Differences in Clinical and Dietary Characteristics, Serum Adipokine Levels, and Metabolomic Profiles between Early- and Late-Onset Gout
Abstract
:1. Introduction
2. Results
2.1. Clinical Features in the Early-Onset and Late-Onset Groups
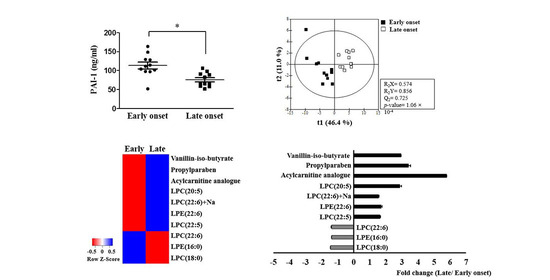
2.2. Serum Adipokines in the Early-Onset and Late-Onset Groups
2.3. Potential Pathophysiologic Mechanism and Markers of Differences between the Early-Onset and Late-Onset Groups
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Serum Adipokine Assay
4.3. Identification of Serum Metabolites by UPLC-Q-TOF-MS
4.4. Chemicals and Reagents
4.5. Sample Preparation
4.6. Metabolic Profiling of Serum Samples by UPLC-Q-TOF-MS
4.7. Data Processing
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gonçalves, J.P.; Oliveira, A.; Severo, M.; Santos, A.C.; Lopes, C. Cross-sectional and longitudinal associations between serum uric acid and metabolic syndrome. Endocrine 2012, 41, 450–457. [Google Scholar] [CrossRef]
- Borghi, C.; Rosei, E.A.; Bardin, T.; Dawson, J.; Dominiczak, A.; Kielstein, J.T.; Manolis, A.J.; Perez-Ruiz, F.; Mancia, G. Serum uric acid and the risk of cardiovascular and renal disease. J. Hypertens. 2015, 33, 1729–1741. [Google Scholar] [CrossRef]
- Zhu, Y.; Pandya, B.J.; Choi, H.K. Prevalence of gout and hyperuricemia in the US general population: The National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum 2011, 63, 3136–3141. [Google Scholar] [CrossRef] [PubMed]
- Kedar, E.; Simkin, P.A. A perspective on diet and gout. Adv. Chronic Kidney Dis. 2012, 19, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Rho, Y.H.; Zhu, Y.; Choi, H.K. The epidemiology of uric acid and fructose. Semin. Nephrol. 2011, 31, 410–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebrahimpour-Koujan, S.; Saneei, P.; Larijani, B.; Esmaillzadeh, A. Consumption of sugar sweetened beverages and dietary fructose in relation to risk of gout and hyperuricemia: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2020, 60, 1–10. [Google Scholar] [CrossRef]
- Dalbeth, N.; Phipps-Green, A.; House, M.E.; Gamble, G.D.; Horne, A.; Stamp, L.K.; Merriman, T.R. Body mass index modulates the relationship of sugar-sweetened beverage intake with serum urate concentrations and gout. Arthritis Res. Ther. 2015, 17, 263. [Google Scholar] [CrossRef] [Green Version]
- Pascart, T.; Norberciak, L.; Ea, H.K.; Guggenbuhl, P.; Lioté, F. Patients with early-onset gout and development of earlier severe joint involvement and metabolic comorbid conditions: Results from a cross-sectional epidemiologic survey. Arthritis Care Res. 2019, 71, 986–992. [Google Scholar] [CrossRef]
- Zhang, B.; Fang, W.; Zeng, X.; Zhang, Y.; Ma, Y.; Sheng, F.; Zhang, X. Clinical characteristics of early-and late-onset gout: A cross-sectional observational study from a Chinese gout clinic. Medicine 2016, 95, e5425. [Google Scholar] [CrossRef]
- Liu, S.C.; Xia, L.; Zhang, J.; Lu, X.H.; Hu, D.K.; Zhang, H.T.; Li, H.J. Gout and risk of myocardial infarction: A systemic review and meta-analysis of cohort studies. PLoS ONE 2015, 10, e0134088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaidi, F.; Narang, R.K.; Phipps-Green, A.; Gamble, G.G.; Tausche, A.K.; So, A.; Riches, P.; Andres, M.; Perez-Ruiz, F.; Doherty, M.; et al. Systematic genetic analysis of early-onset gout: ABCG2 is the only associated locus. Rheumatology 2020, 59, 2544–2549. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, H.; Ichida, K.; Takada, T.; Nakayama, A.; Nakashima, H.; Nakamura, T.; Kawamura, Y.; Takada, Y.; Yamamoto, K.; Inoue, H.; et al. Common dysfunctional variants in ABCG2 are a major cause of early-onset gout. Sci. Rep. 2013, 3, 2014. [Google Scholar] [CrossRef] [Green Version]
- Stiburkova, B.; Pavelcova, K.; Zavada, J.; Petru, L.; Simek, P.; Cepek, P.; Pavlikova, M.; Matsuo, H.; Merriman, T.R.; Pavelka, K. Functional non-synonymous variants of ABCG2 and gout risk. Rheumatology 2017, 56, 1982–1992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelishadi, R.; Mansourian, M.; Heidari-Beni, M. Association of fructose consumption and components of metabolic syndrome in human studies: A systematic review and meta-analysis. Nutrition 2014, 30, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Aeberli, I.; Gerber, P.A.; Hochuli, M.; Kohler, S.; Haile, S.R.; Gouni-Berthold, I.; Berthold, H.K.; Spinas, G.A.; Berneis, K. Low to moderate sugar-sweetened beverage consumption impairs glucose and lipid metabolism and promotes inflammation in healthy young men: A randomized controlled trial. Am. J. Clin. Nutr. 2011, 94, 479–485. [Google Scholar] [CrossRef] [Green Version]
- Lê, K.A.; Faeh, D.; Stettler, R.; Ith, M.; Kreis, R.; Vermathen, P.; Boesch, C.; Ravussin, E.; Tappy, L. A 4-wk high-fructose diet alters lipid metabolism without affecting insulin sensitivity or ectopic lipids in healthy humans. Am. J. Clin. Nutr. 2006, 84, 1374–1379. [Google Scholar] [CrossRef] [Green Version]
- Lê, K.A.; Ith, M.; Kreis, R.; Faeh, D.; Bortolotti, M.; Tran, C.; Boesch, C.; Tappy, L. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am. J. Clin. Nutr. 2009, 89, 1760–1765. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Granda, A.; Damms-Machado, A.; Basrai, M.; Bischoff, S.C. Changes in plasma acylcarnitine and lysophosphatidylcholine levels following a high-fructose diet: A targeted metabolomics study in healthy women. Nutrients 2018, 10, 1254. [Google Scholar] [CrossRef] [Green Version]
- Shireman, P.K.; McCarthy, W.J.; Pearce, W.H.; Patterson, B.K.; Shively, V.P.; Cipollone, M.; Tamarina, N.; Verrusio, E.N.; Kwaan, H.C. Elevated levels of plasminogen-activator inhibitor type 1 in atherosclerotic aorta. J. Vasc. Surg. 1996, 23, 810–817. [Google Scholar] [CrossRef] [Green Version]
- Thögersen, A.M.; Jansson, J.H.; Boman, K.; Nilsson, T.K.; Weinehall, L.; Huhtasaari, F.; Hallmans, G. High plasminogen activator inhibitor and tissue plasminogen activator levels in plasma precede a first acute myocardial infarction in both men and women—evidence for the fibrinolytic system as an independent primary risk factor. Circulation 1998, 98, 2241–2247. [Google Scholar] [CrossRef] [Green Version]
- Jung, R.G.; Motazedian, P.; Ramirez, F.D.; Simard, T.; Di Santo, P.; Visintini, S.; Faraz, M.A.; Labinaz, A.; Jung, Y.; Hibbert, B. Association between plasminogen activator inhibitor-1 and cardiovascular events: A systematic review and meta-analysis. Thromb. J. 2018, 16, 12. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, P.; Reynisdottir, S.; Lönnqvist, F.; Stemme, V.; Hamsten, A.; Arner, P. Adipose tissue secretion of plasminogen activator inhibitor-1 in non-obese and obese individuals. Diabetologia 1998, 41, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Thukkani, A.K.; McHowat, J.; Hsu, F.F.; Brennan, M.L.; Hazen, S.L.; Ford, D.A. Identification of α-chloro fatty aldehydes and unsaturated lysophosphatidylcholine molecular species in human atherosclerotic lesions. Circulation 2003, 108, 3128–3133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu-Wu, Y.; Hurt-Camejo, E.; Wiklund, O. Lysophosphatidylcholine induces the production of IL-1β by human monocytes. Atherosclerosis 1998, 137, 351–357. [Google Scholar] [CrossRef]
- Li, X.; Fang, P.; Li, Y.; Kuo, Y.M.; Andrews, A.J.; Nanayakkara, G.; Johnson, C.; Fu, H.; Shan, H.; Du, F.; et al. Mitochondrial reactive oxygen species mediate lysophosphatidylcholine-induced endothelial cell activation. Arter. Thromb. Vasc. Biol. 2016, 36, 1090–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakiev, E.R.; Sukhorukov, V.N.; Melnichenko, A.A.; Sobenin, I.A.; Ivanova, E.A.; Orekhov, A.N. Lipid composition of circulating multiple-modified low density lipoprotein. Lipids Health Dis. 2016, 15, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, N.D.; Sok, D.E.; Kim, M.R. Prevention of 1-palmitoyl lysophosphatidylcholine-induced inflammation by polyunsaturated acyl lysophosphatidylcholine. Inflamm. Res. 2012, 61, 473–483. [Google Scholar] [CrossRef]
- Kuniyasu, A.; Tokunaga, M.; Yamamoto, T.; Inoue, S.; Obama, K.; Kawahara, K.; Nakayama, H. Oxidized LDL and lysophosphatidylcholine stimulate plasminogen activator inhibitor-1 expression through reactive oxygen species generation and ERK1/2 activation in 3T3-L1 adipocytes. Biochim. Biophys. Acta 2011, 1811, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.K.; Kang, Y.H.; Oh, W.J.; Park, K.; Lee, D.Y.; Choi, D.; Kim, D.K.; Lee, Y.; Rhyu, M.R. Impact of lysophosphatidylcholine on the plasminogen activator system in cultured vascular smooth muscle cells. J. Korean Med. Sci. 2012, 27, 803–810. [Google Scholar] [CrossRef]
- Neogi, T.; Jansen, T.L.; Dalbeth, N.; Fransen, J.; Schumacher, H.R.; Berendsen, D.; Brown, M.; Choi, H.; Edwards, N.L.; Janssens, H.J.; et al. 2015 Gout Classification Criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2015, 74, 1789–1798. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Cho, C.W.; Hwang, J.T.; Son, N.; Choi, J.H.; Shim, G.S.; Han, C.K. LC-MS-based metabolomic analysis of serum and livers from red ginseng-fed rats. J. Ginseng Res. 2013, 37, 371–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Variables | Early-Onset Group (n = 38) | Late-Onset Group (n = 45) | p-Value |
|---|---|---|---|
| Age (years) | 34.5 (29.5–39.3) | 54.0 (49.5–59.0) | <0.001 |
| Age at onset (years) | 29.5 (23.8–34.0) | 50.0 (47.0–54.0) | <0.001 |
| Disease duration (months) | 42.0 (24.0–108.0) | 48.0 (9.0–90.0) | 0.407 |
| Height (cm) | 172.5 (170.0–177.3) | 170.0 (166.5–173.0) | 0.004 |
| Body weight (kg) | 83.5 (74.8–90.8) | 72.0 (65.0–79.0) | <0.001 |
| Body mass index (kg/m2) | 28.0 (25.4–29.8) | 24.8 (23.5–25.9) | <0.001 |
| Comorbidity (yes, %) | 22 (57.9) | 33 (73.3) | 0.138 |
| Tophi (yes, %) | 9 (23.7) | 11 (24.4) | 0.936 |
| Alcohol (yes, %) | 32 (84.2) | 38 (84.4) | 0.977 |
| SSB consumption (yes, %) | 29 (78.4) | 17 (38.6) | <0.001 |
| No | 8 (21.6) | 27 (61.4) | |
| 1–2/month | 11 (29.7) | 4 (9.1) | |
| 1–2/week | 7 (18.9) | 2 (4.5) | |
| 3–4/week | 5 (13.5) | 1 (2.3) | |
| 5–6/week | 2 (5.4) | 7 (15.9) | |
| Every day | 4 (10.8) | 3 (6.8) | |
| Laboratory data | |||
| Uric acid (mg/dL) (3.5–7.2) | 8.3 (6.5–9.6) | 7.3 (5.8–8.0) | 0.010 |
| Fasting glucose (mg/dL) (74–106) | 98.5 (92.0–117.5) | 107.5 (98.0–127.5) | 0.265 |
| Creatinine (mg/dL) (0.67–1.17) | 0.91 (0.85–1.12) | 1.00 (0.86–1.20) | 0.136 |
| eGFR (MDRD) | 108.5 (87.5–115.8) | 93.0 (66.5–101.8) | 0.007 |
| AST (IU/L) (1–37) | 23.5 (17.0–32.5) | 22.0 (18.0–28.8) | 0.432 |
| ALT (IU/L) (0–41) | 30.0 (24.0–51.0) | 27.0 (17.0–32.0) | 0.338 |
| Total cholesterol (mg/dL)(120–200) | 197.0 (179.5–227.5) | 183.5 (157.8–205.5) | 0.055 |
| Triglycerides (mg/dL) (0–150) | 155.0 (112.5–389.0) | 131.0 (90.0–246.3) | 0.304 |
| HDL cholesterol (mg/dL) (40–60) | 43.0 (35.5–55.5) | 42.0 (38.8–60.5) | 0.708 |
| LDL cholesterol (mg/dL) (0–130) | 146.0 (91.5–162.5) | 123.0 (98.5–145.0) | 0.363 |
| C-reactive protein (mg/L) (0.0–5.0) | 0.6 (0.1–1.0) | 0.3 (0.1–1.5) | 0.634 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suh, Y.S.; Noh, H.S.; Kim, H.-J.; Cheon, Y.-H.; Kim, M.; Lee, H.; Kim, H.-O.; Lee, S.-I. Differences in Clinical and Dietary Characteristics, Serum Adipokine Levels, and Metabolomic Profiles between Early- and Late-Onset Gout. Metabolites 2021, 11, 399. https://doi.org/10.3390/metabo11060399
Suh YS, Noh HS, Kim H-J, Cheon Y-H, Kim M, Lee H, Kim H-O, Lee S-I. Differences in Clinical and Dietary Characteristics, Serum Adipokine Levels, and Metabolomic Profiles between Early- and Late-Onset Gout. Metabolites. 2021; 11(6):399. https://doi.org/10.3390/metabo11060399
Chicago/Turabian StyleSuh, Young Sun, Hae Sook Noh, Hyun-Jin Kim, Yun-Hong Cheon, Mingyo Kim, Hanna Lee, Hyun-Ok Kim, and Sang-Il Lee. 2021. "Differences in Clinical and Dietary Characteristics, Serum Adipokine Levels, and Metabolomic Profiles between Early- and Late-Onset Gout" Metabolites 11, no. 6: 399. https://doi.org/10.3390/metabo11060399