Effect of Suppressive Levothyroxine Therapy on Bone Mineral Density in Young Patients with Differentiated Thyroid Carcinoma
Abstract
:1. Introduction
2. Results
2.1. Patients
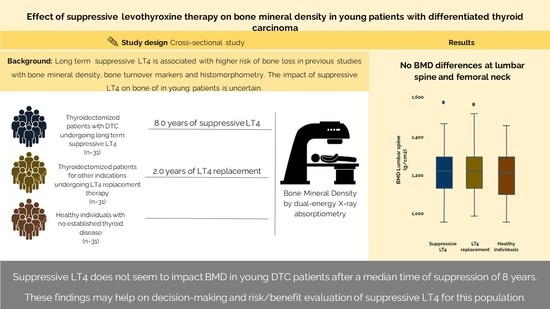
2.2. Bone Mineral Density
2.3. Fractures
3. Discussion
4. Material and Methods
4.1. Patients and Study Design
4.2. Treatment Protocol and Follow-Up
4.3. Outcomes
4.4. Clinical and Oncological Features
4.5. Laboratory Analysis
4.6. Bone Mineral Density
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Enemoto, Y.; Enemoto, K.; Uchino, S.; Shibuya, H.; Watanabe, S.; Noguchi, S. Clinical features, treatment and long-term oucomes of pappilary thyroid cancer in children and adolescents without radiation exposure. World J. Surg. 2012, 36, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Golpanian, S.; Perez, E.A.; Tashiro, J.; Lew, J.I.; Sola, J.E.; Hogan, A.R. Pediatric pappilary thyroid carcinoma: Outcomes and survival predictors in 2504 surgical patients. Pediatr. Surg. Int. 2016, 32, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.L.; Waguespack, S.G.; Bauer, A.J.; Angelos, P.; Benvenga, S.; Cerutti, J.M.; Dinauer, C.A.; Hamilton, J.; Hay, I.D.; Luster, M.; et al. American Thyroid Association Guidelines Task Force. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid 2015, 25, 716–759. [Google Scholar] [CrossRef]
- Mazzafferi, E.L.; Young, R.L.; Oertel, J.E.; Kemmerer, W.T.; Page, C.P. Papillary thyroid carcinoma: The impact of therapy in 576 patients. Medicine 1977, 56, 171–196. [Google Scholar] [CrossRef]
- Pujol, P.; Daures, J.P.; Nsakala, N.; Baldet, L.; Bringer, J.; Jaffiol, C. Degree of thyrotropin suppression as a prognostic determinant in differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 1996, 81, 4318–4323. [Google Scholar] [PubMed]
- Cooper, D.S.; Specker, B.; Ho, M.; Sperling, M.; Ladenson, P.W.; Ross, D.S.; Ain, K.B.; Bigos, S.T.; Brierley, J.D.; Haugen, B.R.; et al. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: Results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid 1998, 8, 737–744. [Google Scholar] [CrossRef]
- Kamel, N.; Güllü, S.; Ilgin, S.D.; Corapcioglu, D.; Cesur, V.T.; Uysal, A.R.; Başkal, N.; Erdoğan, G. Degree of thyrotropin suppression in differentiated thyroid cancer without recurrence or metastases. Thyroid 1999, 9, 1245–1248. [Google Scholar] [CrossRef]
- Klubo-Gwiezinska, J.; Auh, S.; Gershgorn, M.; Daley, B.; Bikas, A.; Burman, K.; Wartofsky, L.; Urken, M.; Dewey, E.; Smallridge, R.; et al. Association of Thyrotropin Suppression with Survival Outcomes in Patients with Intermediate- and High-Risk Differentiated Thyroid Cancer. JAMA Netw. Open 2019, 2, e187754. [Google Scholar] [CrossRef]
- Sugitani, I.; Fugimoto, Y. Does Postoperative Thyrotropin Suppression Therapy Truly Decrease Recurrence in Papillary Thyroid Carcinoma? A Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2010, 95, 4576–4583. [Google Scholar] [CrossRef]
- Briot, K. Non-corticosteroid drug-induced metabolic bone disease. Presse Med. 2006, 35, 1579–1583. [Google Scholar] [CrossRef]
- Batrinos, M.L. The problem of exogenous subclinical hyperthyroidism. Hormones 2006, 5, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Du, F.; Liu, Y.; Wang, X.; Bian, B. Effects of levothyroxine therapy on bone mineral density and bone turnover markers in premenopausal women with thyroid cancer after thyroidectomy. Endokrynol. Pol. 2020, 71, 15–20. [Google Scholar]
- Schneider, R.; Schneider, M.; Reiners, C.; Schneider, P. Effects of levothyroxine on bone mineral density, muscle force, and bone turnover markers: A cohort study. J. Clin. Endocrinol. Metab. 2012, 97, 3926–3934. [Google Scholar] [CrossRef]
- Cardoso, L.F.; Maciel, L.M.Z.; de Paula, F.J.A. The multiple effects of thyroid disorders on bone and mineral metabolism. Arq. Bras. Endocrinol. Metabol. 2014, 58, 452–463. [Google Scholar] [CrossRef]
- Brancatella, A.; Marcocci, C. TSH suppressive therapy and bone. Endocr. Connect. 2020, 9, R158–R172. [Google Scholar] [CrossRef]
- Wang, L.Y.; Smith, A.W.; Palmer, F.L.; Tuttle, R.M.; Mahrous, A.; Nixon, I.J.; Patel, S.G.; Ganly, I.; Fagin, J.A.; Boucai, L. Thyrotropin suppression increases the risk of osteoporosis without decreasing recurrence in ATA low- and intermediate-risk patients with differentiated thyroid carcinoma. Thyroid 2015, 25, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Bonjour, J.P.; Chevalley, T. Pubertal timing, bone acquisition, and risk of fracture throughout life. Endocr. Rev. 2014, 35, 820–847. [Google Scholar] [CrossRef] [PubMed]
- Veldscholte, K.; Barjaktarovic, M.; Trajanoska, K.; Jaddoe, V.W.V.; Visser, T.J.; de Rijke, Y.B.; Peeters, R.B.; Rivadeneira, F.; Korevaar, T.I.M. The association of thyroid function with bone density during childhood. J. Clin. Endocrinol. Metab. 2018, 103, 4125–4134. [Google Scholar] [CrossRef]
- Heaney, R.P.; Abrams, S.; Dawson-Hughes, B.; Looker, A.; Marcus, R.; Matkovic, V.; Weaver, C. Peak Bone Mass. Osteoporos. Int. 2000, 11, 985–1009. [Google Scholar] [CrossRef]
- Mitchell, J.A.; Cousminer, D.L.; Zemel, B.S.; Grant, S.F.; Chesi, A. Genetics of pediatric bone strength. Bonekey Rep. 2016, 5, 823–828. [Google Scholar] [CrossRef]
- Marcocci, C.; Golia, F.; Bruno-Bossio, G.; Vignali, E.; Pinchera, A. Carefully monitored levothyroxine suppressive therapy is not associated with bone loss in premenopausal women. J. Clin. Endocrinol. Metab. 1994, 78, 818–823. [Google Scholar] [PubMed]
- Marcocci, C.; Golia, F.; Vignali, E.; Pinchera, A. Skeletal integrity in men chronically treated with suppressive doses of L-thyroxine. J. Bone Miner. Res. 1997, 12, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Demartini, A.A.; Kulak, C.A.; Borba, V.C.; Cat, M.N.; Dondoni, R.S.; Sandrini, R.; Nesi-França, S.; Filho, L.L. Bone mineral density of children and adolescents with congenital hypothyroidism. Arq. Bras. Endocrinol. Metabol. 2007, 51, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Salama, H.M.; El-Dayem, S.A.; Yousef, H.; Fawzy, A.; Abou-Ismail, L.; El-Lebedy, D. The effects of L-thyroxin replacement therapy on bone minerals and body composition in hypothyroid children. Arch. Med. Sci. 2010, 6, 407–413. [Google Scholar] [CrossRef] [PubMed]
- de Barros, G.M.M.; Madeira, M.; Neto, L.V.; Neto, F.P.P.; Mendonça, L.M.C.; Lima, I.C.B.; Corbo, R.; Farias, M.L.F. Bone mineral density and bone microarchitecture after long-term suppressive levothyroxine treatment of differentiated thyroid carcinoma in young adult patients. J. Bone Miner. Metab. 2015, 34, 417–421. [Google Scholar] [CrossRef]
- Weaver, C.M.; Gordon, C.M.; Janz, K.F.; Kalkwarf, H.J.; Lappe, J.M.; Lewis, R.; O’Karma, M.; Wallace, T.C.; Zemel, B.S. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporos. Int. 2016, 27, 1281–1386. [Google Scholar] [CrossRef]
- Manolagas, S.C.; O’Brien, C.A.; Almeida, M. The role of estrogen and androgen receptors in bone health and disease. Nat. Rev. Endocrinol. 2013, 9, 699–712. [Google Scholar] [CrossRef]
- Scheffel, R.S.; Zanella, A.B.; Dora, J.M.; Maia, A.L. Low recurrence rates in a cohort of differentiated thyroid carcinoma patients: A referral center experience. Thyroid 2015, 25, 883–889. [Google Scholar] [CrossRef]
- Zanella, A.B.; Scheffel, R.S.; Nava, C.F.; Golbert, L.; Meyer, E.L.S.; Punales, M.; Gonçalves, I.; Dora, J.M.; Maia, A.L. Dynamic risk stratification in the follow-up of children and adolescents with differentiated thyroid cancer. Thyroid 2018, 28, 1285–1292. [Google Scholar] [CrossRef]
- Weber, D.R.; Boyce, A.; Gordon, C.; Hogler, W.; Kecsemethy, H.H.; Misra, M.; Swolin-Eide, D.; Tebben, P.; Ward, L.M.; Wasserman, H.; et al. The Utility of DXA Assessment at the Forearm, Proximal Femur, and Lateral Distal Femur, and Vertebral Fracture Assessment in the Pediatric Population: 2019 ISCD Official Position. J. Clin. Densitom. 2019, 22, 567–589. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Morris, L.F.; Haugen, B.; Shah, J.; Sosa, J.A.; Rohren, E. Thyroid-Differentiated and Anaplastic Carcinoma. In AJCC Cancer Staging Manual, 8th ed.; Brierley, J.D., Gospodarowicz, M.K., Wittekind, C., Eds.; Springer International Publishing: New York, NY, USA, 2016. [Google Scholar]
- Haugen, B.R.M.; Alexander, E.K.; Bible, K.C.; Doherty, G.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2015, 26, 1–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Characteristics | s-T4 (n = 31) | L-T4 Replacement (n = 31) | Healthy Individuals (n = 31) | p |
|---|---|---|---|---|
| Age (years) | 28.3 (18–38) | 29.1 (17–42) | 28.6 (20–39) | 0.86 |
| Female sex—n (%) | 24 (77.4) | 24 (77.4) | 24 (77.4) | 1.0 |
| BMI (kg/m2) | 25.5 ± 5.1 | 25.6 ± 4.3 | 24.1 ± 3.6 | 0.33 |
| Age at diagnosis (years) | 19.1 (6–25) | 24.0 (5–41) | 0.01 | |
| Histological type | ||||
| Papillary—n (%) | 30 (96.8) | 7 (22.6) | ||
| Follicular—n (%) | 1 (3.2) | 1 (3.2) | ||
| Medullary—n (%) | 0 (0.0) | 13 (42.0) | ||
| Benign—n (%) | 0 (0.0) | 10 (32.2) | ||
| Tumor size (cm) | 2.0 (1.2–2.7) | 2.5 (2.1–3.5) | ||
| Distant metastasis | 7 (22.5) | |||
| ATA risk | ||||
| Low—n (%) | 10 (32.3) | |||
| Intermediate—n (%) | 14 (45.2) | |||
| High—n (%) | 7 (22.5) | |||
| TSH (mIU/mL) | 0.4 (0.04–6.5) † | 2.7 (0.8–8.5) † | 1.77 (1.14–3.08) | † 0.01 |
| Levothyroxine (mcg/kg) | 2.4 ± 0.6 | 1.6 ± 0.3 | 0.01 | |
| Hypoparathyroidism—n (%) | 8 (25.8) | 5 (16.1) | 0.5 | |
| Lumbar spine BMD (g/cm2) | 1.212 ± 0.142 | 1.231 ± 0.141 | 1.205 ± 0.120 | 0.74 |
| Z-score | 0.4 (−0.6–0.9) | 0.3 (−0.6–1.0) | 0.3 (−0.8–1.0) | 0.74 |
| Femoral neck BMD (g/cm2) | 1.059 ± 0.115 | 1.040 ± 0.170 | 1.041 ± 0.121 | 0.85 |
| Z-score | 0.3 (−0.5–0.8) | 0.0 (−0.5–0.5) | 0.2 (−0.6–0.8) | 0.84 |
| Total femur BMD (g/cm2) | 1.055 ± 0.144 | 1.046 ± 0.162 | 1.041 ± 0.131 | 0.93 |
| Z-score | 0.2 (−1.4–1.3) | 0.1 (−0.3–0.6) | 0.1 (−0.5–0.7) | 0.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanella, A.B.; Marmitt, L.; Fighera, T.M.; Scheffel, R.S.; Spritzer, P.M.; Dora, J.M.; Maia, A.L. Effect of Suppressive Levothyroxine Therapy on Bone Mineral Density in Young Patients with Differentiated Thyroid Carcinoma. Metabolites 2022, 12, 842. https://doi.org/10.3390/metabo12090842
Zanella AB, Marmitt L, Fighera TM, Scheffel RS, Spritzer PM, Dora JM, Maia AL. Effect of Suppressive Levothyroxine Therapy on Bone Mineral Density in Young Patients with Differentiated Thyroid Carcinoma. Metabolites. 2022; 12(9):842. https://doi.org/10.3390/metabo12090842
Chicago/Turabian StyleZanella, André Borsatto, Laura Marmitt, Tayane Muniz Fighera, Rafael Selbach Scheffel, Poli Mara Spritzer, José Miguel Dora, and Ana Luiza Maia. 2022. "Effect of Suppressive Levothyroxine Therapy on Bone Mineral Density in Young Patients with Differentiated Thyroid Carcinoma" Metabolites 12, no. 9: 842. https://doi.org/10.3390/metabo12090842