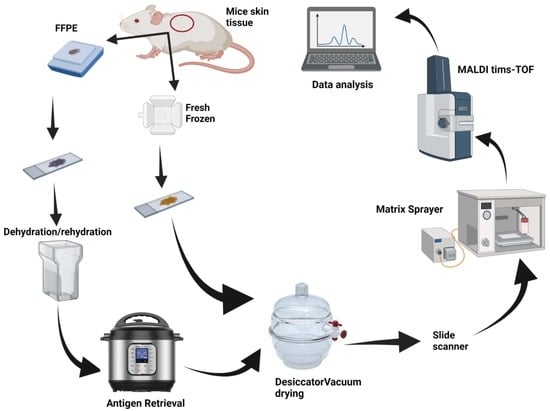
Impact of Skin Tissue Collection Method on Downstream MALDI-Imaging
Abstract
:1. Introduction
2. Results
2.1. MALDI Positive Ion Mode Imaging of FFPE and Fresh Frozen Skin Tissue Samples
2.2. MALDI Negative Ion Mode Imaging of FFPE and Fresh Frozen Skin Tissue Samples
2.3. Spatial Distribution of Metabolites in Skin Tissue via MALD-MSI Combined with Immunohistochemistry
3. Discussion
4. Material and Methods
4.1. Mice
4.2. FFPE Tissue
4.3. Fresh Frozen Tissue
4.4. Matrix Sprayer
4.5. Data Acquisition
4.6. Data Processing and Metabolite Annotations
4.7. Statistical Analysis
4.8. Pathway Analysis
4.9. Immunohistochemistry of Fresh Frozen Tissue
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cacciatore, S.; Zadra, G.; Bango, C.; Penney, K.L.; Tyekucheva, S.; Yanes, O.; Loda, M. Metabolic Profiling in Formalin-Fixed and Paraffin-Embedded Prostate Cancer Tissues. Mol. Cancer Res. 2017, 15, 439–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buck, A.; Ly, A.; Balluff, B.; Sun, N.; Gorzolka, K.; Feuchtinger, A.; Janssen, K.-P.; Kuppen, P.J.K.; van de Velde, C.J.H.; Weirich, G.; et al. High-resolution MALDI-FT-ICR MS imaging for the analysis of metabolites from formalin-fixed, paraffin-embedded clinical tissue samples. J. Pathol. 2015, 237, 123–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorzolka, K.; Walch, A. MALDI mass spectrometry imaging of formalin-fixed paraffin-embedded tissues in clinical research. Histol. Histopathol. 2014, 29, 1365–1376. [Google Scholar] [PubMed]
- Stauber, J.; Lemaire, R.; Franck, J.; Bonnel, D.; Croix†, D.; Day, R.; Wisztorski, M.; Fournier, I.; Salzet, M. MALDI imaging of formalin-fixed paraffin-embedded tissues: Application to model animals of Parkinson disease for biomarker hunting. J. Proteome Res. 2008, 7, 969–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, E. Fresh Frozen Versus Formalin-Fixed Paraffin Embedded for Mass Spectrometry Imaging. Methods Mol. Biol. 2017, 1618, 7–14. [Google Scholar] [PubMed]
- Bien, T.; Bessler, S.; Dreisewerd, K.; Soltwisch, J. Transmission-Mode MALDI Mass Spectrometry Imaging of Single Cells: Optimizing Sample Preparation Protocols. Anal. Chem. 2021, 93, 4513–4520. [Google Scholar] [CrossRef] [PubMed]
- Serafim, V.; Shah, A.; Puiu, M.; Andreescu, N.; Coricovac, D.; Nosyrev, A.; Spandidos, D.A.; Tsatsakis, A.M.; Dehelean, C.; Pinzaru, I. Classification of cancer cell lines using matrix-assisted laser desorption/ionization timeofflight mass spectrometry and statistical analysis. Int. J. Mol. Med. 2017, 40, 1096–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petukhova, V.Z.; Young, A.N.; Wang, J.; Wang, M.; Ladanyi, A.; Kothari, R.; Burdette, J.E.; Sanchez, L.M. Whole Cell MALDI Fingerprinting Is a Robust Tool for Differential Profiling of Two-Component Mammalian Cell Mixtures. J. Am. Soc. Mass Spectrom. 2019, 30, 344–354. [Google Scholar] [CrossRef]
- Spraggins, J.M.; Djambazova, K.V.; Rivera, E.S.; Migas, L.G.; Neumann, E.K.; Fuetterer, A.; Suetering, J.; Goedecke, N.; Ly, A.; van de Plas, R.; et al. High-Performance Molecular Imaging with MALDI Trapped Ion-Mobility Time-of-Flight (timsTOF) Mass Spectrometry. Anal. Chem. 2019, 91, 14552–14560. [Google Scholar] [CrossRef]
- Wisztorski, M.; Franck, J.; Salzet, M.; Fournier, I. MALDI direct analysis and imaging of frozen versus FFPE tissues: What strategy for which sample? Methods Mol. Biol. 2010, 656, 303–322. [Google Scholar] [PubMed]
- De Sio, G.; Smith, A.J.; Galli, M.; Garancini, M.; Chinello, C.; Bono, F.; Pagni, F.; Magn, F. A MALDI-Mass Spectrometry Imaging method applicable to different formalin-fixed paraffin-embedded human tissues. Mol. Biosyst. 2015, 11, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, M.; Chaudhary, P.P.; D’Souza, B.N.; Spathies, J.; Myles, I.A. Impact of Skin Tissue Collection Method on Downstream MALDI-Imaging. Metabolites 2022, 12, 497. https://doi.org/10.3390/metabo12060497
Yadav M, Chaudhary PP, D’Souza BN, Spathies J, Myles IA. Impact of Skin Tissue Collection Method on Downstream MALDI-Imaging. Metabolites. 2022; 12(6):497. https://doi.org/10.3390/metabo12060497
Chicago/Turabian StyleYadav, Manoj, Prem Prashant Chaudhary, Brandon N. D’Souza, Jacquelyn Spathies, and Ian A. Myles. 2022. "Impact of Skin Tissue Collection Method on Downstream MALDI-Imaging" Metabolites 12, no. 6: 497. https://doi.org/10.3390/metabo12060497