Phragmanthera austroarabica A.G.Mill. and J.A.Nyberg Triggers Apoptosis in MDA-MB-231 Cells In Vitro and In Vivo Assays: Simultaneous Determination of Selected Constituents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Extraction Process
2.2. Determination of Gallic Acid, Catechin, and Methyl Gallate in Methanolic Extract of P. austroarabica Using HPTLC Analysis
2.2.1. Preparation of Standard Solutions of Gallic Acid, Catechin, and Methyl Gallate
2.2.2. Analysis Conditions and Construction of Calibration Curves
2.2.3. Plant Sample Assay
2.3. Assessment of In Vitro Cytotoxic Activity of P. austroarabica
2.3.1. MTT Assay
2.3.2. Annexin V/PI Staining and Cell Cycle Flow Cytometry
2.3.3. Gene Expression Analysis Using RT-PCR
2.3.4. Assessment of Caspase 3/7 Activity
2.3.5. Autophagy Evaluation Using Acridine Orange Quantitative Assessment
2.4. In Vivo Study
2.4.1. Animals
2.4.2. Experiment Design
3. Results and Discussion
3.1. Simultaneous Determination of Gallic Acid, Catechin, and Methyl Gallate in a Methanolic Crude Extract of P. austroarabica Using High-Performance Thin Layer Chromatography (HPTLC)
3.1.1. Linearity
3.1.2. System Precision
3.1.3. Method Precision
3.1.4. Accuracy
3.1.5. Limits of Detection and Quantification
3.1.6. Analytical Solution Stability
3.1.7. Sample Analysis
3.2. In Vitro Activities P. austroarabica Extract
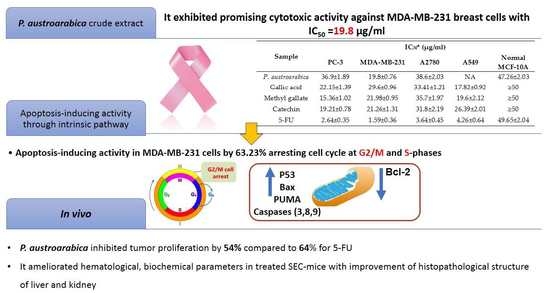
3.2.1. Cytotoxicity of P. austroarabica against PC-3, MDA-MB-231, A2780, and A549 Cancer Cell Lines Using MTT Assay
3.2.2. P. austroarabica Treatment Induced Apoptosis in MDA-MB-231 Cells
3.2.3. P. austroarabica Treatment Affected Gene Expression Analysis of Apoptosis-Related Genes
3.2.4. P. austroarabica Treatment Activated Caspase 3/7 Activity
3.2.5. P. austroarabica Induced MDA-MB-231 Cell Death through Autophagy
3.3. In Vivo Study of P. austroarabica against Solid Ehrlich Carcinoma
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lowy, D.R.; Collins, F.S. Aiming high-changing the trajectory for cancer. N. Engl. J. Med. 2016, 374, 1901–1904. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Kim, Y.H. Cancer prevention from the perspective of global cancer burden patterns. J. Thorac. Dis. 2017, 9, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2020, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Merriel, S.W.D.; Ingle, S.M.; May, M.T.; Martin, R.M. Retrospective cohort study evaluating clinical, biochemical, and pharmacological prognostic factors for prostate cancer progression using primary care data. BMJ 2021, 11, e044420. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.; Massagué, J. Targeting metastatic cancer. Nat. Med. 2021, 27, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Li, S.D. Modifying the tumor microenvironment using nanoparticle therapeutics. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 891–908. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Lu, J.-J.; Ding, J. Natural products in cancer therapy: Past, present and future. Nat. Prod. Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.Y.; Zhang, L.L.; Ding, J.; Lu, J.J. Anticancer drug discovery from Chinese medicinal herbs. Chin. Med. 2018, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L.; Vaishnav, P. Natural products for cancer chemotherapy. Microb. Biotechnol. 2011, 4, 687–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wall, M.E.; Wani, M.C. Camptothecin and taxol: Discovery to clinic--thirteenth Bruce, F. Cain Memorial Award Lecture. Cancer Res. 1995, 55, 753–760. [Google Scholar] [PubMed]
- Wall, M.E. Camptothecin and taxol: Discovery to clinic. Med. Res. Rev. 1998, 18, 299–314. [Google Scholar] [CrossRef]
- Oberlies, N.H.; Kroll, D.J. Camptothecin and taxol: Historic achievements in natural products research. J. Nat. Prod. 2004, 67, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y. Topoisomerase I inhibitors: Camptothecins and beyond. Nat. Rev. Cancer 2006, 6, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Gao, H.; Chen, Y.; Zhu, H.; Cai, Y.; Zhang, X.; Miao, Z.; Jiang, H.; Zhang, J.; Shen, H.; et al. Chimmitecan, a novel 9-substituted camptothecin, with improved anticancer pharmacologic profiles in vitro and in vivo. Clin. Cancer Res. 2007, 13, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Alahdal, F.A.M.; Qashqoosh, M.T.A.; Manea, Y.K.; Salem, M.A.S.; Khan, A.M.T.; Naqvi, S. Eco-friendly synthesis of zinc oxide nanoparticles as nanosensor, nanocatalyst and antioxidant agent using leaf extract of P. austroarabica. OpenNano 2022, 8, 100067. [Google Scholar] [CrossRef]
- Tawfik, M.K.; Badr, J.M. Evaluation of hepatoprotective activity of Plicosepalus acacia and Phragmanthera austroarabica extracts on paracetamol-induced hepatotoxicity in rats. Wulfenia 2012, 19, 325–337. [Google Scholar]
- Nahed, M.; Waly, N.M. Anatomical and statistical analysis of six parasitic Loranthaceae species. Am. J. Res. Commun. 2013, 1, 317–332. [Google Scholar]
- Ibrahim, H.M.; Ajlan, A.A.; Alriany, Y.H.; Al-Gifri, A.N. Correction in Phragmanthera tiegh. (Loranthaceae) in the Flora of AlHujariyah—Taiz Governorate, Yemen. Univ. Aden J. Nat. Appl. Sci. 2014, 18, 449–459. [Google Scholar]
- Hanafy, A.; Badr, J.M. Anti-hyperglycaemic effect of Phragmenthera austroarabica A.G. Mill.& J.A. Nyberg extract in streptozotocin induced diabetes in rats. Nat. Prod. Res. 2014, 28, 2351–2354. [Google Scholar] [CrossRef]
- Almehdar, H.; Abdallah, H.M.; Osman, A.-M.M.; Abdel-Sattar, E.A. In vitro cytotoxic screening of selected Saudi medicinal plants. J. Nat. Med. 2012, 66, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Aldawsari, H.M.; Eid, B.G.; Neamatallah, T.; Zaitone, S.A.; Badr, J.M. Anticonvulsant and neuroprotective activities of Phragmanthera austroarabica extract in pentylenetetrazole-kindled mice. Evid-Based Complement. Altern. Med. 2017, 2017, 5148219. [Google Scholar] [CrossRef] [PubMed]
- Bamane, F.H.; Badr, J.M.; Amin, O.R.M. Antioxidant activities and flavonoid contents of selected plants belonging to Family Loranthaceae. Afr. J. Biotechnol. 2012, 11, 14380–14385. [Google Scholar] [CrossRef]
- Badr, J.M. Chemical constituents of Phragmanthera austroarabica A. G. Mill and J. A. Nyberg with potent antioxidant activity. Pharmacogn. Res. 2014, 7, 335–340. [Google Scholar] [CrossRef]
- Badr, J.M.; Shaala, L.A.; Youssef, D.T.A. Loranthin: A new polyhydroxylated flavanocoumarin from Plicosepalus acacia with significant free radical scavenging and antimicrobial activity. Phytochem. Lett. 2013, 6, 113–117. [Google Scholar] [CrossRef]
- Correa, L.B.; Pádua, T.A.; Seito, L.N.; Costa, T.E.M.M.; Silva, M.A.; Candéa, A.L.P.; Rosas, E.C.; Henriques, M.G. Anti-inflammatory effect of methyl gallate on experimental arthritis: Inhibition of neutrophil recruitment, production of inflammatory mediators, and activation of macrophages. J. Nat. Prod. 2016, 79, 1554–1566. [Google Scholar] [CrossRef]
- BenSaad, L.A.; Kim, K.H.; Quah, C.C.; Kim, W.R.; Shahimi, M. Anti-inflammatory potential of ellagic acid, gallic acid and punicalagin A&B isolated from Punica granatum. BMC Complement. Altern. Med. 2017, 17, 47. [Google Scholar] [CrossRef]
- Fan, F.Y.; Sang, L.X.; Min Jiang, M. Catechins and their therapeutic benefits to inflammatory bowel disease. Molecules 2017, 22, 484. [Google Scholar] [CrossRef]
- Mates, J.M. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 2000, 153, 83–104. [Google Scholar] [CrossRef]
- Rao, Y.K.; Geethangili, M.; Fang, S.-H.; Tzeng, Y.-M. Antioxidant and cytotoxic activities of naturally occurring phenolic and related compounds: A comparative study. Food Chem. Toxicol. 2007, 45, 1770–1776. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell. Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Harput, U.S.; Saracoglu, I.; Inoue, M.; Ogihara, Y. Anti-inflammatory and cytotoxic activities of five Veronica species. Biol. Pharm. Bull. 2002, 25, 483–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemudzivhadi, V.; Masoko, M. In vitro assessment of cytotoxicity, antioxidant, and anti-Inflammatory activities of Ricinus communis (Euphorbiaceae) leaf extracts. Evid. Based Complement. Alternat. Med. 2014, 2014, 625961. [Google Scholar] [CrossRef] [PubMed]
- Kurek, J.; Myszkowski, K.; Okulicz-Kozaryn, I.; Kurant, A.; Kamińska, E.; Szulc, M.; Rubiś, B.; Kaczmarek, M.; Mikołajczak, P.; Murias, M. Cytotoxic, analgesic and anti-inflammatory activity of colchicine and its C-10 sulfur containing derivatives. Sci. Rep. 2021, 11, 9034. [Google Scholar] [CrossRef]
- Elhady, S.S.; Abdelhameed, R.F.A.; Mehanna, E.T.; Wahba, A.S.; Elfaky, M.A.; Koshak, A.E.; Noor, A.O.; Bogari, H.A.; Malatani, R.T.; Goda, M.S. Metabolic profiling, chemical composition, antioxidant capacity, and in vivo hepato- and nephroprotective effects of Sonchus cornutus in mice exposed to cisplatin. Antioxidants 2022, 11, 819. [Google Scholar] [CrossRef]
- Abdel-Hamed, A.R.; Mehanna, E.T.; Hazem, R.M.; Badr, J.M.; Abo-Elmatty, D.M.; Abdel-Kader, M.S.; Goda, M.S. Plicosepalus acacia extract and its major constituents, methyl gallate and quercetin, potentiate therapeutic angiogenesis in diabetic hind limb ischemia: HPTLC quantification and LC-MS/MS metabolic profiling. Antioxidants 2021, 10, 1701. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Chang, Y.-J.; Wei, P.-L.; Hung, C.-S.; Wang, W. Methyl gallate, gallic acid-derived compound, inhibit cell proliferation through increasing ROS production and apoptosis in hepatocellular carcinoma cells. PLoS ONE 2021, 16, e0248521. [Google Scholar] [CrossRef]
- Lee, H.; Lee, H.; Kwon, Y.; Lee, J.-H.; Kim, J.; Shin, M.-K.; Kim, S.-H.; Bae, H. Methyl gallate exhibits potent antitumor activities by inhibiting tumor infiltration of CD4+CD25+ regulatory T cells. J. Immunol. 2010, 185, 6698–6705. [Google Scholar] [CrossRef]
- Subramanian, A.P.; John, A.A.; Vellayappan, M.V.; Balaji, A.; Jaganathan, S.K.; Supriyanto, E.; Yusof, M. Gallic acid: Prospects and molecular mechanisms of its anticancer activity. RSC Adv. 2015, 5, 35608–35621. [Google Scholar] [CrossRef]
- Tsai, C.-L.; Chiu, Y.-M.; Ho, T.-Y.; Hsieh, C.-T.; Shieh, D.-C.; Lee, Y.-J.; Tsay, G.J.; Wu, Y.-Y. Gallic acid induces apoptosis in human gastric adenocarcinoma cells. Anticancer Res. 2018, 38, 2057–2067. [Google Scholar] [PubMed]
- Zhang, T.; Ma, L.; Wu, P.; Li, W.; Li, T.; Gu, R.; Dan, X.; Li, Z.; Fan, X.; Xiao, Z. Gallic acid has anticancer activity and enhances the anticancer effects of cisplatin in non-small cell lung cancer A549 cells via the JAK/STAT3 signaling pathway. Oncol. Rep. 2019, 41, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yin, M.; Hao, D.; Shen, Y. Anti-cancer activity of catechin against A549 lung carcinoma cells by induction of cyclin kinase inhibitor P21 and suppression of cyclin E1 and P–AKT. Appl. Sci. 2020, 10, 2065. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.M.; Heo, H.J. The roles of catechins in regulation of systemic inflammation. Food Sci. Biotechnol. 2022, 31, 957–970. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Nafie, M.S.; Boraei, A.T.A. Exploration of novel VEGFR2 tyrosine kinase inhibitors via design and synthesis of new alkylated indolyl-triazole Schiff bases for targeting breast cancer. Bioorg. Chem. 2022, 122, 105708. [Google Scholar] [CrossRef]
- Nafie, M.S.; Kishk, S.M.; Mahgoub, S.; Amer, A.M. Quinoline-based thiazolidinone derivatives as potent cytotoxic and apoptosis-inducing agents through EGFR inhibition. Chem. Biol. Drug Des. 2022, 99, 547–560. [Google Scholar] [CrossRef]
- Abdelhameed, R.F.A.; Elhady, S.S.; Sirwi, A.; Samir, H.; Ibrahim, E.A.; Thomford, A.K.; El Gindy, A.; Hadad, G.M.; Badr, J.M.; Nafie, M.S. Thonningia sanguinea extract: Antioxidant and cytotoxic activities supported by chemical composition and molecular docking simulations. Plants 2021, 10, 2156. [Google Scholar] [CrossRef]
- Gad, E.M.; Nafie, M.S.; Eltamany, E.H.; Hammad, M.S.A.G.; Barakat, A.; Boraei, A.T.A. Discovery of new apoptosis-inducing agents for breast cancer based on ethyl 2- amino-4,5,6,7-tetra hydrobenzo[b]thiophene-3-carboxylate: Synthesis, in vitro, and in vivo activity evaluation. Molecules 2020, 25, 2523. [Google Scholar] [CrossRef]
- ElZahabi, H.S.A.; Nafie, M.S.; Osman, D.; Elghazawy, N.H.; Soliman, D.H.; EL-Helby, A.A.H.; Arafa, R.K. Design, synthesis, and evaluation of new quinazolin-4-one derivativesas apoptotic enhancers and autophagy inhibitors with potent antitumor activity. Eur. J. Med. Chem. 2021, 222, 113609. [Google Scholar] [CrossRef]
- Goda, M.S.; Nafie, M.S.; Awad, B.M.; Abdel-Kader, M.S.; Ibrahim, A.K.; Badr, J.M.; Eltamany, E.E. In vitro and in vivo studies of anti-lung cancer activity of Artemesia judaica L. crude extract combined with LC-MS/MS metabolic profiling, docking simulation and HPLC-DAD quantification. Antioxidants 2021, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, R.S.; Seif El-Din, A.A.; Abu-Serie, M.; Abd El Rahman, N.M.; El-Demellawy, M.; Metwally, A.M. Investigation of in vitro cytotoxic and potential anticancer activities of flavonoidal aglycones from Egyptian Propolis. Rec. Pharm. Biomed. Sci. 2017, 2, 13–20. [Google Scholar] [CrossRef]
- Abdelhameed, R.F.A.; Habib, E.S.; Goda, M.S.; Fahim, J.R.; Hassanean, H.A.; Eltamany, E.E.; Ibrahim, A.K.; AboulMagd, A.M.; Fayez, S.; El-kader, A.M.A.; et al. Thalassosterol, a new cytotoxic aromatase inhibitor ergosterol derivative from the Red Sea seagrass Thalassodendron ciliatum. Mar. Drugs 2020, 18, 354. [Google Scholar] [CrossRef]
- Xu, H.; Li, Y.; Han, B.; Li, Z.; Wang, B.; Jiang, P.; Zhang, J.; Ma, W.; Zhou, D.; Li, X.; et al. Anti-breast-cancer activity exerted by β-sitosterol-d-glucoside from sweet potato via upregulation of microRNA-10a and via the PI3K-Akt signaling pathway. J. Agric. Food Chem. 2018, 66, 9704–9718. [Google Scholar] [CrossRef]
- Wang, M.; Cui, H.-X.; Sun, C.; Li, G.; Wang, H.-I.; Xia, C.-H.; Wang, Y.-C.; Liu, J.-C. Effect of lupeol on migration and invasion of human breast cancer MDA-MB-231 cells and its mechanism. Acta Pharm. Sin. 2016, 51, 558–562. [Google Scholar] [CrossRef]
- Zhang, X.; TLi, T.; Gong, F.S.; Liu, R.H. Antiproliferative activity of ursolic acid in MDA-MB-231 human breast cancer cells through Nrf2 pathway regulation. J. Agric. Food Chem. 2020, 68, 7404–7415. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Zhou, Q.; Lu, Y.; Zhang, H.; Chen, Q.; Zhao, M.; Su, S. Inhibitory effect of emodin on migration, invasion and metastasis of human breast cancer MDA-MB-231 cells in vitro and in vivo. Oncol. Rep. 2014, 33, 338–346. [Google Scholar] [CrossRef]
- Ren, L.; Li, Z.; Dai, C.; Zhao, D.; Wang, Y.; Ma, C.; Liu, C. Chrysophanol inhibits proliferation and induces apoptosis through NF-κB/cyclin D1 and NF-κB/Bcl-2 signaling cascade in breast cancer cell lines. Mol. Med. Rep. 2018, 17, 4376–4382. [Google Scholar] [CrossRef]
- Ranganathan, S.; Halagowder, D.; Devaraj, N.; Sivasithambaram, N.D. Quercetin suppresses twist to induce apoptosis in MCF-7 breast cancer cells. PLoS ONE 2015, 22, e0141370. [Google Scholar] [CrossRef]
- Devipriya, S.; Vani, G.; Ramamurthy, N.; Shyamaladevi, C.S. Regulation of intracellular calcium levels and urokinase activity in MDA MB 231 cells by quercetin. Chemotherapy 2006, 52, 60–65. [Google Scholar] [CrossRef]
- Chisholm, K.; Bray, B.J.; Rosengren, R.J. Tamoxifen and epigallocatechin gallate are synergistically cytotoxic to MDA-MB-231 human breast cancer cells. Anti-Cancer Drugs 2004, 15, 889–897. [Google Scholar] [CrossRef]
- Schroder, L.; Marahrens, P.; Koch, J.G.; Heidegger, H.; Vilsmeier, T.; Phan-Brehm, T.; Hofmann, S.; Mahner, S.; Jeschke, U.; Richter, D.U. Effects of green tea, matcha tea and their components epigallocatechin gallate and quercetin on MCF 7 and MDA-MB-231 breast carcinoma cell. Oncol. Rep. 2019, 41, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Eltamany, E.E.; Elhady, S.S.; Ahmed, H.A.; Badr, J.M.; Noor, A.O.; Ahmed, S.A.; Nafie, M.S. Chemical profiling, antioxidant, cytotoxic activities and molecular docking simulation of Carrichtera annua DC. (Cruciferae). Antioxidants 2020, 9, 1286. [Google Scholar] [CrossRef] [PubMed]
- Abdelhameed, R.F.A.; Habib, E.S.; Ibrahim, A.K.; Yamada, K.; Abdel-Kader, M.S.; Ibrahim, A.K.; Ahmed, S.A.; Badr, J.M.; Nafie, M.S. Chemical profiling, cytotoxic activities through apoptosis induction in MCF-7 cells and molecular docking of Phyllostachys heterocycla bark nonpolar extract. J. Biomol. Struct. Dyn. 2021, 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nafie, M.S.; Arafa, K.; Sedky, N.K.; Alakhdar, A.A.; Arafa, R.K. Triaryl dicationic DNA minor-groove binders with antioxidant activity display cytotoxicity and induce apoptosis in breast cancer. Chem.-Biol. Interact. 2020, 324, 109087. [Google Scholar] [CrossRef]
- Khalifa, M.M.; Al-Karmalawy, A.A.; Elkaeed, E.B.; Nafie, M.S.; Tantawy, M.A.; Eissa, I.H.; Mahdy, H.A. Topo II Inhibition and DNA Intercalation by New Phthalazine-Based Derivatives as Potent Anticancer Agents: Design, Synthesis, Anti-Proliferative, Docking, and in Vivo Studies. J. Enzym. Inhib. Med. Chem. 2022, 37, 299–314. [Google Scholar] [CrossRef]







| Validation Parameters | Scanned at λ 280 nm | ||
|---|---|---|---|
| Methyl Gallate | Catechin | Gallic Acid | |
| Linearity range (µg/band) | 0.8–9 | 0.4–9 | 0.8–9 |
| Correlation coefficient (R2) | 0.99 | 0.99 | 0.99 |
| Regression equation | Y = 5926.9X + 1684.1 | Y = 2175.9X + 1352.9 | Y = 6831.9X − 1984.2 |
| Limit of detection (µg/band) | 0.1 | 0.09 | 0.11 |
| Limit of quantification | 0.31 | 0.29 | 0.34 |
| System precision [%RSD] | 3.72 | 3.27 | 2.74 |
| Method precision [%RSD] | 3.29 | 1.97 | 3.91 |
| % Recovery | 95.89 | 95.58 | 96.64 |
| Conc. (mg/g extract) | 14.5 | 6.5 | 43.6 |
| Sample | IC50 * (μg/mL) | ||||
|---|---|---|---|---|---|
| PC-3 | MDA-MB-231 | A2780 | A549 | Normal MCF-10A | |
| P. austroarabica | 36.9 ± 1.89 | 19.8 ± 0.76 | 38.6 ± 2.03 | NA | 47.26 ± 2.03 |
| Gallic acid | 22.15 ± 1.39 | 29.6 ± 0.96 | 33.41 ± 1.21 | 17.82 ± 0.92 | ≥50 |
| Methyl gallate | 15.36 ± 1.02 | 21.98 ± 0.95 | 35.7 ± 1.97 | 19.6 ± 2.12 | ≥50 |
| Catechin | 19.21 ± 0.78 | 21.26 ± 1.31 | 31.8 ± 2.19 | 26.39 ± 2.01 | ≥50 |
| 5-FU | 2.64 ± 0.35 | 1.59 ± 0.36 | 3.64 ± 0.45 | 4.26 ± 0.64 | 49.65 ± 2.04 |
| Sample | Gene Expression (Fold Change) * | ||||||
|---|---|---|---|---|---|---|---|
| Pro-Apoptotic Genes | Anti-Apoptotic Gene | ||||||
| P53 | PUMA | Bax | Casp-3 | Casp-8 | Casp-9 | Bcl-2 | |
| Cont./ MDA-MB-231 | 1 | ||||||
| P. austroarabica MDA-MB-231 | 9.28 ± 1.38 | 9.39 ± 1.76 | 7.39 ± 1.38 | 10.36 ± 1.98 | 2.08 ± 0.98 | 12.39 ± 1.67 | 0.53 ± 0.01 |
| Parameters | Normal Control | SEC Control | SEC + P. austroarabica | SEC + 5-FU | |
|---|---|---|---|---|---|
| Anti-tumor potentiality | Tumor weight (mg) | -- | 203.6 ± 4.26 | 96.8 ± 2.34 | 78.3 ± 2.38 |
| Tumor volume (mm3) | -- | 356.9 ± 22.3 | 168.8 ± 19.8 | 126.2 ± 18.6 | |
| Tumor inhibition ratio (TIR%) | -- | -- | 54.26 ± 1.36 | 64.7 ± 1.65 | |
| Hematological parameters | Hb (g/dL) | 8.16 ± 0.67 | 3.69 * ± 0.6 | 7.12 # ± 0.64 | 7.89 # ± 0.54 |
| RBC’s count (×106/μL) | 5.98 ± 0.56 | 2.19 * ± 0.54 | 5.01 # ± 0.56 | 5.21 # ± 0.44 | |
| WBC’s count (×103/μL) | 3.27 ± 0.34 | 6.63 * ± 0.41 | 4.01 # ± 0.55 | 3.69 # ± 0.69 | |
| Liver and kidney parameters | ALT (I/U) | 43.3 ± 1.23 | 66.5 * ± 1.99 | 52.4 # ± 1.4 | 48.5 # ± 1.7 |
| AST (I/U) | 46.5 ± 0.78 | 92.6 * ± 1.45 | 56.1 # ± 2.0 | 50.8 # ± 1.5 | |
| Urea (mg/dL) | 23.2 ± 1.06 | 41.3 * ± 1.01 | 30.3 # ± 1.36 | 30.3 # ± 1.01 | |
| Creatinine (mg/dL) | 0.76 ± 0.02 | 1.01 ± 0.17 | 0.87 ± 0.01 | 0.64 ± 0.06 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goda, M.S.; Elhady, S.S.; Nafie, M.S.; Bogari, H.A.; Malatani, R.T.; Hareeri, R.H.; Badr, J.M.; Donia, M.S. Phragmanthera austroarabica A.G.Mill. and J.A.Nyberg Triggers Apoptosis in MDA-MB-231 Cells In Vitro and In Vivo Assays: Simultaneous Determination of Selected Constituents. Metabolites 2022, 12, 921. https://doi.org/10.3390/metabo12100921
Goda MS, Elhady SS, Nafie MS, Bogari HA, Malatani RT, Hareeri RH, Badr JM, Donia MS. Phragmanthera austroarabica A.G.Mill. and J.A.Nyberg Triggers Apoptosis in MDA-MB-231 Cells In Vitro and In Vivo Assays: Simultaneous Determination of Selected Constituents. Metabolites. 2022; 12(10):921. https://doi.org/10.3390/metabo12100921
Chicago/Turabian StyleGoda, Marwa S., Sameh S. Elhady, Mohamed S. Nafie, Hanin A. Bogari, Raina T. Malatani, Rawan H. Hareeri, Jihan M. Badr, and Marwa S. Donia. 2022. "Phragmanthera austroarabica A.G.Mill. and J.A.Nyberg Triggers Apoptosis in MDA-MB-231 Cells In Vitro and In Vivo Assays: Simultaneous Determination of Selected Constituents" Metabolites 12, no. 10: 921. https://doi.org/10.3390/metabo12100921