The Biosynthesis of Non-Endogenous Apocarotenoids in Transgenic Nicotiana glauca
Abstract
:1. Introduction
2. Results
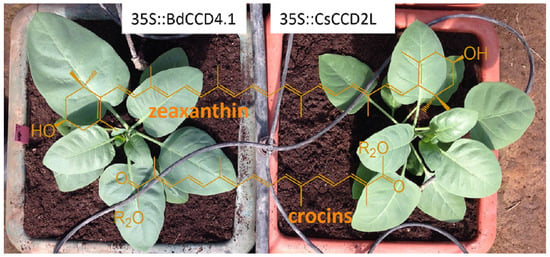
2.1. Generation of N. glauca Plants Expressing BdCCD4.1
2.2. Levels of Crocins in Leaves and Petals of Transgenic N. glauca
2.3. Levels of Endogenous Carotenoids in Leaves and Petals of Transgenic N. glauca Expressing BdCCD4.1
2.4. Stability of Endogenous Apocarotenoids in Leaves and Petals of Transgenic N. glauca
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Vector Construction
4.3. Transformation of Agrobacterium and N. glauca
4.4. Genomic DNA Isolation and PCR conditions
4.5. Carotenoid and Apocarotenoid Extraction and Quantification
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Britton, G. Functions of Carotenoid Metabolites and Breakdown Products. In Carotenoids; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser Verlag: Basel, Switzerland, 2008; pp. 309–323. [Google Scholar]
- Sun, T.; Yuan, H.; Cao, H.; Yazdani, M.; Tadmor, Y.; Li, L. Carotenoid Metabolism in Plants: The Role of Plastids. Mol. Plant 2018, 11, 58–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, T.; Rao, S.; Zhou, X.; Li, L. Plant carotenoids: Recent advances and future perspectives. Mol. Hortic. 2022, 2, 3. [Google Scholar] [CrossRef]
- Barja, M.V.; Ezquerro, M.; Beretta, S.; Diretto, G.; Florez-Sarasa, I.; Feixes, E.; Fiore, A.; Karlova, R.; Fernie, A.R.; Beekwilder, J.; et al. Several geranylgeranyl diphosphate synthase isoforms supply metabolic substrates for carotenoid biosynthesis in tomato. New Phytol. 2021, 231, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Mariutti, L.R.B.; Mercadante, A.Z. Carotenoid esters analysis and occurrence: What do we know so far? Arch. Biochem. Biophys. 2018, 648, 36–43. [Google Scholar] [CrossRef]
- Walter, M.H.; Strack, D. Carotenoids and their cleavage products: Biosynthesis and functions. Nat. Prod. Rep. 2011, 28, 663–692. [Google Scholar] [CrossRef]
- Ahrazem, O.; Gómez-Gómez, L.; Rodrigo, M.J.; Avalos, J.; Limón, M.C. Carotenoid Cleavage Oxygenases from Microbes and Photosynthetic Organisms: Features and Functions. Int. J. Mol. Sci. 2016, 17, 1781. [Google Scholar] [CrossRef] [Green Version]
- Xiong, L.; Zhu, J.K. Regulation of abscisic acid biosynthesis. Plant Physiol. 2003, 133, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Thompson, A.J.; Mulholland, B.J.; Jackson, A.C.; Mckee, J.M.T.; Hilton, H.W.; Symonds, R.C.; Sonneveld, T.; Burbidge, A.; Stevenson, P.; Taylor, I.B. Regulation and manipulation of ABA biosynthesis in roots. Plant Cell Environ. 2007, 30, 67–78. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, Y.; Al-Babili, S. Exploring the Diversity and Regulation of Apocarotenoid Metabolic Pathways in Plants. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef]
- Caceres, L.A.; Lakshminarayan, S.; Yeung, K.C.; McGarvey, B.D.; Hannoufa, A.; Sumarah, M.W.; Benitez, X.; Scott, I.M. Repellent and Attractive Effects of alpha-, beta-, and Dihydro-beta- Ionone to Generalist and Specialist Herbivores. J. Chem. Ecol. 2016, 42, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Ohmiya, A. Diversity of carotenoid composition in petal petals. Jpn. Agric. Res. Q. 2011, 45, 8. [Google Scholar] [CrossRef] [Green Version]
- Ahrazem, O.; Rubio-Moraga, A.; Nebauer, S.G.; Molina, R.V.; Gómez-Gómez, L. Saffron: Its Phytochemistry, Developmental Processes, and Biotechnological Prospects. J. Agric. Food Chem. 2015, 63, 13. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, H.; Li, Y.-X.; Cai, L.; Huang, J.; Zhao, C.; Jia, L.; Buchanan, R.; Yang, T.; Jiang, L.-J. Crocin and geniposide profiles and radical scavenging activity of gardenia fruits (Gardenia jasminoides Ellis) from different cultivars and at the various stages of maturation. Fitoterapia 2010, 81, 269–273. [Google Scholar] [CrossRef]
- Guijarro-Díez, M.; Castro-Puyana, M.; Crego, A.L.; Marina, M.L. Detection of saffron adulteration with gardenia extracts through the determination of geniposide by liquid chromatography–mass spectrometry. J. Food Compos. Anal. 2017, 55, 30–37. [Google Scholar] [CrossRef]
- Liu, T.; Yu, S.; Xu, Z.; Tan, J.; Wang, B.; Liu, Y.-G.; Zhu, Q. Prospects and progress on crocin biosynthetic pathway and metabolic engineering. Comput. Struct. Biotechnol. J. 2020, 18, 3278–3286. [Google Scholar] [CrossRef]
- Ahrazem, O.; Diretto, G.; Argandoña, J.; Rubio-Moraga, Á.; Julve, J.M.; Orzáez, D.; Granell, A.; Gómez-Gómez, L. Evolutionarily distinct carotenoid cleavage dioxygenases are responsible for crocetin production in Buddleja davidii. J. Exp. Bot. 2017, 68, 14. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; He, P.; Zhao, D.; Ye, L.; Dai, L.; Zhang, X.; Sun, Y.; Zheng, J.; Bi, C. Construction of Escherichia coli cell factories for crocin biosynthesis. Microb. Cell Factories 2019, 18, 120. [Google Scholar] [CrossRef]
- Chai, F.; Wang, Y.; Mei, X.; Yao, M.; Chen, Y.; Liu, H.; Xiao, W.; Yuan, Y. Heterologous biosynthesis and manipulation of crocetin in Saccharomyces cerevisiae. Microb. Cell Factories 2017, 16, 54. [Google Scholar] [CrossRef] [Green Version]
- Ahrazem, O.; Rubio-Moraga, A.; Berman, J.; Capell, T.; Christou, P.; Zhu, C.; Gómez-Gómez, L. The carotenoid cleavage dioxygenase CCD2 catalysing the synthesis of crocetin in spring crocuses and saffron is a plastidial enzyme. New Phytol. 2016, 209, 13. [Google Scholar] [CrossRef]
- Ahrazem, O.; Zhu, C.; Huang, X.; Rubio-Moraga, A.; Capell, T.; Christou, P.; Gómez-Gómez, L. Metabolic Engineering of Crocin Biosynthesis in Nicotiana Species. Front. Plant Sci. 2022, 13, 861140. [Google Scholar] [CrossRef] [PubMed]
- Martí, M.; Diretto, G.; Aragonés, V.; Frusciante, S.; Ahrazem, O.; Gómez-Gómez, L.; Daròs, J.-A. Efficient production of saffron crocins and picrocrocin in Nicotiana benthamiana using a virus-driven system. Metab. Eng. 2020, 61, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Ahrazem, O.; Diretto, G.; Rambla, J.L.; Rubio-Moraga, Á.; Lobato-Gómez, M.; Frusciante, S.; Argandoña, J.; Presa, S.; Granell, A.; Gómez-Gómez, L. Engineering high levels of saffron apocarotenoids in tomato. Hortic. Res. 2022, 9, uhac074. [Google Scholar] [CrossRef] [PubMed]
- Diretto, G.; López-Jiménez, A.J.; Ahrazem, O.; Frusciante, S.; Song, J.; Rubio-Moraga, Á.; Gómez-Gómez, L. Identification and characterization of apocarotenoid modifiers and carotenogenic enzymes for biosynthesis of crocins in Buddleja davidii petals. J. Exp. Bot. 2021, 72, 3200–3218. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Gerjets, T.; Sandmann, G. Nicotiana glauca engineered for the production of ketocarotenoids in petals and leaves by expressing the cyanobacterial crtO ketolase gene. Transgenic Res. 2007, 16, 813–821. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Tezara, W.; Rengifo, E.; Herrera, A. Ecophysiological responses to drought and salinity in the cosmopolitan invader Nicotiana glauca. Braz. J. Plant Physiol. 2012, 24, 213–222. [Google Scholar] [CrossRef] [Green Version]
- Mortimer, C.; Bramley, P.M.; Fraser, P.D. The identification and rapid extraction of hydrocarbons from Nicotiana glauca: A potential advanced renewable biofuel source. Phytochem. Lett. 2012, 5, 455–458. [Google Scholar] [CrossRef]
- Usade, B.; Tohge, T.; Scossa, F.; Sierro, N.; Schmidt, M.; Vogel, A.; Bolger, A.; Kozlo, A.; Enfissi, E.M.A.; Morrel, K.; et al. The genome and metabolome of the tobacco tree, Nicotiana glauca: A potential renewable feedstock for the bioeconomy. bioRxiv 2018, 351429. [Google Scholar] [CrossRef] [Green Version]
- Frusciante, S.; Diretto, G.; Bruno, M.; Ferrante, P.; Pietrella, M.; Prado-Cabrero, A.; Rubio-Moraga, A.; Beyer, P.; Gomez-Gomez, L.; Al-Babili, S.; et al. Novel carotenoid cleavage dioxygenase catalyzes the first dedicated step in saffron crocin biosynthesis. Proc. Natl. Acad. Sci. USA 2014, 111, 12246–12251. [Google Scholar] [CrossRef] [Green Version]
- Mortimer, C.L.; Misawa, N.; Perez-Fons, L.; Robertson, F.P.; Harada, H.; Bramley, P.M.; Fraser, P.D. The Formation and Sequestration of Nonendogenous Ketocarotenoids in Transgenic Nicotiana glauca. Plant Physiol. 2017, 173, 1617–1635. [Google Scholar] [CrossRef] [Green Version]
- Tsimidou, M.; Biliaderis, C.G. Kinetic Studies of Saffron (Crocus sativus L.) Quality Deterioration. J. Agric. Food Chem. 1997, 45, 2890–2898. [Google Scholar] [CrossRef]
- Bowles, D.; Lim, E.-K.; Poppenberger, B.; Vaistij, F.E. Glycosyltransferases of lipophilic small molecules. Annu. Rev. Plant Biol. 2006, 57, 567–597. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.M.; Bahrami, I.; Dehnad, D.; Shahidi, S.A. The influence of nanocellulose coating on saffron quality during storage. Carbohydr. Polym. 2018, 181, 536–542. [Google Scholar] [CrossRef]
- Selim, K.; Tsimidou, M.; Biliaderis, C.G. Kinetic studies of degradation of saffron carotenoids encapsulated in amorphous polymer matrices. Food Chem. 2000, 71, 199–206. [Google Scholar] [CrossRef]
- Ordoudi, S.; Cagliani, L.R.; Lalou, S.; Naziri, E.; Tsimidou, M.; Consonni, R. 1H NMR-based metabolomics of saffron reveals markers for its quality deterioration. Food Res. Int. 2015, 70, 1–6. [Google Scholar] [CrossRef]
- Ishwarya, S.P.; Anandharamakrishnan, C.; Stapley, A.G. Spray-freeze-drying: A novel process for the drying of foods and bioproducts. Trends Food Sci. Technol. 2015, 41, 161–181. [Google Scholar] [CrossRef]
- Acar, B.; Sadikoglu, H.; Doymaz, I. Freeze-Drying Kinetics and Diffusion Modeling of Saffron (Crocus sativus L.). J. Food Processing Preserv. 2015, 39, 142–149. [Google Scholar] [CrossRef]
- Chen, D.; Xing, B.; Yi, H.; Li, Y.; Zheng, B.; Wang, Y.; Sha, Q. Effects of different drying methods on appearance, microstructure, bioactive compounds and aroma compounds of saffron (Crocus sativus L.). LWT 2020, 120, 108913. [Google Scholar] [CrossRef]
- Morimoto, S.; Umezaki, Y.; Shoyama, Y.; Saito, H.; Nishi, K.; Irino, N. Post-harvest degradation of carotenoid glucose esters in saffron. Planta Med. 1994, 60, 438–440. [Google Scholar] [CrossRef]
- Sánchez, A.M.; Carmona, M.; Ordoudi, S.A.; Tsimidou, M.Z.; Alonso, G.L. Kinetics of Individual Crocetin Ester Degradation in Aqueous Extracts of Saffron (Crocus sativus L.) upon Thermal Treatment in the Dark. J. Agric. Food Chem. 2008, 56, 1627–1637. [Google Scholar] [CrossRef]
- Khoo, H.-E.; Prasad, N.; Kong, K.-W.; Jiang, Y.; Ismail, A. Carotenoids and Their Isomers: Color Pigments in Fruits and Vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef] [PubMed]
- Bollinedi, H.; Dhakane-Lad, J.; Krishnan, S.G.; Bhowmick, P.; Prabhu, K.; Singh, N. Kinetics of β-carotene degradation under different storage conditions in transgenic Golden Rice® lines. Food Chem. 2019, 278, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Burt, A.J.; Grainger, C.M.; Young, J.C.; Shelp, B.J.; Lee, E.A. Impact of postharvest handling on carotenoid concentration and composition in high-carotenoid maize (Zea mays L.) kernels. J. Agric. Food Chem. 2010, 58, 8286–8292. [Google Scholar] [CrossRef]
- Ortiz, D.; Rocheford, T.; Ferruzzi, M.G. Influence of Temperature and Humidity on the Stability of Carotenoids in Biofortified Maize (Zea mays L.) Genotypes during Controlled Postharvest Storage. J. Agric. Food Chem. 2016, 64, 2727–2736. [Google Scholar] [CrossRef]
- Frusciante, S.; Demurtas, O.C.; Sulli, M.; Mini, P.; Aprea, G.; Diretto, G.; Karcher, D.; Bock, R.; Giuliano, G. Heterologous expression of Bixa orellana cleavage dioxygenase 4-3 drives crocin but not bixin biosynthesis. Plant Physiol. 2022, 188, 1469–1482. [Google Scholar] [CrossRef] [PubMed]
- Horsch, R.B.; Rogers, S.G.; Fraley, R.T. Transgenic plants. Cold Spring Harb. Symp. Quant. Biol. 1985, 50, 433–437. [Google Scholar] [CrossRef]
- Diretto, G.; Ahrazem, O.; Rubio-Moraga, Á.; Fiore, A.; Sevi, F.; Argandoña, J.; Gómez-Gómez, L. UGT709G1: A novel uridine diphosphate glycosyltransferase involved in the biosynthesis of picrocrocin, the precursor of safranal in saffron (Crocus sativus). New Phytol. 2019, 224, 725–740. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Morote, L.; Zhu, C.; Ahrazem, O.; Capell, T.; Christou, P.; Gómez-Gómez, L. The Biosynthesis of Non-Endogenous Apocarotenoids in Transgenic Nicotiana glauca. Metabolites 2022, 12, 575. https://doi.org/10.3390/metabo12070575
Huang X, Morote L, Zhu C, Ahrazem O, Capell T, Christou P, Gómez-Gómez L. The Biosynthesis of Non-Endogenous Apocarotenoids in Transgenic Nicotiana glauca. Metabolites. 2022; 12(7):575. https://doi.org/10.3390/metabo12070575
Chicago/Turabian StyleHuang, Xin, Lucía Morote, Changfu Zhu, Oussama Ahrazem, Teresa Capell, Paul Christou, and Lourdes Gómez-Gómez. 2022. "The Biosynthesis of Non-Endogenous Apocarotenoids in Transgenic Nicotiana glauca" Metabolites 12, no. 7: 575. https://doi.org/10.3390/metabo12070575