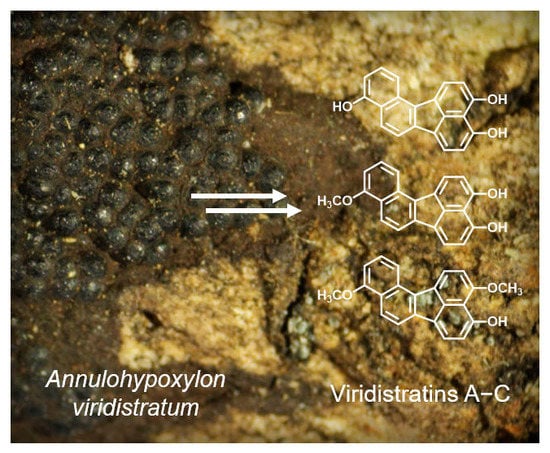
Viridistratins A−C, Antimicrobial and Cytotoxic Benzo[j]fluoranthenes from Stromata of Annulohypoxylon viridistratum (Hypoxylaceae, Ascomycota)
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Fungal Material
2.3. Extraction and Isolation
2.4. Antimicrobial Activity Assay
2.5. Cytotoxicity Assay
2.6. Spectral Data
2.6.1. Viridistratin A (1)
2.6.2. Viridistratin B (2)
2.6.3. Viridistratin C (3)
3. Results
3.1. Structure Elucidation of Viridistratins A−C (1−3)
3.2. Antibacterial, Antifungal and Cytotoxic Activities of Compounds 1−5
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wendt, L.; Sir, E.B.; Kuhnert, E.; Heitkämper, S.; Lambert, C.; Hladki, A.I.; Romero, A.I.; Luangsa-ard, J.J.; Srikitikulchai, P.; Peršoh, D.; et al. Resurrection and emendation of the Hypoxylaceae, recognised from a multigene phylogeny of the Xylariales. Mycol. Prog. 2018, 17, 115–154. [Google Scholar] [CrossRef] [Green Version]
- Kuhnert, E.; Sir, E.B.; Lambert, C.; Hyde, K.D.; Hladki, A.I.; Romero, A.I.; Rohde, M.; Stadler, M. Phylogenetic and chemotaxonomic resolution of the genus Annulohypoxylon (Xylariaceae) including four new species. Fungal Divers. 2017, 85, 1–43. [Google Scholar] [CrossRef]
- Hsieh, H.-M.; Ju, Y.-M.; Rogers, J.D. Molecular phylogeny of Hypoxylon and closely related genera. Mycologia 2017, 97, 844–865. [Google Scholar] [CrossRef]
- Helaly, S.E.; Thongbai, B.; Stadler, M. Diversity of biologically active secondary metabolites from endophytic and saprotrophic fungi of the ascomycete order Xylariales. Nat. Prod. Rep. 2018, 35, 992–1014. [Google Scholar] [CrossRef] [PubMed]
- Stadler, M.; Læssøe, T.; Fournier, J.; Decock, C.; Schmieschek, B.; Tichy, H.-V.; Peršoh, D. A polyphasic taxonomy of Daldinia (Xylariaceae). Stud. Mycol. 2014, 77, 1–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stadler, M.; Fournier, J.; Granmo, A.; Beltrán-Tejera, E. The “red Hypoxylons” of the temperate and subtropical Northern hemisphere. N. Am. Fungi 2008, 3, 73–125. [Google Scholar] [CrossRef] [Green Version]
- Quang, D.N.; Hashimoto, T.; Nomura, Y.; Wollweber, H.; Hellwig, V.; Fournier, J.; Stadler, M.; Asakawa, Y. Cohaerins A and B, azaphilones from the fungus Hypoxylon cohaerens, and comparison of HPLC-based metabolite profiles in Hypoxylon sect. Annulata. Phytochemistry 2005, 66, 797–809. [Google Scholar] [CrossRef]
- Surup, F.; Narmani, A.; Wendt, L.; Pfütze, S.; Kretz, R.; Becker, K.; Menbrivès, C.; Giosa, A.; Elliott, M.; Petit, C.; et al. Identification of fungal fossils and novel azaphilone pigments in ancient carbonised specimens of Hypoxylon fragiforme from forest soils of Châtillon-sur-Seine (Burgundy). Fungal Divers. 2018, 92, 345–356. [Google Scholar] [CrossRef]
- Daranagama, D.A.; Hyde, K.D.; Sir, E.B.; Thambugala, K.M.; Tian, Q.; Samarakoon, M.C.; McKenzie, E.H.C.; Jayasiri, S.C.; Tibpromma, S.; Bhat, J.D.; et al. Towards a natural classification and backbone tree for Graphostromataceae, Hypoxylaceae, Lopadostomataceae and Xylariaceae. Fungal Divers. 2018, 88, 1–165. [Google Scholar] [CrossRef]
- Wibberg, D.; Stadler, M.; Lambert, C.; Bunk, B.; Spröer, C.; Rückert, C.; Kalinowski, J.; Cox, R.J.; Kuhnert, E. High quality genome sequences of thirteen Hypoxylaceae (Ascomycota) strengthen the phylogenetic family backbone and enable the discovery of new taxa. Fungal Divers. 2020, in press. [Google Scholar] [CrossRef]
- Kuhnert, E.; Surup, F.; Halecker, S.; Stadler, M. Minutellins A–D, azaphilones from the stromata of Annulohypoxylon minutellum (Xylariaceae). Phytochemistry 2017, 137, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Sudarman, E.; Kuhnert, E.; Hyde, K.; Sir, E.; Surup, F.; Stadler, M. Truncatones A–D, benzo[j]fluoranthenes from Annulohypoxylon species (Xylariaceae, Ascomycota). Tetrahedron 2016, 72, 6450–6454. [Google Scholar] [CrossRef]
- Koyama, K.; Kuramochi, D.; Kinoshita, K.; Takahashi, K. Hypoxylonols A and B, novel reduced benzo[j]fluoranthene derivatives from the mushroom Hypoxylon truncatum. J. Nat. Prod. 2002, 65, 1489–1490. [Google Scholar] [CrossRef] [PubMed]
- Fukai, M.; Tsukada, M.; Miki, K.; Suzuki, T.; Sugita, T.; Kinoshita, K.; Takahashi, K.; Shiro, M.; Koyama, K. Hypoxylonols C-F, benzo[j]fluoranthenes from Hypoxylon truncatum. J. Nat. Prod. 2011, 75, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Helaly, S.E.; Ashrafi, S.; Teponno, R.B.; Bernecker, S.; Dababat, A.A.; Maier, W.; Stadler, M. Nematicidal cyclic lipodepsipeptides and a xanthocillin derivative from a phaeosphariaceous fungus parasitizing eggs of the plant parasitic nematode Heterodera filipjevi. J. Nat. Prod. 2018, 81, 2228–2234. [Google Scholar] [CrossRef]
- Sandargo, B.; Michehl, M.; Praditya, D.; Steinmann, E.; Stadler, M.; Surup, F. Antiviral meroterpenoid rhodatin and sesquiterpenoids rhodocoranes A-E from the Wrinkled Peach Mushroom, Rhodotus palmatus. Org. Lett. 2019, 21, 3286–3289. [Google Scholar] [CrossRef]
- Eisenman, H.C.; Casadevall, A. Synthesis and assembly of fungal melanin. Appl. Microbiol. Biotechnol. 2012, 93, 931–940. [Google Scholar] [CrossRef] [Green Version]
- Cox, R.J.; Simpson, T.J. Chapter 3 Fungal Type I Polyketide Synthases. In Methods in Enzymology; Elsevier Inc.: Amsterdam, The Netherlands, 2009; Volume 459, pp. 49–78. ISBN 9780123745910. [Google Scholar]
- Stadler, M.; Lambert, C.; Wibberg, D.; Kalinowski, J.; Cox, R.J.; Kolařík, M.; Kuhnert, E. Intragenomic polymorphisms in the ITS region of high-quality genomes of the Hypoxylaceae (Xylariales, Ascomycota). Mycol. Prog. 2020, 19, 235–245. [Google Scholar] [CrossRef] [Green Version]
- Quang, D.N.; Hashimoto, T.; Tanaka, M.; Baumgartner, M.; Stadler, M.; Asakawa, Y. Chemical constituents of the ascomycete Daldinia concentrica. J. Nat. Prod. 2002, 65, 1869–1874. [Google Scholar] [CrossRef]
- Gu, W.; Ge, H.M.; Song, Y.C.; Ding, H.; Zhu, H.L.; Zhao, X.A.; Tan, R.X. Cytotoxic benzo[j]fluoranthene metabolites from Hypoxylon truncatum IFB-18, an endophyte of Artemisia annua. J. Nat. Prod. 2007, 70, 114–117. [Google Scholar] [CrossRef]
- Lee, D.; Choi, P.; Hwang, B.S.; Kim, T.; Kim, Y.; Kim, J.-C.; Song, J.H.; Park, J.S.; Hwang, G.S.; Yamabe, N.; et al. Protective effect of hypoxylonol C and 4,5,4′,5′-tetrahydroxy-1,1′-binaphthyl isolated from Annulohypoxylon annulatum against streptozotocin-induced damage in INS-1 cells. Bioorg. Chem. 2019, 90, 103053. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Hwang, B.S.; Choi, P.; Kim, T.; Kim, Y.; Song, B.G.; Yamabe, N.; Hwang, G.S.; Kang, K.S.; Ham, J. Hypoxylonol F Isolated from Annulohypoxylon annulatum improves insulin secretion by regulating pancreatic β-cell metabolism. Biomolecules 2019, 9, 335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S. Department of Health & Human Services, Public Health Service Agency for Toxic Substances and Disease Registry. Toxicological Profile for Polycyclic Aromatic Hydrocarbons, U.S. Department of Health & Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Washington, D.C., August, 1985. J. Toxicol. Cutan. Ocul. Toxicol. 1999, 18, 141–147. [Google Scholar]
- Bhaganna, P.; Volkers, R.J.M.; Bell, A.N.W.; Kluge, K.; Timson, D.J.; McGrath, J.W.; Ruijssenaars, H.J.; Hallsworth, J.E. Hydrophobic substances induce water stress in microbial cells. Microb. Biotechnol. 2010, 3, 701–716. [Google Scholar] [CrossRef] [Green Version]
- Cray, J.A.; Stevenson, A.; Ball, P.; Bankar, S.B.; Eleutherio, E.C.A.; Ezeji, T.C.; Singhal, R.S.; Thevelein, J.M.; Timson, D.J.; Hallsworth, J.E. Chaotropicity: A key factor in product tolerance of biofuel-producing microorganisms. Curr. Opin. Biotechnol. 2015, 33, 228–259. [Google Scholar] [CrossRef]


| pos 1 | 1 | 2 | 3 | |||||
|---|---|---|---|---|---|---|---|---|
| δC, mult 2 | δH, mult 2 | δC, mult | δH, mult | δC, mult | δH, mult | |||
| 1 | 155.1, C | 156.8, C | 156.2, C | |||||
| 2 | 108.2, CH | 6.90, d (7.53) | 103.6, CH | 6.92, d (7.63) | 104.0, CH | 6.97, m | ||
| 3 | 128.1, CH | 7.42, t (2 × 7.96) | 127.5, CH | 7.52, t (2 × 8.09) | 127.9, CH | 7.56, t (2 × 7.96) | ||
| 4 | 116.4, CH | 8.22, d (8.39) | 116.8, CH | 8.29, d (8.39) | 116.6, CH | 8.30, d (8.39) | ||
| 5 | 132.5, C | 131.5, C | 130.9, C | |||||
| 6 | 133.5, C | 133.0, C | 131.6, C | |||||
| 7 | 129.9, C | 129.3, C | 129.2, C | |||||
| 8 | 127.4, CH | 8.45, m | 126.9, CH | 8.45, d (7.78) | 126.8, CH | 8.56, d (8.17) | ||
| 9 | 111.2, CH | 7.06, d (7.53) | 110.8, CH | 7.06, d (7.78) | 106.7, CH | 7.13, d (7.96) | ||
| 10 | 156.2, C | 155.7, C | 158.1, C | |||||
| 11 | 112.6, C | 112.1, C | 112.7, C | |||||
| 12 | 156.8, C | 156.3, C | 156.8, C | |||||
| 13 | 110.9, CH | 7.01, d (7.53) | 110.5, CH | 7.01, d (7.63) | 112.0, CH | 7.00, m | ||
| 14 | 124.3, CH | 8.06, br s | 123.8, CH | 8.05, m | 124.6, CH | 8.09, m | ||
| 15 | 129.1, C | 128.5, C | 126.7, C | |||||
| 16 | 137.9, C | 137.5, C | 137.3, C | |||||
| 17 | 119.0, CH | 8.06, m | 118.9, CH | 8.06, m | 119.2, CH | 8.09, m | ||
| 18 | 121.8, CH | 8.26, d (8.60) | 120.8, CH | 8.22, m | 120.7, CH | 8.17, d (8.60) | ||
| 19 | 125.4, C | 125.4, C | 124.5, C | |||||
| 20 | 135.2, C | 134.7, C | 134.1, C | |||||
| 21 | 55.5, CH3 | 4.04, s | 56.0, CH3 | 4.02, s | ||||
| 22 | 56.8, CH3 | 4.09, s | ||||||
| Test Organism | Minimum Inhibitory Concentration (MIC) (µg/mL) | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Reference | |
| Bacillus subtilis | 33.3 | 16.7 | >66.7 | >66.7 | 66.7 | 8.3 1 |
| Staphylococcus aureus | 66.7 | 16.7 | >66.7 | >66.7 | >66.7 | 0.4 2 |
| Micrococcus luteus | 16.7 | 8.3 | 66.7 | 33.3 | 16.7 | 0.8 2 |
| Chromobacterium violaceum | 66.7 | 66.7 | >66.7 | >66.7 | >66.7 | 0.1 2 |
| Escherichia coli | >66.7 | >66.7 | >66.7 | >66.7 | >66.7 | 1.7 2 |
| Pseudomonas aeruginosa | >66.7 | >66.7 | >66.7 | >66.7 | >66.7 | 0.4 3 |
| Mycolicibacterium smegmatis | >66.7 | 33.3 | >66.7 | >66.7 | >66.7 | 3.3 4 |
| Candida albicans | >66.7 | >66.7 | >66.7 | >66.7 | >66.7 | 66.7 5 |
| Schizosaccharomyces pombe | 66.7 | 33.3 | >66.7 | >66.7 | >66.7 | 33.3 5 |
| Mucor hiemalis | 66.7 | 4.2 | >66.7 | >66.7 | 66.7 | 66.7 5 |
| Pichia anomala | 66.7 | 33.3 | >66.7 | >66.7 | >66.7 | 66.7 5 |
| Rhodotorula glutinis | 33.3 | 33.3 | >66.7 | >66.7 | 66.7 | 16.7 5 |
| Cell Line | Cytotoxicity (IC50) (µM) | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Reference 1 | ||
| L929 | mouse fibroblasts | 12.7 | 17.2 | 61.0 | 10.4 | 16.3 | 0.00006 |
| KB 3.1 | human endocervical adenocarcinoma (AC) | 28.3 | 17.2 | 85.4 | 44.0 | 30.1 | 0.00079 |
| PC-3 | human prostate AC | 23.7 | 9.9 | n.d. | 44.0 | 25.6 | 0.00008 |
| SK-OV-3 | human ovary AC | 56.7 | 7.3 | n.d. | 66.0 | 33.1 | 0.00034 |
| MCF-7 | human breast AC | 9.7 | 5.1 | n.d. | 8.8 | 7.8 | 0.00012 |
| A431 | human squamous AC | 8.7 | 1.1 | n.d. | 5.7 | 16.3 | 0.00005 |
| A549 | human lung carcinoma | 20.0 | 1.4 | n.d. | 17.0 | 27.1 | 0.00008 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becker, K.; Wessel, A.-C.; Luangsa-ard, J.J.; Stadler, M. Viridistratins A−C, Antimicrobial and Cytotoxic Benzo[j]fluoranthenes from Stromata of Annulohypoxylon viridistratum (Hypoxylaceae, Ascomycota). Biomolecules 2020, 10, 805. https://doi.org/10.3390/biom10050805
Becker K, Wessel A-C, Luangsa-ard JJ, Stadler M. Viridistratins A−C, Antimicrobial and Cytotoxic Benzo[j]fluoranthenes from Stromata of Annulohypoxylon viridistratum (Hypoxylaceae, Ascomycota). Biomolecules. 2020; 10(5):805. https://doi.org/10.3390/biom10050805
Chicago/Turabian StyleBecker, Kevin, Anna-Charleen Wessel, J. Jennifer Luangsa-ard, and Marc Stadler. 2020. "Viridistratins A−C, Antimicrobial and Cytotoxic Benzo[j]fluoranthenes from Stromata of Annulohypoxylon viridistratum (Hypoxylaceae, Ascomycota)" Biomolecules 10, no. 5: 805. https://doi.org/10.3390/biom10050805