1. Introduction
Recently, the World Health Organization (WHO) expected that worldwide death triggered by antibacterial-resistant pathogens would extend to 10 million people annually in 2050 [
1]. A prospective study of hospital-acquired infections within four tertiary care university hospitals in Egypt within 2 years revealed that 86% and 94.6% of Gram-positive and Gram-negative isolates, respectively, were multidrug resistant (MDR) [
2].
Studies indicate that antibiotic resistance is a high priority, markedly in the developing countries, with the exposure to microbial infections at hospitals being a major concern that requires careful study. Recently, the WHO has published a list of “global priority pathogens” (GPP) and indicated the urgent need for the discovery of potent antibacterial agents that can deal with these resistant pathogens [
2]. MDR
Klebsiella pneumoniae, Shiga toxin-producing
Escherichia coli O157 (STEC O157), Methicillin-resistant
Staphylococcus aureus (MRSA), and
Bacillus cereus are considered some of the most serious nosocomial pathogens causing hospital health care problems worldwide. According to the WHO,
K. pneumoniae,
E. coli, and
B. cereus are among the critical pathogens while
S. aureus is among the high priority pathogens [
3].
STEC O157 is one of the major human and animal pathogens worldwide. It can cause several clinical manifestations ranging from stomach cramps and bloody diarrhea to pyelonephritis and hemolytic uremic syndrome (HUS) [
2,
4]. Additionally, based on the STEC O157 infected humans and animals’ similarity analysis isolates, cross-infection between different hosts was obvious [
5]. On the other hand,
K. pneumoniae is the most common clinical pathogen causing nosocomial infections [
6]. Regarding the blood infection pathogens,
K. pneumoniae is second only to STEC O157, and carbapenem-resistant
K. pneumoniae that prompts blood infection has a great death rate [
7].
The antibacterial research community must incessantly search together to develop new and safer medications to resolve the increasing drug resistance, along with toxicity challenges [
8,
9,
10]. Historically, natural compounds have been a rich source of antibacterial therapies [
11,
12,
13,
14]. One class of compounds that should be explored as an anti-bacterial is the alkaloids, such as carbazoles, where the Apocynaceae family presents a high content, particularly in the seeds and latex, and consequently should be explored [
15].
Family Apocynaceae (periwinkle family or dogbane family) is one of the largest plant families, with roughly 400 genera and 1500 species. Apocynaceae is a flowering plants family, with trees, shrubs, herbs, or climbers [
15].
An example of a plant from the Apocynaceae family is
Ochrosia. This genus is of about 36 species, which are used as cytotoxic, anti-inflammatory, antioxidant, and anti-malarial agents.
Ochrosia ellpitica Labill. is a small tropical evergreen shrub native to Oceania in the tribe Vinceae of the subfamily Rauvolfioideae. More than 40 different indole alkaloids have been recognized from the
Ochrosia species [
16,
17]. They exhibited several biological activities, such as central nervous system stimulant, cytotoxic, antiseptic, and hypotensive [
15,
18,
19].
In previous work, we were informed that ellipticine, an alkaloid isolated from
Ochrosia ellpitica Labill. leaves, stopped topoisomerase IV activity, thus killing the multidrug-resistant
E. coli, and revealed broad-spectrum antimicrobial bioactivities against several bacteria (
Staphylococcus aureus,
Klebsiella pneumoniae,
Listeria monocytogenes,
Pseudomonas aeruginosa, with
Salmonella typhi) [
20]. Additionally, ellipticines showed the most potent antibacterial activity against
E. coli and
S. aureus compared to the studied compounds (MIC < 2 mg/L) with an intact efflux mechanism of action, resulting in their development as effective antibacterial drugs [
21]. Among the derivatives of ellipticine tested in this research, ellipticines were discovered with OMe at position 9, which can be beneficial for their antimicrobial action by damaging the bacterial membrane [
21]. In addition, the alkaloidal extract of
Ochrosia oppositifolia was previously examined using disc-diffusion and the calculation of MIC procedures. The results revealed that the highest inhibitory activity against
S. aureus demonstrated by 10 mg/mL of the investigated leaves and stembark extracts (10.0 ± 2.8 mm and 10.5 ± 2.1 mm, respectively) and the roots against MRSA was 14.0 ± 2.8 mm. Furthermore, the MIC of the mentioned samples against
B. subtilis,
Salmonella thyphimurium, and
Serratia marcescens and the root extract against
Vibrio fluvialis were 3.75 mg/mL, 0.94 mg/mL, and 0.12 mg/mL, respectively [
22].
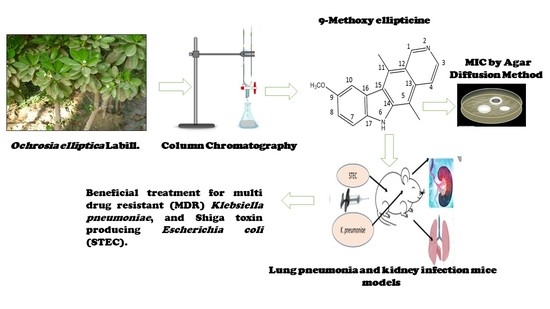
In this work, 9-methoxyellipticine (9-methoxy-5,11-dimethyl-6
H-pyrido [4,3-b]carbazole) (
Figure 1) was isolated from
Ochrosia ellpitica Labill. roots and investigated for its antibacterial potential in vitro towards four of the most common nosocomial agents:
K. pneumoniae, STEC O157, MRSA, and
B. cereus. It is worth mentioning that no previous data have been reported about the antibacterial potential of the isolated compound in vivo against
K. pneumoniae and STEC O157.
3. Results and Discussion
9-methoxyellipticine (C
18H
16N
2O, 276.3) is a naturally occurring carbazole alkaloid isolated from
O. elliptica, which displayed antitumoral, immunosuppressive, and trypanocidal activities [
33]. In this study, 9-methoxyellipticine demonstrated favorable activities against STEC, MRSA, MDR
K. pneumoniae, and MDR
B. cereus either alone or in combination with a commercial antibiotic when tested with the disc diffusion method.
Regarding STEC O157, GEN showed a strong antibacterial activity with an inhibition zone of 20.0 ± 1.00 mm (
Figure 2A and
Table 1), while penicillin (PEN) was almost inactive.
One of the highly beneficial methods to guard against the multidrug-resistant bacterial infection is the drug combination [
34]. Captivatingly, an excellent activity was detected using 9-methoxyellipticine and both antibiotics—GEN and AK—providing 25.0 ± 0.577 mm and 23.0 ± 1.00 mm as zones of inhibition, correspondingly (
Figure 2B and
Table 1). Regarding the MDR
K. pneumoniae, though the strain was resistant to GEN and AK, it was sensitive to 9-methoxyellipticine alone (21 ± 0.577 mm) and in combination with GEN (23.0 ± 1.00 mm) and AK (23.0 ± 1.00 mm) (
Figure 2B and
Table 1). One possible explanation for that effect might be that 9-methoxyellipticine could result in bacterial cell membrane damage [
21], thus improving the efficiency of the antibiotics.
Likewise, 9-methoxyellipticine alone showed an antibacterial inhibition zone of (21 ± 0.577 mm) against MDR
K. pneumoniae which was GEN resistant (
Table 2), while against STEC O157, 9-methoxyellipticine alone showed an inhibition zone of (18 ± 1 mm). On the other hand, 9-methoxyellipticine was ineffective against MRSA and MDR
B. cereus (3 ± 1.00 mm and 5 ± 0.577 mm, respectively).
For MRSA, the combination of 9-methoxyellipticine and VAN revealed a synergetic antibacterial impact as implied from the growth in the inhibitory zone (from 18 mm to 23 mm) (
Table 2). However, the combination of 9-methoxyellipticine with PEN was ineffective in reversing the resistance of both microbes.
The MIC quantities of 9-methoxyellipticine were 512 µg/mL (for
E. coli) and 256 µg/mL (for MDR K.
pneumoniae) (
Table 2). The compound showed weak activity on Gram-positive bacteria, with an MIC higher than 2048 μg/mL for both MRSA and
B. cereus (
Table 2).
Fortunately, the results are promising for the in vitro bacterial susceptibility test, where against STEC O157 and Klebsiella pneumoniae carbapenemase (KPC), 9-methoxyellipticine showed potent bactericidal activity either alone or in combination with commercial antibiotics, such as PEN and GEN, with which 9-methoxyellipticine showed potent synergistic activity.
MIC results for
K. pneumoniae and STEC O157 were lower than those reported previously by Lu et al. [
20] in which MIC ranged from 0.5 and 4 mg/L. This is perhaps due to the difference in chemical structure between 9-methoxyellipticine and ellipticine HCl. This proves that presence of the methoxy moiety is crucial for a potent bactericidal activity.
Upon testing bactericidal activity against MRSA, it was resistant to 9-methoxyellipticine alone but was sensitive when combined with VAN and showed a remarkable synergistic effect by increasing the zone of inhibition from 18.0 ± 1.00 mm to 23.0 ± 1.00 mm. On the other hand, B. cereus was totally resistant to 9-methoxyellipticine either alone or when combined with VAN.
According to our results, VAN is recommended to be co-administered with 9-methoxyellipticine during MRSA treatment, highlighting the importance of drug combination when facing MDR strains.
The most common bacterial infections are signified by inflammation caused by lipopolysaccharide (LPS), which is the principle outer membrane component in Gram-negative bacteria and the main role player in the pathophysiological processes of septicemia, toxic shock syndrome, and inflammation [
35,
36,
37].
Nowadays, each infection treatment protocol must contain antibacterial as well as anti-inflammatory agents [
20,
38]. Previous studies have reported the anti-inflammatory effect of ellipticine against NF-κB [
39]. Moreover, the ellipticine that was isolated from the Ochrosia species was labeled as an anti-HIV, anti-inflammatory, and anticancer agent [
40,
41,
42]. The aforementioned studies demonstrated that ellipticine could notably decrease the bacterial loads and tissue destruction in colistin-resistant
E. coli-infected mice [
20], which is in agreement with our report. In our study, 9-methoxyellipticine could effectively lower the levels of TNF-α and IgM, and as a result it could effectively lower lung and kidney damage in
K. pneumoniae and STEC O157 infected mice, respectively. The positive control groups died prior to the end of the experiment. Analysis experimentation established that all mice were infested with great CFU loads, indicating that mortality rate was triggered by infection.
An assessment of TNF-α and IgM levels was performed to prove that
E. coli and
K. pneumoniae provoked proinflammatory conditions. However, treatment with 9-methoxyellipticine successfully led to normal levels of TNF-α and IgM being exhibited (
Figure 3A–D). In a previous survey, ellipticine notably reduced the levels of pro-inflammatory cytokines and chemokines, resulting in a dramatic reduction in the inflammatory cell infiltration [
43]. These findings agreed with our study, as was revealed from biochemical markers and histopathological images and scoring.
Furthermore, natural compounds that function as immunomodulators are needed for improving the immune system, especially in infectious disorders. Ellipticine was reported to have a potent immunostimulant activity [
44]. Our report documented the role of alkaloids in immunity, where 9-methoxyellipticine increased the levels of IgM to counter their reduced levels after the infection induced by
K. pneumoniae and STEC O157 in mice.
K. pneumoniae pulmonary lesions (
Figure 4a–c) of the untreated positive control exhibited focal hemorrhages in the interstitial tissue and in the alveolar lamina in addition to dilated pulmonary blood vessels. Additionally, inflammatory infiltration composed of the histiocytes and polymorphonuclear sections was seen. In contrast, lungs infected with
K. pneumoniae subsequently treated by 9-methoxyellipticine revealed the moderate infiltration of inflammatory cells with the emphysema of pulmonary alveoli. Sections of
K. pneumoniae infection after treatment with GEN demonstrated an emphysema of pulmonary alveoli and a mild inflammatory cell infiltration in the lung tissue (
Figure 4a–c). Treatment with 9-methoxyellipticine (
Table 3) showed no indication of hemorrhage, no RBCs in alveolar lamina, and no perivascular oedema. More importantly, ellipticine was reported as a suggested treatment for the avoidance of acute death triggered by severe inflammation, with a noteworthy protective activity in
Streptococcus suis serotype 2 strain-infected mice [
45].
One day post
K. pneumoniae inoculation, the infected mice showed indications of quicker respiration, subordinate action, muddled bristle, or coat, and had intensified secretions around the infected eyes (
Figure 5a–c and
Table 4). Furthermore, these symptoms were faster after 48 h of immunization and the animal started to pass away; all animals died within 7 days in the positive control group, showing that
K. pneumoniae inoculation causes a critical pulmonary inflammatory response. However, in the 9-methoxyellipticine-treated group, the pulmonary hemorrhage and interalveolar thickness were significantly decreased.
In the STEC-infected animal renal lesions, (
Figure 5a–c and
Table 4), (untreated group), the manifestation of multi-sized vacuoles in the cytoplasm of tubular epithelium implied necrotic alterations. Additionally, representative of histopathological alteration, coagulation necrosis with karyopyknotic nuclei was also detected. However, the kidney of the animal infected with STEC and treated with 9-methoxyellipticine displayed interstitial inflammatory cell infiltration.
In addition, renal lesions of the animal infected with STEC and treated with GEN showed focal inflammatory cell infiltration in the renal interstitial tissue and deteriorating alterations of the renal tubular epithelium. The histopathological scores (
Table 3 and
Table 4) demonstrated that mice treated with 9-methoxyellipticine had no renal cast, subcapsular and interstitial hemorrhage, necrobiotic alterations of tubular epithelium, and interstitial inflammatory cell infiltration.
The fecal counts of
E. coli in animal feces decreased to 5 × 10
1 after ten days of administration of 9-methoxyellipticine, but those for the GEN-treated animal decreased from 1 × 10
8 to 2 × 10
3. Conversely, 9-methoxyellipticine and GEN can decrease the bacterial loads of
K. pneumoniae from 23 × 10
6 to 3 × 10
2 and from 2 × 10
6 to >220 × 10
2, respectively (
Table 5 and
Table 6). In addition to this, the calculation of colonizing
K. pneumoniae in pulmonary and renal tissues reflected a substantial decline in the bacterial count to 5 × 10
1 in lungs and to total eradication in kidneys. As is known, MDR STEC infection outbreaks in humans or animals cause a vast risk to public health and safety. Over 700,000 deaths per year globally are due to infection with MDR bacteria [
46]. Therefore, finding alternative antibacterial agents with limited side effects has become a must. Lu et al. [
20] who tested ellipticine HCl against multidrug-resistant extraintestinal pathogenic
E. coli reported a MIC nearly half that obtained with 9-methoxy ellipticine (1000 µg /mL for ellipticine HCl and 500 µg /mL for 9- methoxy ellipticine). In addition, the in vivo antibacterial assessment revealed an effective bactericidal activity that was proven by lowering bacterial count to 2 × 10
1 and 5 × 10
1 in the lungs and feces, respectively. Therefore, it can be seen that the isolated compound has powerful bactericidal activity. Furthermore, the scoring of histopathological renal and pulmonary lesions revealed a remarkable enhancement in the severity of tissue damage and this indeed proves the ability of 9-methoxy ellipticine to attenuate bacterial pathogenicity.
The extreme inflammatory responses triggered by the bacterial infection are reported as the main causes of the acute death. Collectively, our study demonstrated that 9-methoxyellipticine could substantially decrease the bacterial loads and tissue destruction in MDR STEC O157 and
K. pneumoniae-infected mice. It also considerably eased the inflammation and pathological injury, with the infiltration of inflammatory cells, alveolar interstitial obstruction, and edema in the pulmonary region and renal tissues of the infected animal. The mechanisms of the antibacterial activity of alkaloids were previously reported [
47,
48,
49].
Finally, 9-methoxyellipticine is a carbazole (nitrogen-containing heterocycle) derivative that showed significant antibacterial activity in our study. As reported, carbazole derivatives exhibit several mechanisms, and can act as antibacterial agents. They act by (1) increasing the membrane permeability through inhibiting specific enzymatic processes and increasing the access of the free radicals, which violates the integrity of the bacterial cells, and (2) interacting with bacterial DNA. Accordingly, various carbazoles are being considered as promising solutions for new antibacterial drugs with two potential actions, thus helping to solve the MDR problems worldwide [
50]. Searching for natural compounds could become a promising way to fight against multi-drug resistant bacteria and meet the emergency demand for discovering highly effective, low toxicity, and low environmental impact antibiotics. New antibacterial compounds with novel mechanisms of action and properties that are different from the currently used drugs could be obtained from ellipticine derivatives. In addition, the application of 9-methoxyellipticine as the lead compound is among the most promising approaches for bioactive drug discovery in the future. Finally, a significant clinical impact with the roles of 9-methoxyellipticine and its derivatives in challenging areas should be of great interest in the future as to produce more potent and targeted analogues of 9-methoxyellipticine. Nevertheless, novel strategies based on the field of nanotechnologies for safer drug delivery are recommended.