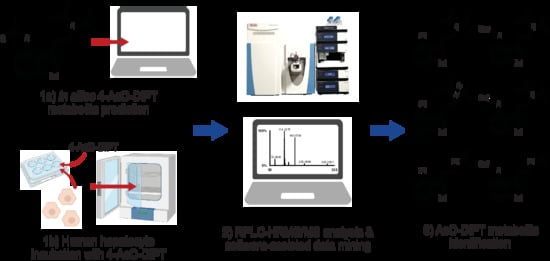
Human Hepatocyte 4-Acetoxy-N,N-Diisopropyltryptamine Metabolite Profiling by Reversed-Phase Liquid Chromatography Coupled with High-Resolution Tandem Mass Spectrometry
Abstract
:1. Introduction
2. Results
2.1. In Silico Metabolite Predictions
2.2. 4-AcO-DiPT Fragmentation Pattern
2.3. Metabolite Identification
2.3.1. Phase I Metabolites
Ester Hydrolysis
Ester Hydrolysis and N-Deisopropylation
Ester Hydrolysis and N-Oxidation
2.3.2. Phase II Metabolites
Ester Hydrolysis and O-Glucuronidation
Ester Hydrolysis and O-Sulfation
Ester Hydrolysis, N-Deisopropylation, and O-Sulfation
3. Discussion
4. Materials and Methods
4.1. In Silico Metabolites Prediction
4.2. Chemicals and Reagents
4.3. Hepatocytes Incubation
4.4. Sample Preparation
4.5. Instrumental Conditions
4.6. Liquid Chromatography
4.7. Mass Spectrometry Conditions
4.8. Final Metabolite Identification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rickli, A.; Moning, O.D.; Hoener, M.C.; Liechti, M.E. Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. Eur. Neuropsychopharmacol. 2016, 26, 1327–1337. [Google Scholar] [CrossRef] [Green Version]
- Baumeister, D.; Barnes, G.; Giaroli, G.; Tracy, D. Classical hallucinogens as antidepressants? A review of pharmacodynamics and putative clinical roles. Ther. Adv. Psychopharmacol. 2014, 4, 156–169. [Google Scholar] [CrossRef] [Green Version]
- Bunzow, J.R.; Sonders, M.S.; Arttamangkul, S.; Harrison, L.M.; Zhang, G.; Quigley, D.I.; Darland, T.; Suchland, K.L.; Pasumamula, S.; Kennedy, J.L.; et al. Amphetamine, 3,4-methylenedioxy methamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol. Pharm. 2001, 60, 1181–1188. [Google Scholar] [CrossRef]
- Cozzi, N.V.; Gopalakrishnan, A.; Anderson, L.L.; Feih, J.T.; Shulgin, A.T.; Daley, P.F.; Ruoho, A.E. Dimethyltryptamine and other hallucinogenic tryptamines exhibit substrate behavior at the serotonin uptake transporter and the vesicle monoamine transporter. J. Neuraltransm. 2009, 116, 1591–1599. [Google Scholar] [CrossRef]
- Fantegrossi, W.E.; Harrington, A.W.; Kiessel, C.L.; Eckler, J.R.; Rabin, R.A.; Winter, J.C.; Coop, A.; Rice, K.C.; Woods, J.H. Hallucinogen-like actions of 5-methoxy-N,N-diisopropyl-tryptamine in mice and rats. Pharmacol. Biochem. Behav. 2006, 83, 122–129. [Google Scholar] [CrossRef]
- McKenna, D.J.; Repke, D.B.; Lo, L.; Peroutka, S.J. Differential interactions of indolealkylamines with 5-hydroxytryptamine receptor subtypes. Neuropharmacology 1990, 29, 193–198. [Google Scholar] [CrossRef]
- Luethi, D.; Liechti, M.E. Monoamine transporter and receptor interaction profiles in vitro predict reported human doses of novel psychoactive stimulants and psychedelics. Int. J. Neuropsychopharmacol. 2018, 21, 926–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Santos, R.G.; Hallak, J.E.C. Therapeutic use of serotoninergic hallucinogens: A review of the evidence and of the biological and psychological mechanisms. Neurosci. Biobehav. Rev. 2020, 108, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Acetoxy-DiPT Legal Status by Erowid. Available online: https://erowid.org/chemicals/4_acetoxy_dipt/4_acetoxy_dipt_law.shtml (accessed on 9 June 2022).
- Malaca, S.; Lo Faro, A.F.; Tamborra, A.; Pichini, S.; Busardò, F.P.; Huestis, M.A. Toxicology and Analysis of Psychoactive Tryptamines. Int. J. Mol. Sci. 2020, 21, 9279. [Google Scholar] [CrossRef] [PubMed]
- Global Drug Survey (GDS). Available online: https://www.globaldrugsurvey.com/wp-content/themes/globaldrugsurvey/results/GDS2019-Exec-Summary.pdf (accessed on 9 June 2022).
- 4-Acetoxy-DiPT Dosage by Erowid. Available online: https://www.erowid.org/chemicals/4_acetoxy_dipt/4_acetoxy_dipt_dose.shtml (accessed on 9 June 2022).
- Zhang, Y.; Yuan, B.; Takagi, N.; Wang, H.; Zhou, Y.; Si, N.; Yang, J.; Wei, X.; Zhao, H.; Bian, B. Comparative Analysis of Hydrophilic Ingredients in Toad Skin and Toad Venom Using the UHPLC-HR-MS/MS and UPLC-QqQ-MS/MS Methods Together with the Anti-Inflammatory Evaluation of Indolealkylamines. Molecules 2018, 24, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swortwood, M.J.; Carlier, J.; Ellefsen, K.N.; Wohlfarth, A.; Diao, X.; Concheiro-Guisan, M.; Kronstrand, R.; Huestis, M.A. In vitro, in vivo and in silico metabolic profiling of α-pyrrolidinopentiothiophenone, a novel thiophene stimulant. Bioanalysis 2016, 8, 65–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diao, X.; Carlier, J.; Zhu, M.; Huestis, M.A. Human Hepatocyte Metabolism of Novel Synthetic Cannabinoids MN-18 and Its 5-Fluoro Analog 5F-MN-18. Clin. Chem. 2017, 63, 1753–1763. [Google Scholar] [CrossRef] [Green Version]
- Caspar, A.T.; Gaab, J.B.; Michely, J.A.; Brandt, S.D.; Meyer, M.R.; Maurer, H.H. Metabolism of the tryptamine-derived new psychoactive substances 5-MeO-2-Me-DALT, 5-MeO-2-Me-ALCHT, and 5-MeO-2-Me-DIPT and their detectability in urine studied by GC-MS, LC-MSn, and LC-HR-MS/MS. Drug Test. Anal. 2018, 10, 184–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlier, J.; Diao, X.; Huestis, M.A. Synthetic cannabinoid BB-22 (QUCHIC): Human hepatocytes metabolism with liquid chromatography-high resolution mass spectrometry detection. J. Pharm. Biomed. Anal. 2018, 157, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Diao, X.; Scheidweiler, K.B.; Wohlfarth, A.; Pang, S.; Kronstrand, R.; Huestis, M.A. In Vitro and In Vivo Human Metabolism of Synthetic Cannabinoids FDU-PB-22 and FUB-PB-22. AAPS J. 2016, 18, 455–464. [Google Scholar] [CrossRef] [Green Version]
- Bruni, P.S.; Grafinger, K.E.; Nussbaumer, S.; König, S.; Schürch, S.; Weinmann, W. Study of the in vitro and in vivo metabolism of 4-HO-MET. Forensic Sci. Int. 2018, 290, 103–110. [Google Scholar] [CrossRef]
- Grafinger, K.E.; Hädener, M.; König, S.; Weinmann, W. Study of the in vitro and in vivo metabolism of the tryptamine 5-MeO-MiPT using human liver microsomes and real case samples. Drug Test. Anal. 2018, 10, 562–574. [Google Scholar] [CrossRef] [Green Version]
- Fabregat-Safont, D.; Barneo-Muñoz, M.; Martinez-Garcia, F.; Sancho, J.V.; Hernández, F.; Ibáñez, M. Proposal of 5-methoxy-N-methyl-N-isopropyltryptamine consumption biomarkers through identification of in vivo metabolites from mice. J. Chromatogr. A 2017, 1508, 95–105. [Google Scholar] [CrossRef]
- Manier, S.K.; Felske, C.; Zapp, J.; Eckstein, N.; Meyer, M.R. Studies on the In Vitro and In Vivo Metabolic Fate of the New Psychoactive Substance N-Ethyl-N-Propyltryptamine for Analytical Purposes. J. Anal. Toxicol. 2021, 45, 195–202. [Google Scholar] [CrossRef]
- Michely, J.A.; Helfer, A.G.; Brandt, S.D.; Meyer, M.R.; Maurer, H.H. Metabolism of the new psychoactive substances N,N-diallyltryptamine (DALT) and 5-methoxy-DALT and their detectability in urine by GC-MS, LC-MSn, and LC-HR-MS-MS. Anal. Bioanal. Chem. 2015, 407, 7831–7842. [Google Scholar] [CrossRef] [Green Version]
- Kamata, T.; Katagi, M.; Kamata, H.T.; Miki, A.; Shima, N.; Zaitsu, K.; Nishikawa, M.; Tanaka, E.; Honda, K.; Tsuchihashi, H. Metabolism of the psychotomimetic tryptamine derivative 5-methoxy-N,N-diisopropyltryptamine in humans: Identification and quantification of its urinary metabolites. Drug Metab. Dispos. 2006, 34, 281–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kops, C.B.; Šícho, M.; Mazzolari, A.; Kirchmair, J. GLORYx: Prediction of the Metabolites Resulting from Phase 1 and Phase 2 Biotransformations of Xenobiotics. Chem. Res. Toxicol. 2021, 34, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Stork, C.; Embruch, G.; Šícho, M.; Kops, C.B.; Chen, Y.; Svozil, D.; Kirchmair, J. NERDD: A web portal providing access to in silico tools for drug discovery. Bioinformatics 2020, 36, 1291–1292. [Google Scholar] [CrossRef] [PubMed]
- Di Trana, A.; Brunetti, P.; Giorgetti, R.; Marinelli, E.; Zaami, S.; Busardò, F.P.; Carlier, J. In silico prediction, LC-HRMS/MS analysis, and targeted/untargeted data-mining workflow for the profiling of phenylfentanyl in vitro metabolites. Talanta 2021, 235, 122740. [Google Scholar] [CrossRef] [PubMed]



| Predicted Metabolite (pM) | Transformation | Elemental Composition | Score (%) | |
|---|---|---|---|---|
| pM1 | N-Hydroxylation | C18H27N2O3 | 63 | |
| pM1.1 | O-Glucuronidation | C25H30N2O8 | 30 | |
| pM1.2 | N-Deisopropylation | C16H21NO3 | 22 | |
| pM1.3 | Hydroxylation | C19H28NO4 | 22 | |
| pM1.4 | Hydroxylation (C1″) | C18H26N04 | 21 | |
| pM2 | Hydroxylation (C2″) | C18H26N2O3 | 63 | |
| pM2.1 | O-Sulfation | C18H24N3O7S | 60 | |
| pM2.2 | O-Glucuronidation | C24H32N2O10 | 39 | |
| pM2.3 | Dealkylation | C17H24N2O2 | 21 | |
| pM2.4 | Hydroxylation (C11) | C19H26N2O4 | 21 | |
| pM2.5 | Carboxylation (C9) | C19H24N2O5 | 21 | |
| pM3 | Deisopropylation | C15H20N2O2 | 63 | |
| pM3.1 | Hydroxylation (C1″) | C16H20N2O3 | 35 | |
| pM3.2 | Depropylation | C13H14O3 | 35 | |
| pM3.3 | N-Hydroxylation | C15H20N2O3 | 35 | |
| pM3.4 | N-Deisopropylation | C12H14N2O2 | 35 | |
| pM4 | Hydroxylation (acetyl) | C18H26N2O3 | 34 | |
| pM4.1 | O-Glucuronidation | C23H32N209 | 28 | |
| pM4.2 | O-Sulfation | C17H24N206S | 24 | |
| pM5 | Carboxylation (acetyl) | C19H26N2O3 | 34 | |
| pM5.1 | O-Glucuronidation | C26H36N2O9 | 31 | |
| pM5.2 | N-Hydroxylation | C18H25N2O5 | 20 | |
| pM5.3 | Deisopropylation | C15H18N204 | 20 | |
| pM5.4 | Hydroxylation (C1″) | C18H24N2O5 | 20 | |
| pM6 | Ester hydrolysis | C19H26N2O | 34 | |
| pM6.1 | O-Sulfation | C16H24N2O4S | 32 | |
| pM6.2 | O-Glucuronidation | C22H32N2O7 | 29 | |
| pM6.3 | Hydroxylation (C1″) | C16H24N2O2 | 20 | |
| pM6.4 | N-Hydroxylation | C16H25N2O2 | 20 | |
| pM7 | Hydroxylation (C2) | C16H24N2O2 | 25 | |
| pM8 | N-Glucuronidation | C12H13NO3 | 20 | |
| pM9 | Deamination (to aldehyde) | C12H13NO3 | 20 | |
| pM10 | Deamination (to alcohol) | C12H11NO3 | 20 | |
| ID | Transformation | RT (min) | [M + H]+ | Elemental Composition | Mass Error (ppm) | Peak Areas (HESI+, HESI−) |
|---|---|---|---|---|---|---|
| M1 | Ester hydrolysis + N-Deisopropylation | 6.31 | 219.1489 | C13H18N2O | −1.21 | 1.10 × 108 |
| M2 | Ester hydrolysis + N-Deisopropylation + O-Sulfation | 7.24 | 299.1057 | C13H18N2O4S | −1.23 | 3.50 × 107, 5.90 × 107 |
| M3 | Ester hydrolysis + O-Glucuronidation | 8.30 | 437.2277 | C22H32N2O7 | −1.45 | 2.94 × 108, 2.60 × 107 |
| M4 | Ester hydrolysis | 11.82 | 261.1955 | C16H24N2O | −2.25 | 4.19 × 109 |
| M5 | Ester hydrolysis + O-Sulfation | 12.11 | 341.1525 | C16H24N2O4S | −1.42 | 4.47 × 107, 2.79 × 107 |
| M6 | Ester hydrolysis + N-Oxidation | 12.53 | 277.1907 | C16H24N2O2 | −1.35 | 7.76 × 106 |
| Parent | no transformation | 15.02 | 303.2059 | C18H26N2O2 | −2.67 | 3.00 × 107 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malaca, S.; Huestis, M.A.; Lattanzio, L.; Marsella, L.T.; Tagliabracci, A.; Carlier, J.; Busardò, F.P. Human Hepatocyte 4-Acetoxy-N,N-Diisopropyltryptamine Metabolite Profiling by Reversed-Phase Liquid Chromatography Coupled with High-Resolution Tandem Mass Spectrometry. Metabolites 2022, 12, 705. https://doi.org/10.3390/metabo12080705
Malaca S, Huestis MA, Lattanzio L, Marsella LT, Tagliabracci A, Carlier J, Busardò FP. Human Hepatocyte 4-Acetoxy-N,N-Diisopropyltryptamine Metabolite Profiling by Reversed-Phase Liquid Chromatography Coupled with High-Resolution Tandem Mass Spectrometry. Metabolites. 2022; 12(8):705. https://doi.org/10.3390/metabo12080705
Chicago/Turabian StyleMalaca, Sara, Marilyn A. Huestis, Leonardo Lattanzio, Luigi T. Marsella, Adriano Tagliabracci, Jeremy Carlier, and Francesco P. Busardò. 2022. "Human Hepatocyte 4-Acetoxy-N,N-Diisopropyltryptamine Metabolite Profiling by Reversed-Phase Liquid Chromatography Coupled with High-Resolution Tandem Mass Spectrometry" Metabolites 12, no. 8: 705. https://doi.org/10.3390/metabo12080705