 | [123] |
| Nanoparticles obtained from iron-oxide-stimulated macrophages | Iron oxide | 4T1 | [125] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, L.; Gu, H. Emerging Nanoparticle Strategies for Modulating Tumor-Associated Macrophage Polarization. Biomolecules 2021, 11, 1912. https://doi.org/10.3390/biom11121912
Shi L, Gu H. Emerging Nanoparticle Strategies for Modulating Tumor-Associated Macrophage Polarization. Biomolecules. 2021; 11(12):1912. https://doi.org/10.3390/biom11121912
Chicago/Turabian StyleShi, Lu, and Hongchen Gu. 2021. "Emerging Nanoparticle Strategies for Modulating Tumor-Associated Macrophage Polarization" Biomolecules 11, no. 12: 1912. https://doi.org/10.3390/biom11121912