Urinary Metabolomics in Young Soccer Players after Winter Training Season
Abstract
:1. Introduction
2. Materials and Methods
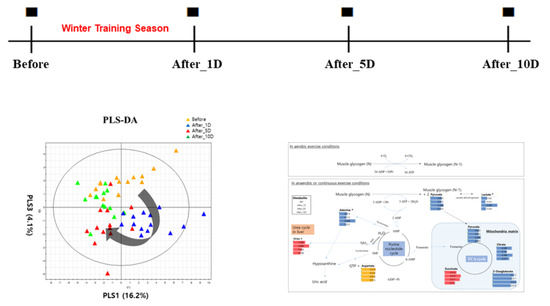
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bloomfield, J.; Polman, R.; O’Donoghue, P. Physical demands of different positions in FA Premier League soccer. J. Sports Sci. Med. 2007, 6, 63–70. [Google Scholar]
- Stølen, T.; Chamari, K.; Castagna, C.; Wisløff, U. Physiology of soccer: An update. Sports Med. 2005, 35, 501–536. [Google Scholar] [CrossRef]
- Yoon, J.H. Effects of Winter season Physical Training on Cardiopulmonary and muscular Function of N league Professional Soccer Players. J. Strength Cond. Res. 2019, 24, 653–660. [Google Scholar]
- Lloyd, R.S.; Oliver, J.L.; Faigenbaum, A.D.; Myer, G.D.; De Ste Croix, M.B. Chronological age vs. biological maturation: Implications for exercise programming in youth. J. Strength Cond. Res. 2014, 28, 1454–1464. [Google Scholar] [CrossRef]
- Romero, S.A.; Minson, C.T.; Halliwill, J.R. The cardiovascular system after exercise. J. Appl. Physiol. 2017, 122, 925–932. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, J.R. Potential causes, mechanisms, and implications of post exercise hypotension. J. Hum. Hypertens. 2002, 16, 225–236. [Google Scholar] [CrossRef] [Green Version]
- Halliwill, J.R. Mechanisms and clinical implications of post-exercise hypotension in humans. Exerc. Sport Sci. Rev. 2001, 29, 65–70. [Google Scholar] [CrossRef]
- Lee, J.D.; Kim, H.Y.; Park, J.J.; Oh, S.B.; Goo, H.; Cho, K.J.; Kim, S.; Kim, K.-B. Metabolomics approach to biomarkers of dry eye disease using 1H-NMR in rats. J. Toxicol. Environ. Health A 2021, 84, 313–333. [Google Scholar] [CrossRef]
- Lee, J.D.; Kim, H.Y.; Kang, K.; Jeong, H.G.; Song, M.-K.; Tae, I.H.; Lee, S.H.; Kim, H.R.; Lee, K.; Chae, S.; et al. Integration of transcriptomics, proteomics and metabolomics identifies biomarkers for pulmonary injury by polyhexamethylene guanidine phosphate (PHMG-p), a humidifier disinfectant, in rats. Arch. Toxicol. 2020, 94, 887–909. [Google Scholar] [CrossRef]
- Kim, H.; Yoo, S.; Lee, J.-D.; Kim, H.-Y.; Kim, S.; Kim, K.-B. A metabolomics approach to sulforaphane efficacy in secondhand smoking-induced pulmonary samage in mice. Metabolites 2022, 12, 518. [Google Scholar] [CrossRef]
- Kim, K.-B.; Lee, B.-M. Metabolomics, a new promising technology for toxicological research. Toxicol. Res. 2009, 25, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Worley, B.; Powers, R. Multivariate Analysis in Metabolomics. Curr. Metab. 2013, 1, 92–107. [Google Scholar] [CrossRef]
- Van Renterghem, P.; Sottas, P.E.; Saugy, M.; Van Eenoo, P. Statistical discrimination of steroid profiles in doping control with support vector machines. Anal. Chim. Acta 2013, 20, 768:41–768:48. [Google Scholar] [CrossRef] [PubMed]
- Persson, F.; Rossing, P. Diagnosis of diabetic kidney disease: State of the art and future perspective. Kidney Int. Suppl. 2018, 8, 2–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Ling, Z.; Xiao, Y.; Yang, Q.; Wang, B.; Zheng, L.; Jiang, P.; Li, L.; Wang, W. Alterations of Urinary Microbiota in Type 2 Diabetes Mellitus with Hypertension and/or Hyperlipidemia. Front. Physiol. 2017, 8, 126. [Google Scholar] [CrossRef] [Green Version]
- Quintas, G.; Reche, X.; Sanjuan-Herráez, J.D.; Martínez, H.; Herrero, M.; Valle, X.; Masa, M.; Rodas, G. Urine metabolomic analysis for monitoring internal load in professional football players. Metabolomics 2020, 16, 45. [Google Scholar] [CrossRef]
- Vike, N.L.; Bari, S.; Stetsiv, K.; Talavage, T.M.; Nauman, E.A.; Papa, L.; Slobounov, S.; Breiter, H.C.; Cornelis, M.C. Metabolomic response to collegiate football participation: Pre- and Post-season analysis. Sci. Rep. 2022, 12, 3091. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Kim, S.; Ryu, H.Y.; Cha, K.S.; Sung, D.J. Exercise-induced rhabdomyolysis mechanisms and prevention: A literature review. J. Sport Health Sci. 2016, 5, 324–333. [Google Scholar] [CrossRef] [Green Version]
- Jang, H.J.; Lee, J.D.; Jeon, H.S.; Kim, A.R.; Kim, S.; Lee, H.S.; Kim, K.B. Metabolic Profiling of Eccentric Exercise-Induced Muscle Damage in Human Urine. Toxicol. Res. 2018, 34, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Gamble, P. Physical preparation for elite-level rugby union football. Strength Cond. J. 2004, 26, 10–23. [Google Scholar] [CrossRef]
- Sternlicht, E.; Rugg, S.G.; Bernstein, M.D.; Armstrong, S.D. Electromyographical analysis and comparison of selected abdominal training devices with a traditional crunch. J. Strength Cond. Res. 2005, 19, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Kidney Int. Suppl. 2020, 2, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Mutch, B.J.; Banister, E.W. Ammonia metabolism in exercise and fatigue: A review. Med. Sci. Sports Exerc. 1983, 15, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Ipata, P.L.; Pesi, R. Metabolic interaction between purine nucleotide cycle and oxypurine cycle during skeletal muscle contraction of different intensities: A biochemical reappraisal. Metabolomics 2018, 14, 42. [Google Scholar] [CrossRef]
- Walker, V. Ammonia toxicity and its prevention in inherited defects of the urea cycle. Diabetes Obes. Metab. 2009, 11, 823–835. [Google Scholar] [CrossRef]
- Refsum, H.E.; Strömme, S.B. Urea and Creatinine Production and Excretion in Urine during and after Prolonged Heavy Exercise. Scand. J. Clin. Lab. Investig. 1974, 33, 247–254. [Google Scholar] [CrossRef]
- Henriksson, J. Effect of exercise on amino acid concentrations in skeletal muscle and plasma. J. Exp. Biol. 1991, 160, 149–165. [Google Scholar] [CrossRef]
- Ishikura, K.; Ra, S.G.; Ohmori, H. Exercise-induced changes in amino acid levels in skeletal muscle and plasma. Phys. Fit. Sports Med. 2013, 2, 301–310. [Google Scholar] [CrossRef] [Green Version]
- Lau, A.; Tymianski, M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflug. Arch. 2010, 460, 525–542. [Google Scholar] [CrossRef]
- Rainesalo, S.; Keränen, T.; Palmio, J.; Peltola, J.; Oja, S.S.; Saransaari, P. Plasma and cerebrospinal fluid amino acids in epileptic patients. Neurochem. Res. 2004, 29, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Miulli, D.E.; Norwell, D.Y.; Schwartz, F.N. Plasma concentrations of glutamate and its metabolites in patients with Alzheimer’s disease. J. Am. Osteopath. Assoc. 1993, 93, 670–676. [Google Scholar] [PubMed]
- Cairns, B.E.; Gambarota, G.; Svensson, P.; Arendt-Nielsen, L.; Berde, C.B. Glutamate-induced sensitization of rat masseter muscle fibers. Neuroscience 2002, 109, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Kim, D.M.; Kim, K.B.; Park, J.W.; Choi, J.Y.; Oh, J.H.; Song, K.D.; Kim, S.; Cho, B.W. Analysis of metabolomic patterns in thoroughbreds before and after exercise. Asian-Australas. J. Anim. Sci. 2017, 30, 1633–1642. [Google Scholar] [CrossRef] [PubMed]
- Wibom, R.; Hultman, E.; Johansson, M.; Matherei, K.; Constantin-Teodosiu, D.; Schantz, P.G. Adaptation of mitochondrial ATP production in human skeletal muscle to endurance training and detraining. J. Appl. Physiol. 1992, 73, 2004–2010. [Google Scholar] [CrossRef]
- Voss, C.M.; Arildsen, L.; Nissen, J.D.; Waagepetersen, H.S.; Schousboe, A.; Maechler, P.; Ott, P.; Vilstrup, H.; Walls, A.B. Glutamate Dehydrogenase Is Important for Ammonia Fixation and Amino Acid Homeostasis in Brain During Hyperammonemia. Front. Neurosci. 2021, 15, 646291. [Google Scholar] [CrossRef]
- Hisanaga, S.; Ueno, N.; Inagaki, H.; Tokura, T.; Uezono, S.; Yokota, N.; Fujimoto, S.; Eto, T. Exercise-induced acute renal failure associated with renal vasoconstriction. Nihon Jinzo Gakkai Shi. 1999, 4, 406–412. (In Japanese) [Google Scholar]
- Gundlapalli, S.; Gaur, Y.; Rao, M.V.; Bande, S.R.; Sandhya, P. Renal Hypouricemia with Exercise Induced Acute Kidney Injury-A Case Report. Indian J. Nephrol. 2021, 31, 307–310. [Google Scholar] [CrossRef]
- Bain, M.A. Accumulation of trimethylamine and trimethylamine-N-oxide in end-stage renal disease patients undergoing haemodialysis. Nephrol. Dial. Transplant. 2006, 21, 1300–1304. [Google Scholar] [CrossRef] [Green Version]
- Riphagen, I.J.; Minović, I.; Groothof, D.; Post, A.; Eggersdorfer, M.L.; Kootstra-Ros, J.E.; de Borst, M.H.; Navis, G.; Muskiet, F.A.J.; Kema, I.P.; et al. Methylmalonic acid, vitamin B12, renal function, and risk of all-cause mortality in the general population: Results from the prospective Lifelines-MINUTHE study. BMC Med. 2020, 18, 380. [Google Scholar] [CrossRef]
- Walter, J.H.; Thompson, G.N.; Leonard, J.V.; Bartlett, K.; Halliday, D. Contribution of amino acid catabolism to propionate production in methylmalonic acidaemia. Lancet 1989, 1, 1298–1299. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, P.; Sklar, R.; Murrell, M.; Swanson, R.A.; Sharp, F.R. Methylmalonyl-CoA mutase induction by cerebral ischemia and neurotoxicity of the mitochondrial toxin methylmalonic acid. J. Neurosci. 1996, 16, 7336–7346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.H.; Wei, F.; Vaziri, N.D.; Cheng, X.L.; Bai, X.; Lin, R.C.; Zhao, Y.Y. Metabolomics insights into chronic kidney disease and modulatory effect of rhubarb against tubulointerstitial fibrosis. Sci. Rep. 2015, 5, 14472. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Liu, L.; Fu, T.; Zhou, Q.; Zhou, D.; Xiao, L.; Liu, J.; Kong, Y.; Xie, H.; Yi, F.; et al. Exercise Inducible Lactate Dehydrogenase B Regulates Mitochondrial Function in Skeletal Muscle. J. Biol. Chem. 2016, 291, 25306–25318. [Google Scholar] [CrossRef] [Green Version]
- Ishii, H.; Nishida, Y. Effect of Lactate Accumulation during Exercise-induced Muscle Fatigue on the Sensorimotor Cortex. J. Phys. Ther. Sci. 2013, 25, 1637–1642. [Google Scholar] [CrossRef] [Green Version]
- Lamb, G.D.; Stephenson, D.G.; Bangsbo, J.; Juel, C. Point:Counterpoint: Lactic acid accumulation is an advantage/disadvantage during muscle activity. J. Appl. Physiol. 2006, 100, 1410–1414. [Google Scholar] [CrossRef]
- Brooks, G.A. The lactate shuttle during exercise and recovery. Med. Sci. Sports Exerc. 1986, 18, 360–368. [Google Scholar] [CrossRef] [Green Version]
- Pavletic, A.J.; Pao, M. Exercise-induced elevation of liver enzymes in a healthy female research volunteer. Psychosomatics 2015, 56, 604–606. [Google Scholar] [CrossRef] [Green Version]
- Clifford, T.; Ventress, M.; Allerton, D.M.; Stansfield, S.; Tang, J.C.Y.; Fraser, W.D.; Vanhoecke, B.; Prawitt, J.; Stevenson, E. The effects of collagen peptides on muscle damage, inflammation and bone turnover following exercise: A randomized, controlled trial. Amino Acids. 2019, 51, 691–704. [Google Scholar] [CrossRef] [Green Version]
- Landaas, S.; Pettersen, J.E. Clinical conditions associated with urinary excretion of 2-hydroxybutyric acid. Scand. J. Clin. Lab. Investig. 1975, 35, 259–266. [Google Scholar] [CrossRef]
- Siopi, A.; Deda, O.; Manou, V.; Kellis, S.; Kosmidis, I.; Komninou, D.; Raikos, N.; Christoulas, K.; Theodoridis, G.A.; Mougios, V. Effects of Different Exercise Modes on the Urinary Metabolic Fingerprint of Men with and without Metabolic Syndrome. Metabolites 2017, 7, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettersson, J.; Hindorf, U.; Persson, P.; Bengtsson, T.; Malmqvist, U.; Werkström, V.; Ekelund, M. Muscular exercise can cause highly pathological liver function tests in healthy men. Br. J. Clin. Pharmacol. 2008, 65, 253–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahlin, K.; Broberg, S. Adenine Nucleotide Depletion in Human Muscle During Exercise: Causality and Significance of AMP Deamination. Int. J. Sports Med. 1990, 11, S62–S67. [Google Scholar] [CrossRef]
- Davison, G.; Vinaixa, M.; McGovern, R.; Beltran, A.; Novials, A.; Correig, X.; McClean, C. Metabolomic Response to Acute Hypoxic Exercise and Recovery in Adult Males. Front. Physiol. 2018, 9, 1682. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Karaca, T.; Al Suleimani, Y.; Al Za’abi, M.; Al Kalbani, J.; Ashique, M.; Nemmar, A. The effect of swimming exercise on adenine-induced kidney disease in rats, and the influence of curcumin or lisinopril thereon. PLoS ONE 2017, 12, e0176316. [Google Scholar] [CrossRef]
- Yokozawa, T.; Zheng, P.D.; Oura, H.; Koizumi, F. Animal model of adenine-induced chronic renal failure in rats. Nephron 1986, 44, 230–234. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-Y.; Lee, J.-D.; Lee, Y.-H.; Seo, S.-W.; Lee, H.-S.; Kim, S.; Kim, K.-B. Urinary Metabolomics in Young Soccer Players after Winter Training Season. Metabolites 2022, 12, 1283. https://doi.org/10.3390/metabo12121283
Kim H-Y, Lee J-D, Lee Y-H, Seo S-W, Lee H-S, Kim S, Kim K-B. Urinary Metabolomics in Young Soccer Players after Winter Training Season. Metabolites. 2022; 12(12):1283. https://doi.org/10.3390/metabo12121283
Chicago/Turabian StyleKim, Hyang-Yeon, Jung-Dae Lee, Yun-Hwan Lee, Sang-Won Seo, Ho-Seong Lee, Suhkmann Kim, and Kyu-Bong Kim. 2022. "Urinary Metabolomics in Young Soccer Players after Winter Training Season" Metabolites 12, no. 12: 1283. https://doi.org/10.3390/metabo12121283