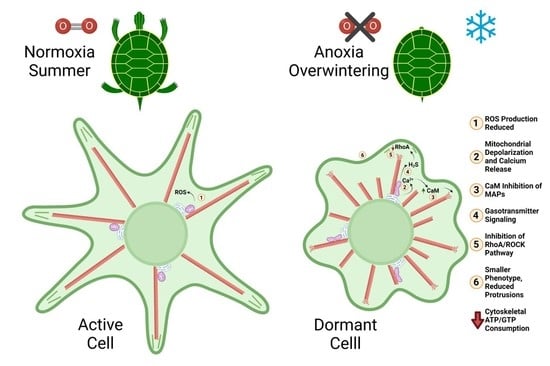
Cytoskeletal Arrest: An Anoxia Tolerance Mechanism
Abstract
:1. The Anoxia-Tolerant Western Painted Turtle
2. Cytoskeletal Dynamics Consume ATP
3. The Turtle Cytoskeleton
4. Cytoskeletal Shrinkage in Overwintering Animals
5. Calcium Signalling Is Associated with Cytoskeletal Inhibition
6. Cytosolic Calcium Increases Marginally in Anoxic Turtle Cells
7. Reduced ROS Production in Anoxic Animals May Protect Microtubules from Catastrophic Loss of Structure
8. Gasotransmitters Involved in Anoxic Metabolic Rate Depression and Their Impact on Cytoskeletal Dynamics
9. MMP Depolarizes Marginally in Anoxic Turtle Cells
10. Mitochondrial Distribution and Dynamics on the Anoxic Cytoskeleton
11. Summary of Cytoskeletal Arrest Theory
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, W.F.; Garg, S.; Zimorski, V. Endosymbiotic Theories for Eukaryote Origin. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storey, K.B. Adventures in Oxygen Metabolism. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2004, 139, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Scholander, P.F.; Irving, L.; Grinnell, S.W. On the Temperature and Metabolism of the Seal during Diving. J. Cell. Comp. Physiol. 1942, 19, 67–78. [Google Scholar] [CrossRef]
- Jackson, D.C. Metabolic Depression and Oxygen Depletion in the Diving Turtle. J. Appl. Physiol. 1968, 24, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.C. Living without Oxygen: Lessons from the Freshwater Turtle. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2000, 125, 299–315. [Google Scholar] [CrossRef]
- Milton, S.L.; Prentice, H.M. Beyond Anoxia: The Physiology of Metabolic Downregulation and Recovery in the Anoxia-Tolerant Turtle. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 147, 277–290. [Google Scholar] [CrossRef] [Green Version]
- Buck, L.T.; Pamenter, M.E. The Hypoxia-Tolerant Vertebrate Brain: Arresting Synaptic Activity. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Fraser, K.P.; Houlihan, D.F.; Lutz, P.L.; Leone-Kabler, S.; Manuel, L.; Brechin, J.G. Complete Suppression of Protein Synthesis during Anoxia with No Post-Anoxia Protein Synthesis Debt in the Red-Eared Slider Turtle Trachemys Scripta Elegans. J. Exp. Biol. 2001, 204, 4353–4360. [Google Scholar] [CrossRef]
- Keenan, S.W.; Hill, C.A.; Kandoth, C.; Buck, L.T.; Warren, D.E. Transcriptomic Responses of the Heart and Brain to Anoxia in the Western Painted Turtle. PLoS ONE 2015, 10, e0131669. [Google Scholar] [CrossRef]
- Bickler, P.E.; Buck, L.T. Hypoxia Tolerance in Reptiles, Amphibians, and Fishes: Life with Variable Oxygen Availability. Annu. Rev. Physiol. 2007, 69, 145–170. [Google Scholar] [CrossRef]
- Buck, L.T.; Land, S.C.; Hochachka, P.W. Anoxia-Tolerant Hepatocytes: Model System for Study of Reversible Metabolic Suppression. Am. J. Physiol. 1993, 265, R49–R56. [Google Scholar] [CrossRef]
- Hochachka, P.W.; Buck, L.T.; Doll, C.J.; Land, S.C. Unifying Theory of Hypoxia Tolerance: Molecular/Metabolic Defense and Rescue Mechanisms for Surviving Oxygen Lack. Proc. Natl. Acad. Sci. USA 1996, 93, 9493–9498. [Google Scholar] [CrossRef] [Green Version]
- Pollard, T.D.; Goldman, R.D. Overview of the Cytoskeleton from an Evolutionary Perspective. Cold Spring Harb. Perspect. Biol. 2018, 10, a030288. [Google Scholar] [CrossRef] [PubMed]
- Goodson, H.V.; Jonasson, E.M. Microtubules and Microtubule-Associated Proteins. Cold Spring Harb. Perspect. Biol. 2018, 10, a022608. [Google Scholar] [CrossRef] [PubMed]
- Kadzik, R.S.; Homa, K.E.; Kovar, D.R. F-Actin Cytoskeleton Network Self-Organization Through Competition and Cooperation. Annu. Rev. Cell Dev. Biol. 2020, 36, 35–60. [Google Scholar] [CrossRef]
- Fletcher, D.A.; Mullins, R.D. Cell Mechanics and the Cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svitkina, T. The Actin Cytoskeleton and Actin-Based Motility. Cold Spring Harb. Perspect. Biol. 2018, 10, a018267. [Google Scholar] [CrossRef] [Green Version]
- Percipalle, P.; Vartiainen, M. Cytoskeletal Proteins in the Cell Nucleus: A Special Nuclear Actin Perspective. MBoC 2019, 30, 1781–1785. [Google Scholar] [CrossRef]
- Moore, A.S.; Wong, Y.C.; Simpson, C.L.; Holzbaur, E.L.F. Dynamic Actin Cycling through Mitochondrial Subpopulations Locally Regulates the Fission–Fusion Balance within Mitochondrial Networks. Nat. Commun. 2016, 7, 1–13. [Google Scholar] [CrossRef]
- Titus, M.A. Myosin-Driven Intracellular Transport. Cold Spring Harb. Perspect. Biol. 2018, 10, a021972. [Google Scholar] [CrossRef]
- Stricker, J.; Falzone, T.; Gardel, M.L. Mechanics of the F-Actin Cytoskeleton. J. Biomech. 2010, 43, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Murrell, M.; Oakes, P.W.; Lenz, M.; Gardel, M.L. Forcing Cells into Shape: The Mechanics of Actomyosin Contractility. Nat. Rev. Mol. Cell Biol. 2015, 16, 486–498. [Google Scholar] [CrossRef]
- Etienne-Manneville, S. Cytoplasmic Intermediate Filaments in Cell Biology. Annu. Rev. Cell Dev. Biol. 2018, 34, 1–28. [Google Scholar] [CrossRef]
- Thuillier, R.; Hauet, T. Impact of Hypothermia and Oxygen Deprivation on the Cytoskeleton in Organ Preservation Models. BioMed Res. Int. 2018, 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonder, E.M.; Fishkind, D.J.; Mooseker, M.S. Direct Measurement of Critical Concentrations and Assembly Rate Constants at the Two Ends of an Actin Filament. Cell 1983, 34, 491–501. [Google Scholar] [CrossRef]
- Hyman, A.A.; Salser, S.; Drechsel, D.N.; Unwin, N.; Mitchison, T.J. Role of GTP Hydrolysis in Microtubule Dynamics: Information from a Slowly Hydrolyzable Analogue, GMPCPP. MBoC 1992, 3, 1155–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanghvi-Shah, R.; Weber, G.F. Intermediate Filaments at the Junction of Mechanotransduction, Migration, and Development. Front. Cell Dev. Biol. 2017, 5, 81. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, D.F.; Brown, G.C. Cellular Energy Utilization and Molecular Origin of Standard Metabolic Rate in Mammals. Physiol. Rev. 1997, 77, 731–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolomeisky, A.B. Motor Proteins and Molecular Motors: How to Operate Machines at Nanoscale. J. Phys. Condens. Matter 2013, 25, 463101. [Google Scholar] [CrossRef] [Green Version]
- Lavine, M.S. Cell Spreading Affects Energy Consumption. Science 2020, 370, 806. [Google Scholar] [CrossRef]
- Xie, J.; Bao, M.; Hu, X.; Koopman, W.J.H.; Huck, W.T.S. Energy Expenditure during Cell Spreading Influences the Cellular Response to Matrix Stiffness. Biomaterials 2021, 267, 120494. [Google Scholar] [CrossRef]
- Rosenmund, C.; Westbrook, G.L. Calcium-Induced Actin Depolymerization Reduces NMDA Channel Activity. Neuron 1993, 10, 805–814. [Google Scholar] [CrossRef]
- Fernie, A.R.; Zhang, Y.; Sampathkumar, A. Cytoskeleton Architecture Regulates Glycolysis Coupling Cellular Metabolism to Mechanical Cues. Trends Biochem. Sci. 2020, 45, 637–638. [Google Scholar] [CrossRef] [PubMed]
- Cantiello, H.F. Role of Actin Filament Organization in Cell Volume and Ion Channel Regulation. J. Exp. Zool. 1997, 279, 425–435. [Google Scholar] [CrossRef]
- Engl, E.; Attwell, D. Non-Signalling Energy Use in the Brain. J. Physiol. (Lond.) 2015, 593, 3417–3429. [Google Scholar] [CrossRef] [Green Version]
- Rangaraju, V.; Calloway, N.; Ryan, T.A. Activity-Driven Local ATP Synthesis Is Required for Synaptic Function. Cell 2014, 156, 825–835. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, B.W.; Bamburg, J.R. Actin-ATP Hydrolysis Is a Major Energy Drain for Neurons. J. Neurosci. 2003, 23, 1–6. [Google Scholar] [CrossRef]
- Kim, C.-H.; Lisman, J.E. A Role of Actin Filament in Synaptic Transmission and Long-Term Potentiation. J. Neurosci. 1999, 19, 4314–4324. [Google Scholar] [CrossRef] [Green Version]
- Edson, K.J.; Lim, S.S.; Borisy, G.G.; Letourneau, P.C. FRAP Analysis of the Stability of the Microtubule Population along the Neurites of Chick Sensory Neurons. Cell Motil. Cytoskelet. 1993, 25, 59–72. [Google Scholar] [CrossRef]
- Mitchison, T.J. Compare and Contrast Actin Filaments and Microtubules. MBoC 1992, 3, 1309–1315. [Google Scholar] [CrossRef] [Green Version]
- Star, E.N.; Kwiatkowski, D.J.; Murthy, V.N. Rapid Turnover of Actin in Dendritic Spines and Its Regulation by Activity. Nat. Neurosci. 2002, 5, 239–246. [Google Scholar] [CrossRef]
- Engl, E.; Jolivet, R.; Hall, C.N.; Attwell, D. Non-Signalling Energy Use in the Developing Rat Brain. J. Cereb. Blood Flow Metab. 2017, 37, 951–966. [Google Scholar] [CrossRef] [Green Version]
- Harris, J.J.; Attwell, D. The Energetics of CNS White Matter. J. Neurosci. 2012, 32, 356–371. [Google Scholar] [CrossRef]
- Miles, A.R.; Hawrysh, P.J.; Hossein-Javaheri, N.; Buck, L.T. Taurine Activates Glycine and GABAA Receptor Currents in Anoxia-Tolerant Painted Turtle Pyramidal Neurons. J. Exp. Biol. 2018, 221, jeb181529. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Moore, J.K. Microtubule Dynamics at Low Temperature: Evidence That Tubulin Recycling Limits Assembly. Mol. Biol. Cell 2020, 31, 1154–1166. [Google Scholar] [CrossRef] [PubMed]
- Zieseniss, A. Hypoxia and the Modulation of the Actin Cytoskeleton—Emerging Interrelations. Hypoxia (Auckl.) 2014, 2, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kloc, M.; Li, X.C.; Ghobrial, R.M. RhoA Cytoskeletal Pathway to Transplantation. J. Immunol. Clin. Res. 2014, 2, 1012–2014. [Google Scholar]
- Cao, H.; Yu, D.; Yan, X.; Wang, B.; Yu, Z.; Song, Y.; Sheng, L. Hypoxia Destroys the Microstructure of Microtubules and Causes Dysfunction of Endothelial Cells via the PI3K/Stathmin1 Pathway. Cell Biosci. 2019, 9, 20. [Google Scholar] [CrossRef] [Green Version]
- Copple, B.L. Hypoxia Stimulates Hepatocyte Epithelial to Mesenchymal Transition by Hypoxiainducible Factor- and Transforming Growth Factor-β-Dependent Mechanisms. Liver Int. 2010, 30, 669–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, S.J.; Hosford, M.A.; Molitoris, B.A. Mechanism of Actin Polymerization in Cellular ATP Depletion. J. Biol. Chem. 2004, 279, 5194–5199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, C.-Y.; Xiong, T.-Q.; Tan, B.-H.; Gui, Y.; Ye, N.; Li, S.-L.; Li, Y.-C. The Temporal and Spatial Changes of Actin Cytoskeleton in the Hippocampal CA1 Neurons Following Transient Global Ischemia. Brain Res. 2019, 1720, 146297. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.E.; Chow, E.J.; Haddad, G.G. State of Actin Filaments Is Changed by Anoxia in Cultured Rat Neocortical Neurons. Neuroscience 1998, 82, 421–427. [Google Scholar] [CrossRef]
- Gisselsson, L.; Toresson, H.; Ruscher, K.; Wieloch, T. Rho Kinase Inhibition Protects CA1 Cells in Organotypic Hippocampal Slices during in Vitro Ischemia. Brain Res. 2010, 1316, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Welch, M.D. The World According to Arp: Regulation of Actin Nucleation by the Arp2/3 Complex. Trends Cell Biol. 1999, 9, 423–427. [Google Scholar] [CrossRef]
- Smith, R.W.; Cash, P.; Hogg, D.W.; Buck, L.T. Proteomic Changes in the Brain of the Western Painted Turtle (Chrysemys Picta Bellii) during Exposure to Anoxia. Proteomics 2015, 15, 1587–1597. [Google Scholar] [CrossRef]
- Gremm, D.; Wegner, A. Gelsolin as a Calcium-Regulated Actin Filament-Capping Protein. Eur. J. Biochem. 2000, 267, 4339–4345. [Google Scholar] [CrossRef]
- Odorizzi, G. The Multiple Personalities of Alix. J. Cell Sci. 2006, 119, 3025–3032. [Google Scholar] [CrossRef] [Green Version]
- Pan, S.; Wang, R.; Zhou, X.; He, G.; Koomen, J.; Kobayashi, R.; Sun, L.; Corvera, J.; Gallick, G.E.; Kuang, J. Involvement of the Conserved Adaptor Protein Alix in Actin Cytoskeleton Assembly. J. Biol. Chem. 2006, 281, 34640–34650. [Google Scholar] [CrossRef] [Green Version]
- Laporte, M.H.; Chatellard, C.; Vauchez, V.; Hemming, F.J.; Deloulme, J.-C.; Vossier, F.; Blot, B.; Fraboulet, S.; Sadoul, R. Alix Is Required during Development for Normal Growth of the Mouse Brain. Sci. Rep. 2017, 7, 44767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabezas, A.; Bache, K.G.; Brech, A.; Stenmark, H. Alix Regulates Cortical Actin and the Spatial Distribution of Endosomes. J. Cell Sci. 2005, 118, 2625–2635. [Google Scholar] [CrossRef] [Green Version]
- Lutz, P.L.; Milton, S.L. Negotiating Brain Anoxia Survival in the Turtle. J. Exp. Biol. 2004, 207, 3141–3147. [Google Scholar] [CrossRef] [Green Version]
- Milton, S.L.; Thompson, J.W.; Lutz, P.L. Mechanisms for Maintaining Extracellular Glutamate Levels in the Anoxic Turtle Striatum. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, R1317–R1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Land, S.C.; Hochachka, P.W. Protein Turnover during Metabolic Arrest in Turtle Hepatocytes: Role and Energy Dependence of Proteolysis. Am. J. Physiol.-Cell Physiol. 1994, 266, C1028–C1036. [Google Scholar] [CrossRef] [PubMed]
- Land, S.C.; Buck, L.T.; Hochachka, P.W. Response of Protein Synthesis to Anoxia and Recovery in Anoxia-Tolerant Hepatocytes. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1993, 265, R41–R48. [Google Scholar] [CrossRef] [PubMed]
- Alderman, S.L.; Riggs, C.L.; Bullingham, O.; Gillis, T.E.; Warren, D.E. Cold-Acclimation Induces Life Stage-Specific Responses in the Cardiac Proteome of Western Painted Turtles (Chrysemys Picta Bellii): Implications for Anoxia Tolerance. bioRxiv 2021. [Google Scholar] [CrossRef]
- Cantiello, H.F. Actin Filaments Stimulate the Na(+)-K(+)-ATPase. Am. J. Physiol. 1995, 269, F637–F643. [Google Scholar] [CrossRef]
- Denker, S.P.; Barber, D.L. Ion Transport Proteins Anchor and Regulate the Cytoskeleton. Curr. Opin. Cell Biol. 2002, 14, 214–220. [Google Scholar] [CrossRef]
- Mills, J.W.; Schwiebert, E.M.; Stanton, B.A. The Cytoskeleton and Membrane Transport. Curr. Opin. Nephrol. Hypertens. 1994, 3, 529–534. [Google Scholar] [CrossRef]
- Liu, T.; Guevara, O.E.; Warburton, R.R.; Hill, N.S.; Gaestel, M.; Kayyali, U.S. Regulation of Vimentin Intermediate Filaments in Endothelial Cells by Hypoxia. Am. J. Physiol. Cell Physiol. 2010, 299, C363–C373. [Google Scholar] [CrossRef] [Green Version]
- Virtanen, I.; Lehto, V.P.; Lehtonen, E.; Badley, R.A. Organization of Intermediate Filaments in Cultured Fibroblasts upon Disruption of Microtubules by Cold Treatment. Eur. J. Cell Biol. 1980, 23, 80–84. [Google Scholar]
- Pamenter, M.E.; Hogg, D.W.; Ormond, J.; Shin, D.S.; Woodin, M.A.; Buck, L.T. Endogenous GABA(A) and GABA(B) Receptor-Mediated Electrical Suppression Is Critical to Neuronal Anoxia Tolerance. Proc. Natl. Acad. Sci. USA 2011, 108, 11274–11279. [Google Scholar] [CrossRef] [Green Version]
- Myrka, A.; Frost, R.; Distefano, D.; Plotnikov, S.; Buck, L. Evidence of Cold Induced Cytoskeletal Arrest in Hepatocytes of the Western Painted Turtle. FASEB J. 2021, 35. [Google Scholar] [CrossRef]
- Myrka, A.; Frost, R.; Distefano, D.; Plotnikov, S.V.; Buck, L.T. Cold Stimulated Cytoskeletal Arrest in Western Painted Turtle Hepatocytes. Available online: https://sicb.burkclients.com/meetings/2021/schedule/abstractdetails.php?id=1282 (accessed on 30 July 2021).
- Stoll, B.; Gerok, W.; Lang, F.; Häussinger, D. Liver Cell Volume and Protein Synthesis. Biochem. J. 1992, 287, 217–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, K.; Jyrwa, L.M.; Häussinger, D.; Saha, N. Influence of Cell Volume Changes on Protein Synthesis in Isolated Hepatocytes of Air-Breathing Walking Catfish (Clarias Batrachus). Fish Physiol. Biochem. 2010, 36, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Espelt, M.V.; Mut, P.N.; Amodeo, G.; Krumschnabel, G.; Schwarzbaum, P.J. Volumetric and Ionic Responses of Goldfish Hepatocytes to Anisotonic Exposure and Energetic Limitation. J. Exp. Biol. 2003, 206, 513–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Der Linden, A.V.; Verhoye, M.; Nilsson, G.E. Does Anoxia Induce Cell Swelling in Carp Brains? In Vivo MRI Measurements in Crucian Carp and Common Carp. J. Neurophysiol. 2001, 85, 125–133. [Google Scholar] [CrossRef]
- Lefevre, S.; Stecyk, J.A.W.; Torp, M.-K.; Løvold, L.Y.; Sørensen, C.; Johansen, I.B.; Stensløkken, K.-O.; Couturier, C.S.; Sloman, K.A.; Nilsson, G.E. Re-Oxygenation after Anoxia Induces Brain Cell Death and Memory Loss in the Anoxia-Tolerant Crucian Carp. J. Exp. Biol. 2017, 220, 3883–3895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hindle, A.G.; Martin, S.L. Cytoskeletal Regulation Dominates Temperature-Sensitive Proteomic Changes of Hibernation in Forebrain of 13-Lined Ground Squirrels. PLoS ONE 2013, 8, e71627. [Google Scholar] [CrossRef]
- Lázaro, J.; Hertel, M.; Sherwood, C.C.; Muturi, M.; Dechmann, D.K.N. Profound Seasonal Changes in Brain Size and Architecture in the Common Shrew. Brain Struct. Funct. 2018, 223, 2823–2840. [Google Scholar] [CrossRef] [Green Version]
- Magariños, A.M.; McEwen, B.S.; Saboureau, M.; Pevet, P. Rapid and Reversible Changes in Intrahippocampal Connectivity during the Course of Hibernation in European Hamsters. Proc. Natl. Acad. Sci. USA 2006, 103, 18775–18780. [Google Scholar] [CrossRef] [Green Version]
- von der Ohe, C.G.; Darian-Smith, C.; Garner, C.C.; Heller, H.C. Ubiquitous and Temperature-Dependent Neural Plasticity in Hibernators. J. Neurosci. 2006, 26, 10590–10598. [Google Scholar] [CrossRef]
- Popov, V.I.; Bocharova, L.S.; Bragin, A.G. Repeated Changes of Dendritic Morphology in the Hippocampus of Ground Squirrels in the Course of Hibernation. Neuroscience 1992, 48, 45–51. [Google Scholar] [CrossRef]
- Popov, V.I.; Medvedev, N.I.; Patrushev, I.V.; Ignat’ev, D.A.; Morenkov, E.D.; Stewart, M.G. Reversible Reduction in Dendritic Spines in CA1 of Rat and Ground Squirrel Subjected to Hypothermia-Normothermia in Vivo: A Three-Dimensional Electron Microscope Study. Neuroscience 2007, 149, 549–560. [Google Scholar] [CrossRef]
- Ray, S.; Li, M.; Koch, S.P.; Mueller, S.; Boehm-Sturm, P.; Wang, H.; Brecht, M.; Naumann, R.K. Seasonal Plasticity in the Adult Somatosensory Cortex. Proc. Natl. Acad. Sci. USA 2020, 117, 32136–32144. [Google Scholar] [CrossRef]
- Stieler, J.T.; Bullmann, T.; Kohl, F.; Tøien, Ø.; Brückner, M.K.; Härtig, W.; Barnes, B.M.; Arendt, T. The Physiological Link between Metabolic Rate Depression and Tau Phosphorylation in Mammalian Hibernation. PLoS ONE 2011, 6, e14530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drew, K.L. Hypoxia Tolerance in Mammalian Heterotherms. J. Exp. Biol. 2004, 207, 3155–3162. [Google Scholar] [CrossRef] [Green Version]
- von der Ohe, C.G.; Garner, C.C.; Darian-Smith, C.; Heller, H.C. Synaptic Protein Dynamics in Hibernation. J. Neurosci. 2007, 27, 84–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonntag, M.; Arendt, T. Neuronal Activity in the Hibernating Brain. Front. Neuroanat. 2019, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, J.M.; Horwitz, B.A. Extreme Neuroplasticity of Hippocampal CA1 Pyramidal Neurons in Hibernating Mammalian Species. Front. Neuroanat. 2019, 13, 9. [Google Scholar] [CrossRef] [Green Version]
- Arendt, T.; Bullmann, T. Neuronal Plasticity in Hibernation and the Proposed Role of the Microtubule-Associated Protein Tau as a “Master Switch” Regulating Synaptic Gain in Neuronal Networks. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R478–R489. [Google Scholar] [CrossRef] [Green Version]
- Arendt, T.; Stieler, J.; Strijkstra, A.M.; Hut, R.A.; Rüdiger, J.; der Zee, E.A.V.; Harkany, T.; Holzer, M.; Härtig, W. Reversible Paired Helical Filament-Like Phosphorylation of Tau Is an Adaptive Process Associated with Neuronal Plasticity in Hibernating Animals. J. Neurosci. 2003, 23, 6972–6981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallas-Bazarra, N.; Draffin, J.; Cuadros, R.; Antonio Esteban, J.; Avila, J. Tau Is Required for the Function of Extrasynaptic NMDA Receptors. Sci. Rep. 2019, 9, 9116. [Google Scholar] [CrossRef]
- Storey, K.B. Molecular Mechanisms of Anoxia Tolerance. Int. Congr. Ser. 2004, 1275, 47–54. [Google Scholar] [CrossRef]
- Storey, K.B.; Storey, J.M. Metabolic Rate Depression in Animals: Transcriptional and Translational Controls. Biol. Rev. 2004, 79, 207–233. [Google Scholar] [CrossRef]
- Brooks, S.P.; Storey, K.B. De Novo Protein Synthesis and Protein Phosphorylation during Anoxia and Recovery in the Red-Eared Turtle. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1993, 265, R1380–R1386. [Google Scholar] [CrossRef]
- Pucek, Z. Seasonal and Age Changes in the Weight of Internal Organs of Shrews. Acta Theriol. 1965, 10, 369–438. [Google Scholar] [CrossRef] [Green Version]
- Lazaro, J.; Dechmann, D. Dehnel’s Phenomenon. Curr. Biol. 2021, 31, R463–R465. [Google Scholar] [CrossRef]
- Baudier, J.; Cole, R.D. Phosphorylation of Tau Proteins to a State like That in Alzheimer’s Brain Is Catalyzed by a Calcium/Calmodulin-Dependent Kinase and Modulated by Phospholipids. J. Biol. Chem. 1987, 262, 17577–17583. [Google Scholar] [CrossRef]
- Kadavath, H.; Hofele, R.V.; Biernat, J.; Kumar, S.; Tepper, K.; Urlaub, H.; Mandelkow, E.; Zweckstetter, M. Tau Stabilizes Microtubules by Binding at the Interface between Tubulin Heterodimers. Proc. Natl. Acad. Sci. USA 2015, 112, 7501–7506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gattoni, G.; Bernocchi, G. Calcium-Binding Proteins in the Nervous System during Hibernation: Neuroprotective Strategies in Hypometabolic Conditions? Int. J. Mol. Sci. 2019, 20, 2364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emirbekov, E.Z.; Pashaeva, M.E. Expression of Cytoskeleton Proteins in Hypothalamic Cells in Winter Sleeping Ground Squirrels Citellus Pygmaeus Pallas during Hibernation. Neurochem. J. 2014, 8, 178–183. [Google Scholar] [CrossRef]
- Pisu, M.B.; Scherini, E.; Bernocchi, G. Immunocytochemical Changes of Cytoskeleton Components and Calmodulin in the Frog Cerebellum and Optic Tectum during Hibernation. J. Chem. Neuroanat. 1998, 15, 63–73. [Google Scholar] [CrossRef]
- Holenweg, A.-K.; Reyer, H.-U. Hibernation Behavior of Rana Lessonae and R. Esculenta in Their Natural Habitat. Oecologia 2000, 123, 41–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vezzoli, A.; Gussoni, M.; Greco, F.; Zetta, L.; Cerretelli, P. Quantitative Analysis of Anaerobic Metabolism in Resting Anoxic Muscle by 31P and 1H MRS. Biochim. Et Biophys. Acta (BBA)—Bioenerg. 1997, 1322, 195–207. [Google Scholar] [CrossRef] [Green Version]
- Welker, A.F.; Moreira, D.C.; Hermes-Lima, M. Roles of Catalase and Glutathione Peroxidase in the Tolerance of a Pulmonate Gastropod to Anoxia and Reoxygenation. J. Comp. Physiol. B 2016, 186, 553–568. [Google Scholar] [CrossRef]
- Vorhaben, J.E.; Klotz, A.V.; Campbell, J.W. Activity and Oxidative Metabolism of the Land Snail Helix Aspersa. Physiol. Zool. 1984, 57, 357–365. [Google Scholar] [CrossRef]
- Vignola, C.; Fenoglio, C.; Scherini, E.; Bernocchi, G. The Cerebral Neurons of Helix Aspersa during Hibernation. Changes in the Cytochemical Detection of Calmodulin, Cytoskeletal Components and Phosphatases. Tissue Cell 1995, 27, 185–196. [Google Scholar] [CrossRef]
- Gattoni, G.; Insolia, V.; Bernocchi, G. Hibernation Induces Changes in the Metacerebral Neurons of Cornu Aspersum: Distribution and Co-Localization of Cytoskeletal and Calcium-Binding Proteins. Invert. Neurosci. 2018, 18, 13. [Google Scholar] [CrossRef]
- Regalado-Reyes, M.; Benavides-Piccione, R.; Fernaud-Espinosa, I.; DeFelipe, J.; León-Espinosa, G. Effect of Phosphorylated Tau on Cortical Pyramidal Neuron Morphology during Hibernation. Cereb. Cortex Commun. 2020, 1, tgaa018. [Google Scholar] [CrossRef]
- Bullmann, T.; Seeger, G.; Stieler, J.; Hanics, J.; Reimann, K.; Kretzschmann, T.P.; Hilbrich, I.; Holzer, M.; Alpár, A.; Arendt, T. Tau Phosphorylation-Associated Spine Regression Does Not Impair Hippocampal-Dependent Memory in Hibernating Golden Hamsters. Hippocampus 2016, 26, 301–318. [Google Scholar] [CrossRef]
- Härtig, W.; Stieler, J.; Boerema, A.S.; Wolf, J.; Schmidt, U.; Weissfuss, J.; Bullmann, T.; Strijkstra, A.M.; Arendt, T. Hibernation Model of Tau Phosphorylation in Hamsters: Selective Vulnerability of Cholinergic Basal Forebrain Neurons—Implications for Alzheimer’s Disease. Eur. J. Neurosci. 2007, 25, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Hossein-Javaheri, N.; Wilkie, M.P.; Lado, W.E.; Buck, L.T. Stellate and Pyramidal Neurons in Goldfish Telencephalon Respond Differently to Anoxia and GABA Receptor Inhibition. J. Exp. Biol. 2017, 220, 695–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, L.M.; Ebner, F.F.; Colonnier, M. The Thalamocortical Projection in Pseudemys Turtles: A Quantitative Electron Microscopic Study. J. Comp. Neurol. 1980, 190, 445–461. [Google Scholar] [CrossRef]
- Hawrysh, P.J.; Buck, L.T. Oxygen-Sensitive Interneurons Exhibit Increased Activity and GABA Release during ROS Scavenging in the Cerebral Cortex of the Western Painted Turtle. J. Neurophysiol. 2019, 122, 466–479. [Google Scholar] [CrossRef]
- Perrot, R.; Berges, R.; Bocquet, A.; Eyer, J. Review of the Multiple Aspects of Neurofilament Functions, and Their Possible Contribution to Neurodegeneration. Mol. Neurobiol. 2008, 38, 27–65. [Google Scholar] [CrossRef]
- Grant, P.; Pant, H.C. Neurofilament Protein Synthesis and Phosphorylation. J. Neurocytol. 2000, 29, 843–872. [Google Scholar] [CrossRef]
- Yuan, A.; Rao, M.V.; Veeranna; Nixon, R.A. Neurofilaments and Neurofilament Proteins in Health and Disease. Cold Spring Harb. Perspect. Biol. 2017, 9, a018309. [Google Scholar] [CrossRef] [Green Version]
- Nixon, R.A.; Shea, T.B. Dynamics of Neuronal Intermediate Filaments: A Developmental Perspective. Cell Motil. 1992, 22, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Ackerley, S.; Thornhill, P.; Grierson, A.J.; Brownlees, J.; Anderton, B.H.; Leigh, P.N.; Shaw, C.E.; Miller, C.C.J. Neurofilament Heavy Chain Side Arm Phosphorylation Regulates Axonal Transport of Neurofilaments. J. Cell Biol. 2003, 161, 489–495. [Google Scholar] [CrossRef]
- Roda, E.; Bottone, M.G.; Insolia, V.; Barni, S.; Bernocchi, G. Changes in the Cerebellar Cytoarchitecture of Hibernating Hedgehog Erinaceus Europaeus L. (Mammalia): An Immunocytochemical Approach. Eur. Zool. J. 2017, 84, 496–511. [Google Scholar] [CrossRef] [Green Version]
- Elie, A.; Prezel, E.; Guérin, C.; Denarier, E.; Ramirez-Rios, S.; Serre, L.; Andrieux, A.; Fourest-Lieuvin, A.; Blanchoin, L.; Arnal, I. Tau Co-Organizes Dynamic Microtubule and Actin Networks. Sci. Rep. 2015, 5, 9964. [Google Scholar] [CrossRef] [Green Version]
- Dehmelt, L.; Halpain, S. The MAP2/Tau Family of Microtubule-Associated Proteins. Genome Biol. 2004, 6, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedrotti, B.; Colombo, R.; Islam, K. Interactions of Microtubule-Associated Protein MAP2 with Unpolymerized and Polymerized Tubulin and Actin Using a 96-Well Microtiter Plate Solid-Phase Immunoassay. Biochemistry 1994, 33, 8798–8806. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.; John, A. Microtubule-Associated Proteins as Direct Crosslinkers of Actin Filaments and Microtubules. IUBMB Life 2015, 67, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Kalil, K. The Microtubule-Associated Protein Tau Mediates the Organization of Microtubules and Their Dynamic Exploration of Actin-Rich Lamellipodia and Filopodia of Cortical Growth Cones. J. Neurosci. 2018, 38, 291–307. [Google Scholar] [CrossRef]
- Pimm, M.L.; Henty-Ridilla, J.L. New Twists in Actin–Microtubule Interactions. MBoC 2021, 32, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Cabrales Fontela, Y.; Kadavath, H.; Biernat, J.; Riedel, D.; Mandelkow, E.; Zweckstetter, M. Multivalent Cross-Linking of Actin Filaments and Microtubules through the Microtubule-Associated Protein Tau. Nat. Commun. 2017, 8, 1981. [Google Scholar] [CrossRef]
- Sattilaro, R.F. Interaction of Microtubule-Associated Protein 2 with Actin Filaments. Biochemistry 1986, 25, 2003–2009. [Google Scholar] [CrossRef] [PubMed]
- Heng, Y.-W.; Koh, C.-G. Actin Cytoskeleton Dynamics and the Cell Division Cycle. Int. J. Biochem. Cell Biol. 2010, 42, 1622–1633. [Google Scholar] [CrossRef]
- DeWane, G.; Salvi, A.M.; DeMali, K.A. Fueling the Cytoskeleton—Links between Cell Metabolism and Actin Remodeling. J. Cell Sci. 2021, 134, jcs248385. [Google Scholar] [CrossRef] [PubMed]
- Loranger, A.; Tuchweber, B.; Youseff, I.; Marceau, N. Biliary Secretion and Actin-Cytokeratin Filament Distribution in Rat Hepatocytes during Phalloidin-Induced Cholestasis. Biochem. Cell Biol. 1995, 73, 641–649. [Google Scholar] [CrossRef]
- Prentki, M.; Chaponnier, C.; Jeanrenaud, B.; Gabbiani, G. Actin Microfilaments, Cell Shape, and Secretory Processes in Isolated Rat Hepatocytes. Effect of Phalloidin and Cytochalasin D. J. Cell Biol. 1979, 81, 592–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, M.T.; Schempp, R.; Lutz, A.; Felder, T.; Felder, E.; Miklavc, P. Interaction of Microtubules and Actin during the Post-Fusion Phase of Exocytosis. Sci. Rep. 2019, 9, 11973. [Google Scholar] [CrossRef] [Green Version]
- Kitamura, T.; Brauneis, U.; Gatmaitan, Z.; Arias, I.M. Extracellular ATP, Intracellular Calcium and Canalicular Contraction in Rat Hepatocyte Doublets. Hepatology 1991, 14, 640–647. [Google Scholar] [CrossRef]
- Oka, M.; Fujisaki, N.; Maruko-Otake, A.; Ohtake, Y.; Shimizu, S.; Saito, T.; Hisanaga, S.-I.; Iijima, K.M.; Ando, K. Ca2+/Calmodulin-Dependent Protein Kinase II Promotes Neurodegeneration Caused by Tau Phosphorylated at Ser262/356 in a Transgenic Drosophila Model of Tauopathy. J. Biochem. 2017, 162, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.-P.; Ye, J.-W.; Wang, X.; Zhu, L.-P.; Hu, Q.-H.; Wang, Q.; Ke, D.; Tian, Q.; Wang, J.-Z. Tau-Induced Ca2+/Calmodulin-Dependent Protein Kinase-IV Activation Aggravates Nuclear Tau Hyperphosphorylation. Neurosci. Bull. 2018, 34, 261–269. [Google Scholar] [CrossRef]
- Lee, Y.C.; Wolff, J. Calmodulin Binds to Both Microtubule-Associated Protein 2 and Tau Proteins. J. Biol. Chem. 1984, 259, 1226–1230. [Google Scholar] [CrossRef]
- Kakiuchi, S.; Sobue, K. Ca2+- and Calmodulin-Dependent Flip—Flop Mechanism in Microtubule Assembly—Disassembly. FEBS Lett. 1981, 132, 141–143. [Google Scholar] [CrossRef] [Green Version]
- Padilla, R.; Maccioni, R.B.; Avila, J. Calmodulin Binds to a Tubulin Binding Site of the Microtubule-Associated Protein Tau. Mol Cell Biochem. 1990, 97, 35–41. [Google Scholar] [CrossRef]
- Hashimoto, R.; Nakamura, Y.; Komai, S.; Kashiwagi, Y.; Tamura, K.; Goto, T.; Aimoto, S.; Kaibuchi, K.; Shiosaka, S.; Takeda, M. Site-Specific Phosphorylation of Neurofilament-L Is Mediated by Calcium/Calmodulin-Dependent Protein Kinase II in the Apical Dendrites During Long-Term Potentiation. J. Neurochem. 2000, 75, 373–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henrich, M.; Buckler, K.J. Effects of Anoxia and Aglycemia on Cytosolic Calcium Regulation in Rat Sensory Neurons. J. Neurophysiol. 2008, 100, 456–473. [Google Scholar] [CrossRef] [Green Version]
- Nayler, W.G.; Poole-Wilson, P.A.; Williams, A. Hypoxia and Calcium. J. Mol. Cell. Cardiol. 1979, 11, 683–706. [Google Scholar] [CrossRef]
- Choi, D.W. Calcium: Still Center-Stage in Hypoxic-Ischemic Neuronal Death. Trends Neurosci. 1995, 18, 58–60. [Google Scholar] [CrossRef]
- Piper, H.M.; Siegmund, B.; Ladilov, Y.V.; Schlüter, K.-D. Calcium and Sodium Control in Hypoxic-Reoxygenated Cardiomyocytes. Basic Res. Cardiol. 1993, 88, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Snowdowne, K.W.; Freudenrich, C.C.; Borle, A.B. The Effects of Anoxia on Cytosolic Free Calcium, Calcium Fluxes, and Cellular ATP Levels in Cultured Kidney Cells. J. Biol. Chem. 1985, 260, 11619–11626. [Google Scholar] [CrossRef]
- Wold, R.M.; Kondratiev, T.; Tveita, T. Myocardial Calcium Overload during Graded Hypothermia and after Rewarming in an in Vivo Rat Model. Acta Physiol. 2013, 207, 460–469. [Google Scholar] [CrossRef]
- Oliver, A.E.; Tablin, F.; Walker, N.J.; Crowe, J.H. The Internal Calcium Concentration of Human Platelets Increases during Chilling. Biochim. Biophys. Acta 1999, 1416, 349–360. [Google Scholar] [CrossRef] [Green Version]
- Polderman, K.H. Of Ions and Temperature: The Complicated Interplay of Temperature, Fluids, and Electrolytes on Myocardial Function. Crit. Care 2013, 17, 1018. [Google Scholar] [CrossRef] [Green Version]
- Waters, L.; Padula, M.P.; Marks, D.C.; Johnson, L. Calcium Chelation: A Novel Approach to Reduce Cryopreservation-Induced Damage to Frozen Platelets. Transfusion 2020, 60, 1552–1563. [Google Scholar] [CrossRef]
- Duchen, M.R. Mitochondria and Calcium: From Cell Signalling to Cell Death. J. Physiol. 2000, 529, 57–68. [Google Scholar] [CrossRef]
- Cerella, C.; Diederich, M.; Ghibelli, L. The Dual Role of Calcium as Messenger and Stressor in Cell Damage, Death, and Survival. Int. J. Cell Biol. 2010, 2010, e546163. [Google Scholar] [CrossRef] [Green Version]
- Hori, M.; Sato, H.; Inoue, M. Cytoskeletal Changes in the Calcium-Overloaded Heart. In New Horizons for Failing Heart Syndrome; Sasayama, S., Ed.; Springer: Tokyo, Japan, 1996; pp. 45–65. ISBN 978-4-431-66945-6. [Google Scholar]
- Piper, H.M.; Noll, T.; Muhs, A.; Besselmann, M.; Kuhne, W.; Watanabe, H. Cytosolic Ca2+ Overload and Macromolecule Permeability of Endothelial Monolayers. Herz 1992, 17, 277–283. [Google Scholar] [PubMed]
- Bickler, P.E.; Buck, L.T. Adaptations of Vertebrate Neurons to Hypoxia and Anoxia: Maintaining Critical Ca2+ Concentrations. J. Exp. Biol. 1998, 201, 1141–1152. [Google Scholar] [CrossRef] [PubMed]
- Krumschnabel, G.; Schwarzbaum, P.J.; Biasi, C.; Dorigatti, M.; Wieser, W. Effects of Energy Limitation on Ca2+ and K+ Homeostasis in Anoxia-Tolerant and Anoxia-Intolerant Hepatocytes. Am. J. Physiol. 1997, 273, R307–R316. [Google Scholar] [CrossRef] [PubMed]
- Janmey, P.A. Phosphoinositides and Calcium as Regulators of Cellular Actin Assembly and Disassembly. Annu. Rev. Physiol. 1994, 56, 169–191. [Google Scholar] [CrossRef]
- Furukawa, K.; Fu, W.; Li, Y.; Witke, W.; Kwiatkowski, D.J.; Mattson, M.P. The Actin-Severing Protein Gelsolin Modulates Calcium Channel and NMDA Receptor Activities and Vulnerability to Excitotoxicity in Hippocampal Neurons. J. Neurosci. 1997, 17, 8178–8186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bickler, P.E.; Donohoe, P.H.; Buck, L.T. Hypoxia-Induced Silencing of NMDA Receptors in Turtle Neurons. J. Neurosci. 2000, 20, 3522–3528. [Google Scholar] [CrossRef]
- Rycroft, B.K.; Gibb, A.J. Regulation of Single NMDA Receptor Channel Activity by Alpha-Actinin and Calmodulin in Rat Hippocampal Granule Cells. J. Physiol. 2004, 557, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Hawrysh, P.J.; Buck, L.T. Anoxia-Mediated Calcium Release through the Mitochondrial Permeability Transition Pore Silences NMDA Receptor Currents in Turtle Neurons. J. Exp. Biol. 2013, 216, 4375–4387. [Google Scholar] [CrossRef] [Green Version]
- Carey, H.V.; Andrews, M.T.; Martin, S.L. Mammalian Hibernation: Cellular and Molecular Responses to Depressed Metabolism and Low Temperature. Physiol. Rev. 2003, 83, 1153–1181. [Google Scholar] [CrossRef] [Green Version]
- Bickler, P.E. Cerebral Anoxia Tolerance in Turtles: Regulation of Intracellular Calcium and PH. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1992, 263, R1298–R1302. [Google Scholar] [CrossRef]
- Buck, L.T.; Bickler, P.E. Role of Adenosine in NMDA Receptor Modulation in the Cerebral Cortex of an Anoxia-Tolerant Turtle (Chrysemys Picta Belli). J. Exp. Biol. 1995, 198, 1621. [Google Scholar] [CrossRef] [PubMed]
- Buck, L.T.; Bickler, P.E. Adenosine and Anoxia Reduce N-Methyl-D-Aspartate Receptor Open Probability in Turtle Cerebrocortex. J. Exp. Biol. 1998, 201, 289–297. [Google Scholar] [CrossRef]
- Centritto, R.; Buck, L. Anoxia-Induced Release of Adenosine by Turtle Hepatocytes. FASEB J. 2001, 15, A91. [Google Scholar]
- Krumschnabel, G.; Biasi, C.; Wieser, W. Action of Adenosine on Energetics, Protein Synthesis and K(+) Homeostasis in Teleost Hepatocytes. J. Exp. Biol. 2000, 203, 2657–2665. [Google Scholar] [CrossRef]
- Pamenter, M.E.; Shin, D.S.-H.; Cooray, M.; Buck, L.T. Mitochondrial ATP-Sensitive K+ Channels Regulate NMDAR Activity in the Cortex of the Anoxic Western Painted Turtle. J. Physiol. 2008, 586, 1043–1058. [Google Scholar] [CrossRef]
- Paredes, R.M.; Etzler, J.C.; Watts, L.T.; Lechleiter, J.D. Chemical Calcium Indicators. Methods 2008, 46, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Patergnani, S.; Suski, J.M.; Agnoletto, C.; Bononi, A.; Bonora, M.; De Marchi, E.; Giorgi, C.; Marchi, S.; Missiroli, S.; Poletti, F.; et al. Calcium Signaling around Mitochondria Associated Membranes (MAMs). Cell Commun. Signal. 2011, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Chandel, N.S.; Maltepe, E.; Goldwasser, E.; Mathieu, C.E.; Simon, M.C.; Schumacker, P.T. Mitochondrial Reactive Oxygen Species Trigger Hypoxia-Induced Transcription. Proc. Natl. Acad. Sci. USA 1998, 95, 11715–11720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.; Lai, U.H.; Zhu, L.; Singh, A.; Ahmed, M.; Forsyth, N.R. Reactive Oxygen Species Formation in the Brain at Different Oxygen Levels: The Role of Hypoxia Inducible Factors. Front. Cell Dev. Biol. 2018, 6, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, S.S.; Marcondes, M.-C.G.; Bajova, H.; Dugan, L.L.; Conti, B. Metabolic Depression and Increased Reactive Oxygen Species Production by Isolated Mitochondria at Moderately Lower Temperatures. J. Biol. Chem. 2010, 285, 32522–32528. [Google Scholar] [CrossRef] [Green Version]
- Hendriks, K.D.W.; Brüggenwirth, I.M.A.; Maassen, H.; Gerding, A.; Bakker, B.; Porte, R.J.; Henning, R.H.; Leuvenink, H.G.D. Renal Temperature Reduction Progressively Favors Mitochondrial ROS Production over Respiration in Hypothermic Kidney Preservation. J. Transl. Med. 2019, 17, 265. [Google Scholar] [CrossRef] [PubMed]
- Schaible, N.; Han, Y.S.; Tveita, T.; Sieck, G.C. Role of Superoxide Ion Formation in Hypothermia/Rewarming Induced Contractile Dysfunction in Cardiomyocytes. Cryobiology 2018, 81, 57–64. [Google Scholar] [CrossRef]
- Selivanov, V.A.; Votyakova, T.V.; Pivtoraiko, V.N.; Zeak, J.; Sukhomlin, T.; Trucco, M.; Roca, J.; Cascante, M. Reactive Oxygen Species Production by Forward and Reverse Electron Fluxes in the Mitochondrial Respiratory Chain. PLoS Comput. Biol. 2011, 7, e1001115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, T.; Prather, E.R.; Garrison, D.E.; Zuo, L. Interplay between ROS and Antioxidants during Ischemia-Reperfusion Injuries in Cardiac and Skeletal Muscle. Int. J. Mol. Sci. 2018, 19, 417. [Google Scholar] [CrossRef] [Green Version]
- Görlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A Mutual Interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, C.; González-Billault, C. Regulation of Cytoskeletal Dynamics by Redox Signaling and Oxidative Stress: Implications for Neuronal Development and Trafficking. Front. Cell Neurosci. 2015, 9, 381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, E.T.; Salmon, E.D.; Erickson, H.P. How Calcium Causes Microtubule Depolymerization. Cell Motil. 1997, 36, 125–135. [Google Scholar] [CrossRef]
- Na, N.; Chandel, N.S.; Litvan, J.; Ridge, K.M. Mitochondrial Reactive Oxygen Species Are Required for Hypoxia-Induced Degradation of Keratin Intermediate Filaments. FASEB J. 2010, 24, 799–809. [Google Scholar] [CrossRef]
- Ou, J.; Ball, J.M.; Luan, Y.; Zhao, T.; Miyagishima, K.J.; Xu, Y.; Zhou, H.; Chen, J.; Merriman, D.K.; Xie, Z.; et al. IPSCs from a Hibernator Provide a Platform for Studying Cold Adaptation and Its Potential Medical Applications. Cell 2018, 173, 851–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, J.C.L.; Chung, D.J.; Belgrave, K.R.; Staples, J.F. Mitochondrial Metabolic Suppression and Reactive Oxygen Species Production in Liver and Skeletal Muscle of Hibernating Thirteen-Lined Ground Squirrels. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R15–R28. [Google Scholar] [CrossRef] [Green Version]
- Hendriks, K.D.W.; Lupi, E.; Hardenberg, M.C.; Hoogstra-Berends, F.; Deelman, L.E.; Henning, R.H. Differences in Mitochondrial Function and Morphology during Cooling and Rewarming between Hibernator and Non-Hibernator Derived Kidney Epithelial Cells. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Abramov, A.Y.; Scorziello, A.; Duchen, M.R. Three Distinct Mechanisms Generate Oxygen Free Radicals in Neurons and Contribute to Cell Death during Anoxia and Reoxygenation. J. Neurosci. 2007, 27, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Turrens, J.F. Mitochondrial Formation of Reactive Oxygen Species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Larsen, G.A.; Skjellegrind, H.K.; Berg-Johnsen, J.; Moe, M.C.; Vinje, M.L. Depolarization of Mitochondria in Isolated CA1 Neurons during Hypoxia, Glucose Deprivation and Glutamate Excitotoxicity. Brain Res. 2006, 1077, 153–160. [Google Scholar] [CrossRef]
- Andersson, B.S.; Aw, T.Y.; Jones, D.P. Mitochondrial Transmembrane Potential and PH Gradient during Anoxia. Am. J. Physiol.-Cell Physiol. 1987, 252, C349–C355. [Google Scholar] [CrossRef]
- Pamenter, M.E.; Richards, M.D.; Buck, L.T. Anoxia-Induced Changes in Reactive Oxygen Species and Cyclic Nucleotides in the Painted Turtle. J. Comp. Physiol. B 2007, 177, 473–481. [Google Scholar] [CrossRef]
- Dukoff, D.J.; Hogg, D.W.; Hawrysh, P.J.; Buck, L.T. Scavenging ROS Dramatically Increase NMDA Receptor Whole-Cell Currents in Painted Turtle Cortical Neurons. J. Exp. Biol. 2014, 217, 3346–3355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milton, S.L.; Nayak, G.; Kesaraju, S.; Kara, L.; Prentice, H.M. Suppression of Reactive Oxygen Species Production Enhances Neuronal Survival in Vitro and in Vivo in the Anoxia-Tolerant Turtle Trachemys Scripta. J. Neurochem. 2007, 101, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Hogg, D.W.; Pamenter, M.E.; Dukoff, D.J.; Buck, L.T. Decreases in Mitochondrial Reactive Oxygen Species Initiate GABAA Receptor-Mediated Electrical Suppression in Anoxia-Tolerant Turtle Neurons. J. Physiol. 2015, 593, 2311–2326. [Google Scholar] [CrossRef] [Green Version]
- Pillai, V.; Buck, L.; Lari, E. Scavenging of Reactive Oxygen Species Mimics the Anoxic Response in Goldfish Pyramidal Neurons. J. Exp. Biol. 2021, 224, jeb238147. [Google Scholar] [CrossRef] [PubMed]
- Bundgaard, A.; James, A.M.; Gruszczyk, A.V.; Martin, J.; Murphy, M.P.; Fago, A. Metabolic Adaptations during Extreme Anoxia in the Turtle Heart and Their Implications for Ischemia-Reperfusion Injury. Sci. Rep. 2019, 9, 2850. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Fabuel, I.; Le Douce, J.; Logan, A.; James, A.M.; Bonvento, G.; Murphy, M.P.; Almeida, A.; Bolaños, J.P. Complex I Assembly into Supercomplexes Determines Differential Mitochondrial ROS Production in Neurons and Astrocytes. Proc. Natl. Acad. Sci. USA 2016, 113, 13063–13068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bundgaard, A.; Qvortrup, K.; Rasmussen, L.J.; Fago, A. Turtles Maintain Mitochondrial Integrity but Reduce Mitochondrial Respiratory Capacity in the Heart after Cold Acclimation and Anoxia. J. Exp. Biol. 2019, 222, jeb200410. [Google Scholar] [CrossRef] [Green Version]
- Willmore, W.G.; Storey, K.B. Antioxidant Systems and Anoxia Tolerance in a Freshwater Turtle Trachemys Scripta Elegans. Mol. Cell Biochem. 1997, 170, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Krivoruchko, A.; Storey, K.B. Forever Young: Mechanisms of Natural Anoxia Tolerance and Potential Links to Longevity. Oxid. Med. Cell. Longev. 2010, 3, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.E.; Lee, E.J.; Choy, Y. High Levels of Ascorbic Acid, Not Glutathione, in the CNS of Anoxia-Tolerant Reptiles Contrasted with Levels in Anoxia-Intolerant Species. J. Neurochem. 1995, 64, 1790–1799. [Google Scholar] [CrossRef]
- Redondo, J.; Hares, K.; Wilkins, A.; Scolding, N.; Kemp, K. Reductions in Kinesin Expression Are Associated with Nitric Oxide-Induced Axonal Damage. J. Neurosci. Res. 2015, 93, 882–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, C.; Terman, J.R.; González-Billault, C.; Ahmed, G. Actin Filaments—A Target for Redox Regulation. Cytoskeleton (Hoboken) 2016, 73, 577–595. [Google Scholar] [CrossRef] [Green Version]
- Balta, E.; Kramer, J.; Samstag, Y. Redox Regulation of the Actin Cytoskeleton in Cell Migration and Adhesion: On the Way to a Spatiotemporal View. Front. Cell Dev. Biol. 2020, 8, 618261. [Google Scholar] [CrossRef]
- Korshunov, S.S.; Skulachev, V.P.; Starkov, A.A. High Protonic Potential Actuates a Mechanism of Production of Reactive Oxygen Species in Mitochondria. FEBS Lett. 1997, 416, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Suski, J.M.; Lebiedzinska, M.; Bonora, M.; Pinton, P.; Duszynski, J.; Wieckowski, M.R. Relation Between Mitochondrial Membrane Potential and ROS Formation. In Mitochondrial Bioenergetics: Methods and Protocols; Palmeira, C.M., Moreno, A.J., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; pp. 183–205. ISBN 978-1-61779-382-0. [Google Scholar]
- Mustafa, A.K.; Gadalla, M.M.; Snyder, S.H. Signaling by Gasotransmitters. Sci. Signal. 2009, 2, re2. [Google Scholar] [CrossRef] [Green Version]
- Bundgaard, A.; Jensen, B.S.; Jensen, F.B.; Fago, A. Exploring Pathways of NO and H2S Signaling in Metabolic Depression: The Case of Anoxic Turtles. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2021, 253, 110857. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, K.D.; Maassen, H.; van Dijk, P.R.; Henning, R.H.; van Goor, H.; Hillebrands, J.-L. Gasotransmitters in Health and Disease: A Mitochondria-Centered View. Curr. Opin. Pharmacol. 2019, 45, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Waltz, P.K.; Kautza, B.; Luciano, J.; Dyer, M.; Stolz, D.B.; Loughran, P.; Neal, M.D.; Sperry, J.L.; Rosengart, M.R.; Zuckerbraun, B.S. Heme Oxygenase-2 Localizes to Mitochondria and Regulates Hypoxic Responses in Hepatocytes. Oxidative Med. Cell. Longev. 2018, 2018, e2021645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tengan, C.H.; Moraes, C.T. NO Control of Mitochondrial Function in Normal and Transformed Cells. Biochim. Biophys. Acta 2017, 1858, 573–581. [Google Scholar] [CrossRef]
- Teng, H.; Wu, B.; Zhao, K.; Yang, G.; Wu, L.; Wang, R. Oxygen-Sensitive Mitochondrial Accumulation of Cystathionine β-Synthase Mediated by Lon Protease. Proc. Natl. Acad. Sci. USA 2013, 110, 12679–12684. [Google Scholar] [CrossRef] [Green Version]
- Cooper, C.E.; Brown, G.C. The Inhibition of Mitochondrial Cytochrome Oxidase by the Gases Carbon Monoxide, Nitric Oxide, Hydrogen Cyanide and Hydrogen Sulfide: Chemical Mechanism and Physiological Significance. J. Bioenerg. Biomembr. 2008, 40, 533. [Google Scholar] [CrossRef]
- Hartmann, C.; Nussbaum, B.; Calzia, E.; Radermacher, P.; Wepler, M. Gaseous Mediators and Mitochondrial Function: The Future of Pharmacologically Induced Suspended Animation? Front. Physiol. 2017, 8, 691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, Z.; Storey, K.B. Heme Oxygenase Expression and Nrf2 Signaling during Hibernation in Ground Squirrels. Can. J. Physiol. Pharm. 2010, 88, 379–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, B.; Pardue, S.; Duffy, B.; Kevil, C.; Staples, J.; Fago, A. Suppression of Mitochondrial Respiration by Hydrogen Sulfide in Hibernating 13-Lined Ground Squirrels. Free Radic. Biol. Med. 2021, 169, 181–186. [Google Scholar] [CrossRef]
- Walewska, A.; Szewczyk, A.; Koprowski, P. Gas Signaling Molecules and Mitochondrial Potassium Channels. Int. J. Mol. Sci. 2018, 19, 3227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G. Gasotransmitters and Protein Post-Translational Modifications. MOJ Proteom. Bioinform. 2017, 5. [Google Scholar] [CrossRef] [Green Version]
- Nalli, A.D.; Rajagopal, S.; Mahavadi, S.; Grider, J.R.; Murthy, K.S. Inhibition of RhoA-Dependent Pathway and Contraction by Endogenous Hydrogen Sulfide in Rabbit Gastric Smooth Muscle Cells. Am. J. Physiol. Cell Physiol. 2015, 308, C485–C495. [Google Scholar] [CrossRef] [Green Version]
- Semiz, A.T.; Teker, A.B.; Yapar, K.; Doğan, B.S.U.; Takır, S. Hydrogen Sulfide Dilates the Isolated Retinal Artery Mainly via the Activation of Myosin Phosphatase. Life Sci. 2020, 255, 117834. [Google Scholar] [CrossRef]
- Nalli, A.D.; Wang, H.; Bhattacharya, S.; Blakeney, B.A.; Murthy, K.S. Inhibition of RhoA/Rho Kinase Pathway and Smooth Muscle Contraction by Hydrogen Sulfide. Pharm. Res. Perspect. 2017, 5, e00343. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, J.; Chen, Z. H2S Protects Hippocampal Neurons against Hypoxia-Reoxygenation Injury by Promoting RhoA Phosphorylation at Ser188. Cell Death Discov. 2021, 7, 1–15. [Google Scholar] [CrossRef]
- Zuckerbraun, B.S.; Stoyanovsky, D.A.; Sengupta, R.; Shapiro, R.A.; Ozanich, B.A.; Rao, J.; Barbato, J.E.; Tzeng, E. Nitric Oxide-Induced Inhibition of Smooth Muscle Cell Proliferation Involves S-Nitrosation and Inactivation of RhoA. Am. J. Physiol. Cell Physiol. 2007, 292, C824–C831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, L.; Yusuf, M.Z.; Aburima, A.; Ahmed, Y.; Thomas, S.G.; Naseem, K.M.; Calaminus, S.D.J. Reversal of Stress Fibre Formation by Nitric Oxide Mediated RhoA Inhibition Leads to Reduction in the Height of Preformed Thrombi. Sci. Rep. 2018, 8, 3032. [Google Scholar] [CrossRef]
- Lin, L.; Xu, C.; Carraway, M.S.; Piantadosi, C.A.; Whorton, A.R.; Li, S. RhoA Inactivation by S-Nitrosylation Regulates Vascular Smooth Muscle Contractive Signaling. Nitric Oxide 2018, 74, 56–64. [Google Scholar] [CrossRef]
- Raines, K.W.; Bonini, M.G.; Campbell, S.L. Nitric Oxide Cell Signaling: S-Nitrosation of Ras Superfamily GTPases. Cardiovasc. Res. 2007, 75, 229–239. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Decker, N.K.; Chatterjee, S.; Yao, J.; Friedman, S.; Shah, V. Mechanisms of Nitric Oxide Interplay with Rho GTPase Family Members in Modulation of Actin Membrane Dynamics in Pericytes and Fibroblasts. Am. J. Pathol. 2005, 166, 1861–1870. [Google Scholar] [CrossRef] [Green Version]
- Awede, B.; Lemaire, M.-C.; Hyvelin, J.-M.; Halimi, J.-M.; Bonnet, P.; Eder, V. Hemin, a Carbon Monoxide Donor, Improves Systemic Vascular Compliance by Inhibiting the RhoA–Rhokinase Pathway in Spontaneous Hypertensive Rats. Eur. J. Pharmacol. 2010, 626, 256–261. [Google Scholar] [CrossRef]
- Inoue, K.; Patterson, E.K.; Capretta, A.; Lawendy, A.R.; Fraser, D.D.; Cepinskas, G. Carbon Monoxide–Releasing Molecule-401 Suppresses Polymorphonuclear Leukocyte Migratory Potential by Modulating F-Actin Dynamics. Am. J. Pathol. 2017, 187, 1121–1133. [Google Scholar] [CrossRef] [Green Version]
- Paez, A.; Vazquez, E.; Gueron, G. Heme Oxygenase 1 Governs the Cytoskeleton at Filopodia: Pulling the Brakes on the Migratory Capacity of Prostate Tumoral Cells. Cell Death Discov. 2017, 3, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Jensen, B.; Pardue, S.; Kevil, C.G.; Fago, A. Tissue-Dependent Variation of Hydrogen Sulfide Homeostasis in Anoxic Freshwater Turtles. J. Exp. Biol. 2019, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melleby, A.O.; Sandvik, G.K.; Couturier, C.S.; Nilsson, G.E.; Stecyk, J.A.W. H2S-Producing Enzymes in Anoxia-Tolerant Vertebrates: Effects of Cold Acclimation, Anoxia Exposure and Reoxygenation on Gene and Protein Expression. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2020, 243–244, 110430. [Google Scholar] [CrossRef] [PubMed]
- Patang, M. H2S as a Potential O2 Sensor in C. Picta Cerebral Cortex. Master’s Thesis, University of Toronto, Toronto, ON, Canada, 2021. [Google Scholar]
- Jacobsen, S.; Hansen, M.; Jensen, F.; Skovgaard, N.; Wang, T.; Fago, A. Circulating Nitric Oxide Metabolites and Cardiovascular Changes in the Turtle Trachemys Scripta during Normoxia, Anoxia and Reoxygenation. J. Exp. Biol. 2012, 215, 2560–2566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pamenter, M.E.; Hogg, D.W.; Buck, L.T. Endogenous Reductions in N -Methyl- d -Aspartate Receptor Activity Inhibit Nitric Oxide Production in the Anoxic Freshwater Turtle Cortex. FEBS Lett. 2008, 582, 1738–1742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.L.; Zhu, X.; Rivera, P.M.; Tøien, Ø.; Barnes, B.M.; LaManna, J.C.; Smith, M.A.; Drew, K.L. Absence of Cellular Stress in Brain after Hypoxia Induced by Arousal from Hibernation in Arctic Ground Squirrels. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2005, 289, R1297–R1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandvik, G.K.; Nilsson, G.E.; Jensen, F.B. Dramatic Increase of Nitrite Levels in Hearts of Anoxia-Exposed Crucian Carp Supporting a Role in Cardioprotection. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R468–R477. [Google Scholar] [CrossRef] [Green Version]
- Williams, B.L.; Wiebler, J.M.; Lee, R.E.; Costanzo, J.P. Nitric Oxide Metabolites in Hypoxia, Freezing, and Hibernation of the Wood Frog, Rana Sylvatica. J. Comp. Physiol. B 2018, 188, 957–966. [Google Scholar] [CrossRef] [Green Version]
- Kesaraju, S.; Schmidt-Kastner, R.; Prentice, H.M.; Milton, S.L. Modulation of Stress Proteins and Apoptotic Regulators in the Anoxia Tolerant Turtle Brain. J. Neurochem. 2009, 109, 1413–1426. [Google Scholar] [CrossRef] [Green Version]
- Tzaneva, V.; Perry, S.F. Heme Oxygenase-1 (HO-1) Mediated Respiratory Responses to Hypoxia in the Goldfish, Carassius Auratus. Respir. Physiol. Neurobiol. 2014, 199, 1–8. [Google Scholar] [CrossRef]
- Al-Dajani, F. Mechanisms of Anoxia Tolerance in Non-Excitable Cells: Investigating the Role of the Mitochondria in Western Painted Turtle Hepatocytes. Master’s Thesis, University of Toronto, Toronto, ON, Canada, 2018. [Google Scholar]
- McClintock, D.S.; Santore, M.T.; Lee, V.Y.; Brunelle, J.; Budinger, G.R.S.; Zong, W.-X.; Thompson, C.B.; Hay, N.; Chandel, N.S. Bcl-2 Family Members and Functional Electron Transport Chain Regulate Oxygen Deprivation-Induced Cell Death. Mol. Cell Biol. 2002, 22, 94–104. [Google Scholar] [CrossRef] [Green Version]
- Buck, L.T. Succinate and Alanine as Anaerobic End-Products in the Diving Turtle (Chrysemys Picta Bellii). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2000, 126, 409–413. [Google Scholar] [CrossRef]
- Churchill, T.A.; Busza, A.L.; Fuller, B.J. Energy Metabolism in Liver of Anoxia-Tolerant Turtle Species (Pseudemys Scripta): A Model for Studying Hepatic Tolerance to Cold Hypoxia. Cryobiology 1997, 35, 14–19. [Google Scholar] [CrossRef]
- Devaux, J.B.L.; Hickey, A.J.R.; Renshaw, G.M.C. Mitochondrial Plasticity in the Cerebellum of Two Anoxia-Tolerant Sharks: Contrasting Responses to Anoxia/Re-Oxygenation. J. Exp. Biol. 2019, 222, jeb191353. [Google Scholar] [CrossRef] [Green Version]
- Hawrysh, P.J.; Buck, L.T. Mitochondrial Matrix PH Acidifies during Anoxia and Is Maintained by the F1Fo-ATPase in Anoxia-Tolerant Painted Turtle Cortical Neurons. FEBS Open Bio 2019, 9, 571–581. [Google Scholar] [CrossRef] [Green Version]
- Pamenter, M.E.; Gomez, C.R.; Richards, J.G.; Milsom, W.K. Mitochondrial Responses to Prolonged Anoxia in Brain of Red-Eared Slider Turtles. Biol. Lett. 2016, 12, 20150797. [Google Scholar] [CrossRef]
- Al-Mehdi, A.-B.; Pastukh, V.M.; Swiger, B.M.; Reed, D.J.; Patel, M.R.; Bardwell, G.C.; Pastukh, V.V.; Alexeyev, M.F.; Gillespie, M.N. Perinuclear Mitochondrial Clustering Creates an Oxidant-Rich Nuclear Domain Required for Hypoxia-Induced Transcription. Sci. Signal. 2012, 5, ra47. [Google Scholar] [CrossRef] [Green Version]
- Thomas, L.W.; Ashcroft, M. Exploring the Molecular Interface between Hypoxia-Inducible Factor Signalling and Mitochondria. Cell. Mol. Life Sci. 2019, 76, 1759–1777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, K.; Chacko, L.A.; Chug, M.K.; Jhunjhunwala, S.; Ananthanarayanan, V. Association of Mitochondria with Microtubules Inhibits Mitochondrial Fission by Precluding Assembly of the Fission Protein Dnm1. J. Biol. Chem. 2019, 294, 3385–3396. [Google Scholar] [CrossRef] [Green Version]
- Saxton, W.M.; Hollenbeck, P.J. The Axonal Transport of Mitochondria. J. Cell Sci. 2012, 125, 2095–2104. [Google Scholar] [CrossRef] [Green Version]
- Maday, S.; Twelvetrees, A.E.; Moughamian, A.J.; Holzbaur, E.L.F. Axonal Transport: Cargo-Specific Mechanisms of Motility and Regulation. Neuron 2014, 84, 292–309. [Google Scholar] [CrossRef] [Green Version]
- Schroer, T.A. Dynactin. Annu. Rev. Cell Dev. Biol. 2004, 20, 759–779. [Google Scholar] [CrossRef]
- Zhang, H.; Bosch-Marce, M.; Shimoda, L.A.; Tan, Y.S.; Baek, J.H.; Wesley, J.B.; Gonzalez, F.J.; Semenza, G.L. Mitochondrial Autophagy Is an HIF-1-Dependent Adaptive Metabolic Response to Hypoxia. J. Biol. Chem. 2008, 283, 10892–10903. [Google Scholar] [CrossRef] [Green Version]
- Zuo, W.; Zhang, S.; Xia, C.-Y.; Guo, X.-F.; He, W.-B.; Chen, N.-H. Mitochondria Autophagy Is Induced after Hypoxic/Ischemic Stress in a Drp1 Dependent Manner: The Role of Inhibition of Drp1 in Ischemic Brain Damage. Neuropharmacology 2014, 86, 103–115. [Google Scholar] [CrossRef]
- Westermann, B. Bioenergetic Role of Mitochondrial Fusion and Fission. Biochim. Et Biophys. Acta (BBA)—Bioenerg. 2012, 1817, 1833–1838. [Google Scholar] [CrossRef] [Green Version]
- Twig, G.; Shirihai, O.S. The Interplay between Mitochondrial Dynamics and Mitophagy. Antioxid. Redox Signal. 2011, 14, 1939–1951. [Google Scholar] [CrossRef] [Green Version]
- Mishra, P.; Chan, D.C. Metabolic Regulation of Mitochondrial Dynamics. J. Cell Biol. 2016, 212, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, K.; Grimm, A.; Dallmann, R.; Oettinghaus, B.; Restelli, L.M.; Witzig, M.; Ishihara, N.; Mihara, K.; Ripperger, J.A.; Albrecht, U.; et al. Circadian Control of DRP1 Activity Regulates Mitochondrial Dynamics and Bioenergetics. Cell Metab. 2018, 27, 657–666. [Google Scholar] [CrossRef] [Green Version]
- Horbay, R.; Bilyy, R. Mitochondrial Dynamics during Cell Cycling. Apoptosis 2016, 21, 1327–1335. [Google Scholar] [CrossRef]
- Ježek, J.; Cooper, K.F.; Strich, R. Reactive Oxygen Species and Mitochondrial Dynamics: The Yin and Yang of Mitochondrial Dysfunction and Cancer Progression. Antioxidants 2018, 7, 13. [Google Scholar] [CrossRef]
- Ghose, P.; Park, E.C.; Tabakin, A.; Salazar-Vasquez, N.; Rongo, C. Anoxia-Reoxygenation Regulates Mitochondrial Dynamics through the Hypoxia Response Pathway, SKN-1/Nrf, and Stomatin-Like Protein STL-1/SLP-2. PLoS Genet. 2013, 9, e1004063. [Google Scholar] [CrossRef] [Green Version]
- Toyama, E.Q.; Herzig, S.; Courchet, J.; Lewis, T.L.; Losón, O.C.; Hellberg, K.; Young, N.P.; Chen, H.; Polleux, F.; Chan, D.C.; et al. AMP-Activated Protein Kinase Mediates Mitochondrial Fission in Response to Energy Stress. Science 2016, 351, 275–281. [Google Scholar] [CrossRef] [Green Version]
- Rider, M.H.; Hussain, N.; Dilworth, S.M.; Storey, K.B. Phosphorylation of Translation Factors in Response to Anoxia in Turtles, Trachemys Scripta Elegans: Role of the AMP-Activated Protein Kinase and Target of Rapamycin Signalling Pathways. Mol. Cell. Biochem. 2009, 332, 207–213. [Google Scholar] [CrossRef]
- Stensløkken, K.-O.; Ellefsen, S.; Stecyk, J.A.W.; Dahl, M.B.; Nilsson, G.E.; Vaage, J. Differential Regulation of AMP-Activated Kinase and AKT Kinase in Response to Oxygen Availability in Crucian Carp (Carassius Carassius). Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2008, 295, R1803–R1814. [Google Scholar] [CrossRef] [Green Version]
- Brustovetsky, N.N.; Egorova, M.V.; Iljasova, E.N.; Bakeeva, L.E. Relationship between Structure and Function of Liver Mitochondria from Hibernating and Active Ground Squirrels, Citellus Undulatus. Comp. Biochem. Physiol. B 1993, 106, 125–130. [Google Scholar] [CrossRef]
- Brustovetsky, N.N.; Egorova, M.V.; Gnutov, D.Y. The Mechanism of Calcium-Dependent Activation of Respiration of Liver Mitochondria from Hibernating Ground Squirrels, Citellus Undulatus. Comp. Biochem. Physiol. B 1993, 106, 423–426. [Google Scholar] [CrossRef]
- Bailey, J.R.; Driedzic, W.R. Decreased Total Ventricular and Mitochondrial Protein Synthesis during Extended Anoxia in Turtle Heart. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1996, 271, R1660–R1667. [Google Scholar] [CrossRef]
- Picard, M.; McManus, M.J.; Csordás, G.; Várnai, P.; Dorn Ii, G.W.; Williams, D.; Hajnóczky, G.; Wallace, D.C. Trans-Mitochondrial Coordination of Cristae at Regulated Membrane Junctions. Nat. Commun. 2015, 6, 6259. [Google Scholar] [CrossRef] [Green Version]
- Farhat, E.; Cheng, H.; Romestaing, C.; Pamenter, M.; Weber, J.-M. Goldfish Response to Chronic Hypoxia: Mitochondrial Respiration, Fuel Preference and Energy Metabolism. Metabolites 2021, 11, 187. [Google Scholar] [CrossRef]
- Onishi, M.; Yamano, K.; Sato, M.; Matsuda, N.; Okamoto, K. Molecular Mechanisms and Physiological Functions of Mitophagy. EMBO J. 2021, 40, e104705. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myrka, A.; Buck, L. Cytoskeletal Arrest: An Anoxia Tolerance Mechanism. Metabolites 2021, 11, 561. https://doi.org/10.3390/metabo11080561
Myrka A, Buck L. Cytoskeletal Arrest: An Anoxia Tolerance Mechanism. Metabolites. 2021; 11(8):561. https://doi.org/10.3390/metabo11080561
Chicago/Turabian StyleMyrka, Alexander, and Leslie Buck. 2021. "Cytoskeletal Arrest: An Anoxia Tolerance Mechanism" Metabolites 11, no. 8: 561. https://doi.org/10.3390/metabo11080561