Metabolomics of Photosynthetically Active Tissues in White Grapes: Effects of Light Microclimate and Stress Mitigation Strategies
Abstract
:1. Introduction
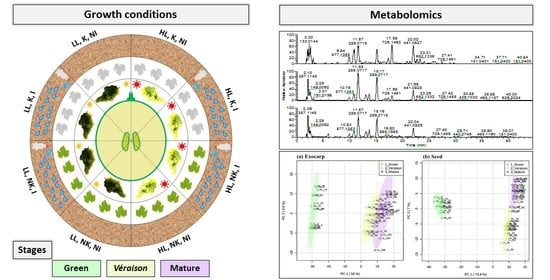
2. Results and Discussion
2.1. Global Effects on Grape Exocarp and Seed Metabolome
2.1.1. Specific Effects of Microclimate
2.1.2. Specific Effects of Irrigation
2.2. Changes in Carbon Skeletons
2.3. Lipid-Soluble Antioxidants and Lipid Oxidation in Photosynthetically-Active Grape Tissues
2.4. Effects on Grape Quality-Related Compounds: Total Phenolics and Sugars in Mature Fruit
3. Materials and Methods
3.1. Grapevine Field Conditions and Sampling
3.2. Untargeted Metabolomics by Liquid Chromatography Mass Spectrometry (LCMS) and Gas Chromatography Mass Spectrometry (GCMS)
3.2.1. LCMS Analysis
3.2.2. GCMS Analysis
3.2.3. Untargeted Data Processing and Multivariate Statistical Analysis
3.3. Targeted Analysis
3.3.1. Tocopherols
3.3.2. Sugars
3.3.3. Total Soluble Phenolics
3.3.4. Lipid Peroxidation Products
3.3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Estreicher, S.K. The beginning of wine and viticulture. Phys. Status Solidi C 2017, 14. [Google Scholar] [CrossRef]
- Ali, K.; Maltese, F.; Choi, Y.H.; Verpoorte, R. Metabolic constituents of grapevine and grape-derived products. Phytochem. Rev. 2010, 9, 357–378. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural bioactive compounds from winery by-products as health promoters: A review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef] [Green Version]
- OIV International Organisation of Vine and Wine. Statistical Report on World Vitiviniculture; OIV: Paris, France, 2018. [Google Scholar]
- Leeuwen, V.C.; Seguin, G. The concept of terroir in viticulture. J. Wine Res. 2006, 17, 1–10. [Google Scholar] [CrossRef]
- Blancquaert, E.H.; Oberholster, A.; Ricardo-da-Silva, J.M.; Deloire, A.J. Effects of abiotic factors on phenolic compounds in the grape berry—A review. S. Afr. J. Enol. Vitic. 2019, 40, 1–14. [Google Scholar] [CrossRef]
- Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Santos, J.A. Future scenarios for viticultural zoning in Europe: Ensemble projections and uncertainties. Int. J. Biometeorol. 2013, 57, 909–925. [Google Scholar] [CrossRef] [PubMed]
- Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Jones, G.V.; Alves, F.; Pinto, J.G.; Santos, J.A. Very high resolution bioclimatic zoning of Portuguese wine regions: Present and future scenarios. Reg. Environ. Chang. 2014, 14, 295–306. [Google Scholar] [CrossRef]
- Orduna, R.M. Climate change associated effects on grape and wine quality and production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Fraga, H.; Pinto, J.G.; Santos, J.A. Climate change projections for chilling and heat forcing conditions in European vineyards and olive orchards: A multi-model assessment. Clim. Chang. 2019, 152, 179–193. [Google Scholar] [CrossRef]
- Bernardo, S.; Dinis, L.T.; Machado, N.; Moutinho-Pereira, J. Grapevine abiotic stress assessment and search for sustainable adaptation strategies in Mediterranean-like climates. A review. Agron. Sustain. Dev. 2018, 38, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Duchêne, E. How can grapevine genetics contribute to the adaptation to climate change? Oeno One 2016, 50, 113–114. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, A.G. Viticultural and vineyard management practices and their effects on grape and wine quality. In Managing Wine Quality: Viticulture and Wine Quality; Woodhead Publishing: Cambridge, UK, 2010; pp. 365–444. [Google Scholar]
- Caravia, L.; Collins, C.; Petrie, P.R.; Tyerman, S.D. Application of shade treatments during Shiraz berry ripening to reduce the impact of high temperature. Aust. J. Grape Wine Res. 2016, 22, 422–437. [Google Scholar] [CrossRef]
- Parpinello, G.P.; Ricci, A.; Rombolà, A.D.; Nigro, G.; Versari, A. Comparison of Sangiovese wines obtained from stabilized organic and biodynamic vineyard management systems. Food Chem. 2019, 283, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Fraga, H.; García de Cortázar Atauri, I.; Santos, J.A. Viticultural irrigation demands under climate change scenarios in Portugal. Agric. Water Manag. 2018, 196, 66–74. [Google Scholar] [CrossRef]
- Conde, A.; Neves, A.; Breia, R.; Pimentel, D.; Dinis, L.T.; Bernardo, S.; Correia, C.M.; Cunha, A.; Gerós, H.; Moutinho-Pereira, J. Kaolin particle film application stimulates photoassimilate synthesis and modifies the primary metabolome of grape leaves. J. Plant Physiol. 2018, 223, 47–56. [Google Scholar] [CrossRef]
- Garrido, A.; Serôdio, J.; De Vos, R.; Conde, A.; Cunha, A. Influence of foliar kaolin application and irrigation on photosynthetic activity of grape berries. Agronomy 2019, 9, 685. [Google Scholar] [CrossRef] [Green Version]
- Dinis, L.T.; Ferreira, H.; Pinto, G.; Bernardo, S.; Correia, C.M.; Moutinho-Pereira, J. Kaolin-based, foliar reflective film protects photosystem II structure and function in grapevine leaves exposed to heat and high solar radiation. Photosynthetica 2016, 54, 47–55. [Google Scholar] [CrossRef]
- Dinis, L.T.; Malheiro, A.C.; Luzio, A.; Fraga, H.; Ferreira, H.; Gonçalves, I.; Pinto, G.; Correia, C.M.; Moutinho-Pereira, J. Improvement of grapevine physiology and yield under summer stress by kaolin-foliar application: Water relations, photosynthesis and oxidative damage. Photosynthetica 2018, 56, 641–651. [Google Scholar] [CrossRef]
- Conde, A.; Pimentel, D.; Neves, A.; Dinis, L.-T.; Bernardo, S.; Correia, C.M.; Gerós, H.; Moutinho-Pereira, J. Kaolin Foliar Application Has a Stimulatory Effect on Phenylpropanoid and Flavonoid Pathways in Grape Berries. Front. Plant Sci. 2016, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Dinis, L.T.; Bernardo, S.; Conde, A.; Pimentel, D.; Ferreira, H.; Félix, L.; Gerós, H.; Correia, C.M.; Moutinho-Pereira, J. Kaolin exogenous application boosts antioxidant capacity and phenolic content in berries and leaves of grapevine under summer stress. J. Plant Physiol. 2016, 191, 45–53. [Google Scholar] [CrossRef]
- Garrido, A.; Breia, R.; Serôdio, J.; Cunha, A. Impact of the Light Microclimate on Photosynthetic Activity of Grape Berry (Vitis vinifera): Insights for Radiation Absorption Mitigations’ Measures. In Theory and Practice of Climate Adaptation; Springer: Cham, Switzerland, 2018; pp. 419–441. [Google Scholar]
- Reshef, N.; Walbaum, N.; Agam, N.; Fait, A. Sunlight modulates fruit metabolic profile and shapes the spatial pattern of compound accumulation within the grape cluster. Front. Plant Sci. 2017, 8, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reshef, N.; Agam, N.; Fait, A. Grape Berry Acclimation to Excessive Solar Irradiance Leads to Repartitioning between Major Flavonoid Groups. J. Agric. Food Chem. 2018, 66, 3624–3636. [Google Scholar] [CrossRef] [PubMed]
- Reshef, N.; Fait, A.; Agam, N. Grape berry position affects the diurnal dynamics of its metabolic profile. Plant Cell Environ. 2019, 42, 1897–1912. [Google Scholar] [CrossRef]
- Brazel, A.J.; Ó’Maoileídigh, D.S. Photosynthetic activity of reproductive organs. J. Exp. Bot. 2019, 70, 1737–1753. [Google Scholar] [CrossRef]
- Breia, R.; Vieira, S.; da Silva, J.M.; Gerós, H.; Cunha, A. Mapping grape berry photosynthesis by chlorophyll fluorescence imaging: The effect of saturating pulse intensity in different tissues. Photochem. Photobiol. 2013, 89, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Wünsche, J.-N.; Lombardini, L.; Greer, D.H.; Palmer, J.W. “Surround” particle film applications—The effect on whole canopy physiology of apple. In XXVI International Horticultural Congress: Key Processes in the Growth and Cropping of Deciduous Fruit and Nut Trees, Proceedings of the XXVI International Horticultural Congress, Toronto, Canada, 11–17 August, 2002; ISHS: Toronto, ON, Canada, 2004; Volume 636, pp. 565–571. [Google Scholar]
- Keller, M.; Romero, P.; Gohil, H.; Smithyman, R.P.; Riley, W.R.; Casassa, L.F.; Harbertson, J.F. Deficit irrigation alters grapevine growth, physiology, and fruit microclimate. Am. J. Enol. Vitic. 2016, 67, 426–435. [Google Scholar] [CrossRef]
- Conde, C.; Silva, P.; Fontes, N.; Dias, A.C.P.; Tavares, R.M.; Sousa, M.J.; Agasse, A.; Delrot, S.; Gerós, H. Biochemical changes throughout grape berry development and fruit and wine quality. Food 2007, 1, 1–22. [Google Scholar]
- Garrido, J.; Borges, F. Wine and grape polyphenols—A chemical perspective. Food Res. Int. 2013, 54, 1844–1858. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, A.; Eiras-Dias, J.; Castellarin, S.D.; Gerós, H. Berry phenolics of grapevine under challenging environments. Int. J. Mol. Sci. 2013, 14, 18711–18739. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, G.J.T.; Pilbrow, J.R.; Hutton, D.R.; Hewitt, D.; Hunter, C.R.; Ristic, R.; Iland, P.G.; Jones, G.P. Development of seed polyphenols in berries from Vitis vinifera L. cv. Shiraz. Aust. J. Grape Wine Res. 2000, 6, 244–254. [Google Scholar] [CrossRef]
- Hassanein, M.M.M.; Abedel-Razek, A.G. Chromatographic quantitation of some bioactive minor components in oils of wheat germ and grape seeds produced as by-products. J. Oleo Sci. 2009, 58, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Cocaliadis, M.F.; Fernández-Muñoz, R.; Pons, C.; Orzaez, D.; Granell, A. Increasing tomato fruit quality by enhancing fruit chloroplast function. A double-edged sword? J. Exp. Bot. 2014, 65, 4589–4598. [Google Scholar] [CrossRef] [Green Version]
- Obiadalla-Ali, H.; Fernie, A.R.; Lytovchenko, A.; Kossmann, J.; Lloyd, J.R. Inhibition of chloroplastic fructose 1,6-bisphosphatase in tomato fruits leads to decreased fruit size, but only small changes in carbohydrate metabolism. Planta 2004, 219, 533–540. [Google Scholar] [CrossRef]
- Lytovchenko, A.; Eickmeier, I.; Pons, C.; Osorio, S.; Szecowka, M.; Lehmberg, K.; Arrivault, S.; Tohge, T.; Pineda, B.; Anton, M.T.; et al. Tomato Fruit Photosynthesis Is Seemingly Unimportant in Primary Metabolism and Ripening But Plays a Considerable Role in Seed Development. Plant Physiol. 2011, 157, 1650–1663. [Google Scholar] [CrossRef] [Green Version]
- Ollat, N.; Gaudillere, J.P. Carbon balance in developing grapevine berries. In Proceedings of the V International Symposium on Grapevine Physiology, Jerusalem, Israel, 25 May 1997; ISHS: Jerusalem, Israel, 2000; Volume 526, pp. 345–350. [Google Scholar]
- Deluc, L.G.; Grimplet, J.; Wheatley, M.D.; Tillett, R.L.; Quilici, D.R.; Osborne, C.; Schooley, D.A.; Schlauch, K.A.; Cushman, J.C.; Cramer, G.R. Transcriptomic and metabolite analyses of Cabernet Sauvignon grape berry development. BMC Genom. 2007, 8, 429. [Google Scholar] [CrossRef] [Green Version]
- Degu, A.; Hochberg, U.; Sikron, N.; Venturini, L.; Buson, G.; Ghan, R.; Plaschkes, I.; Batushansky, A.; Chalifa-Caspi, V.; Mattivi, F.; et al. Metabolite and transcript profiling of berry skin during fruit development elucidates differential regulation between Cabernet Sauvignon and Shiraz cultivars at branching points in the polyphenol pathway. BMC Plant Biol. 2014, 14, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Du Plessis, K.; Young, P.R.; Eyéghé-Bickong, H.A.; Vivier, M.A. The transcriptional responses and metabolic consequences of acclimation to elevated light exposure in grapevine berries. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Sun, X.; Weiszmann, J.; Weckwerth, W. System-Level and Granger Network Analysis of Integrated Proteomic and Metabolomic Dynamics Identifies Key Points of Grape Berry Development at the Interface of Primary and Secondary Metabolism. Front. Plant Sci. 2017, 8, 1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Z.W.; Léon, C.; Feil, R.; Lunn, J.E.; Delrot, S.; Gomès, E. Metabolic profiling reveals coordinated switches in primary carbohydrate metabolism in grape berry (Vitis vinifera L.), a non-climacteric fleshy fruit. J. Exp. Bot. 2013, 64, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, A.; di Carli, M.; Guzzo, F.; Stocchero, M.; Zenoni, S.; Ferrarini, A.; Tononi, P.; Toffali, K.; Desiderio, A.; Lilley, K.S.; et al. Identification of putative stage-specific grapevine berry biomarkers and omics data integration into networks. Plant Physiol. 2010, 154, 1439–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano, A.; Espinoza, C.; Armijo, G.; Inostroza-blancheteau, C.; Poblete, E.; Meyer-regueiro, C.; Arce, A.; Parada, F.; Santibáñez, C.; Arce-johnson, P.; et al. Omics Approaches for Understanding Grapevine Berry Development: Regulatory Networks Associated with Endogenous Processes and Environmental Responses. Front. Plant Sci. 2017, 8, 1486. [Google Scholar] [CrossRef] [Green Version]
- Jordão, A.M.; Ricardo-da-Silva, J.M.; Laureano, O. Evolution of catechins and oligomeric procyanidins during grape maturation of Castelão Francês and Touriga Francesa. Am. J. Enol. Vitic. 2001, 52, 230–234. [Google Scholar]
- Jeandet, P.; Bessis, R.; Gautheron, B. The production of resveratrol (3, 5, 4’-trihydroxystilbene) by grape berries in different developmental stages. Am. J. Enol. Vitic. 1991, 42, 41–46. [Google Scholar]
- Hasan, M.M.; Bae, H. An overview of stress-induced resveratrol synthesis in grapes: Perspectives for resveratrol-enriched grape products. Molecules 2017, 22, 294. [Google Scholar] [CrossRef] [PubMed]
- Downey, M.O.; Harvey, J.S.; Robinson, S.P. Synthesis of flavonols and expression of flavonol synthase genes in the developing grape berries of Shiraz and Chardonnay (Vitis vinifera L.). Aust. J. Grape Wine Res. 2003, 9, 110–121. [Google Scholar] [CrossRef]
- Song, J.; Shellie, K.C.; Wang, H.; Qian, M.C. Influence of deficit irrigation and kaolin particle film on grape composition and volatile compounds in Merlot grape (Vitis vinifera L.). Food Chem. 2012, 134, 841–850. [Google Scholar] [CrossRef]
- Cooley, N.M.; Glenn, D.M.; Clingeleffer, P.R.; Walker, R.R. The Effects of Water Deficit and Particle Film Technology Interactions on Cabernet Sauvignon Grape Composition. Acta Hortic. 2008, 193–200. [Google Scholar] [CrossRef]
- Friedel, M.; Stoll, M.; Patz, C.D.; Will, F.; Dietrich, H. Impact of light exposure on fruit composition of white “Riesling” grape berries (Vitis vinifera L.). Vitis J. Grapevine Res. 2015, 54, 107–116. [Google Scholar]
- Joubert, C.; Young, P.R.; Eyéghé-Bickong, H.A.; Vivier, M.A. Field-grown grapevine berries use carotenoids and the associated xanthophyll cycles to acclimate to UV exposure differentially in high and low light (Shade) conditions. Front. Plant Sci. 2016, 7, 786. [Google Scholar] [CrossRef] [Green Version]
- Koyama, K.; Ikeda, H.; Poudel, P.R.; Goto-Yamamoto, N. Light quality affects flavonoid biosynthesis in young berries of Cabernet Sauvignon grape. Phytochemistry 2012, 78, 54–64. [Google Scholar] [CrossRef]
- Agati, G.; Tattini, M. Multiple functional roles of flavonoids in photoprotection. New Phytol. 2010, 186, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Guo, A.; Zhang, Y.; Wang, H.; Liu, Y.; Li, H. A review on astringency and bitterness perception of tannins in wine. Trends Food Sci. Technol. 2014, 40, 6–19. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Peter Constabel, C. Tannins in plant-herbivore interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef]
- Rocchi, L. Physiological Responses of White Grape Berries to Sunlight Exposure. Ph.D. Thesis, Università degli Studi di Milano, Milan, Italy, 15 December 2015. [Google Scholar]
- Fujita, A.; Soma, N.; Goto-Yamamoto, N.; Mizuno, A.; Kiso, K.; Hashizume, K. Effect of shading on proanthocyanidin biosynthesis in the grape berry. J. Jpn. Soc. Hortic. Sci. 2007, 72, 112–119. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J.J. Naturally lignan-rich foods: A dietary tool for health promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef] [Green Version]
- Lewis, N.G.; Davin, L.B.; Sarkanen, S. Lignin and Lignan Biosynthesis: Distinctions and Reconciliations. ACS Symp. Ser. 1998. [Google Scholar] [CrossRef] [Green Version]
- Cadot, Y.; Miñana-Castelló, M.T.; Chevalier, M. Anatomical, Histological, and Histochemical Changes in Grape Seeds from Vitis vinifera L. cv Cabernet franc during Fruit Development. J. Agric. Food Chem. 2006, 54, 9206–9215. [Google Scholar] [CrossRef]
- Ananga, A.; Obuya, J.; Ochieng, J. Grape Seed Nutraceuticals for Disease Prevention: Current and Future Prospects. In Phenolic Compounds-Biological Activity; BoD—Books on Demand: Norderstedt, Germany, 2017; pp. 119–137. [Google Scholar]
- Duarte, N.; Ramalhete, C.; Rijo, P.; Reis, M.A.; Ferreira, M.-J.U. Stilbenoids in Grapes and Wine. In Handbook of Dietary Phytochemicals; Springer: Singapore, 2020; pp. 1–28. [Google Scholar]
- Genebra, T.; Santos, R.R.; Francisco, R.; Pinto-Marijuan, M.; Brossa, R.; Serra, A.T.; Duarte, C.M.M.; Chaves, M.M.; Zarrouk, O. Proanthocyanidin accumulation and biosynthesis are modulated by the irrigation regime in tempranillo seeds. Int. J. Mol. Sci. 2014, 15, 11862–11877. [Google Scholar] [CrossRef]
- Castellarin, S.D.; Matthews, M.A.; Di Gaspero, G.; Gambetta, G.A. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 2007, 227, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Koundouras, S.; Hatzidimitriou, E.; Karamolegkou, M.; Dimopoulou, E.; Kallithraka, S.; Tsialtas, J.T.; Zioziou, E.; Nikolaou, N.; Kotseridis, Y. Irrigation and rootstock effects on the phenolic concentration and aroma potential of Vitis vinifera L. cv. Cabernet Sauvignon grapes. J. Agric. Food Chem. 2009, 57, 7805–7813. [Google Scholar] [CrossRef] [PubMed]
- Kyraleou, M.; Kotseridis, Y.; Koundouras, S.; Chira, K.; Teissedre, P.L.; Kallithraka, S. Effect of irrigation regime on perceived astringency and proanthocyanidin composition of skins and seeds of Vitis vinifera L. cv. Syrah grapes under semiarid conditions. Food Chem. 2016, 203, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Yamaki, S. Metabolism and accumulation of sugars translocated to fruit and their regulation. J. Jpn. Soc. Hortic. Sci. 2010, 79, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Vallarino, J.G.; Osorio, S. Organic acids. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2018; p. 207. [Google Scholar]
- Chidi, B.S.; Bauer, F.F.; Rossouw, D. Organic acid metabolism and the impact of fermentation practices on wine acidity—A review. S. Afr. J. Enol. Vitic. 2018. [Google Scholar] [CrossRef] [Green Version]
- Sweetman, C.; Deluc, L.G.; Cramer, G.R.; Ford, C.M.; Soole, K.L. Regulation of malate metabolism in grape berry and other developing fruits. Phytochemistry 2009, 70, 1329–1344. [Google Scholar] [CrossRef]
- Cholet, C.; Claverol, S.; Claisse, O.; Rabot, A.; Osowsky, A.; Dumot, V.; Ferrari, G.; Gény, L. Tartaric acid pathways in Vitis vinifera L. (cv. Ugni blanc): A comparative study of two vintages with contrasted climatic conditions. BMC Plant Biol. 2016, 16, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sattler, S.E.; Cahoon, E.B.; Coughlan, S.J.; Dellapenna, D. Characterization of tocopherol cyclases from higher plants and cyanobacteria. Evolutionary implications for tocopherol synthesis and function. Plant Physiol. 2003, 132, 2184–2195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilati, S.; Brazzale, D.; Guella, G.; Milli, A.; Ruberti, C.; Biasioli, F.; Zottini, M.; Moser, C. The onset of grapevine berry ripening is characterized by ROS accumulation and lipoxygenase-mediated membrane peroxidation in the skin. BMC Plant Biol. 2014, 14, 87. [Google Scholar] [CrossRef] [Green Version]
- Sattler, S.E.; Gilliland, L.U.; Magallanes-lundback, M.; Pollard, M.; Dellapenna, D. Vitamin E is Essential for Seed Longevity and for Preventing Lipid Peroxidation during Germination. The Plant Cell 2004, 16, 1419–1432. [Google Scholar] [CrossRef] [Green Version]
- Kadir, S.; von Weihe, M.; Al-Khatib, K. Photochemical Efficiency and Recovery of Photosystem II in Grapes After Exposure to Sudden and Gradual Heat Stress. J. Am. Soc. Hortic. Sci. 2007, 132, 764–769. [Google Scholar] [CrossRef] [Green Version]
- Bouhamidi, R.; Prévost, V.; Nouvelot, A. High protection by grape seed proanthocyanidins (GSPC) of polyunsaturated fatty acids against UV-C induced peroxidation. Comptes Rendus Acad. Sci. 1998, 321, 31–38. [Google Scholar] [CrossRef]
- Bernardo, S.; Dinis, L.T.; Luzio, A.; Pinto, G.; Meijón, M.; Valledor, L.; Conde, A.; Gerós, H.; Correia, C.M.; Moutinho-Pereira, J. Kaolin particle film application lowers oxidative damage and DNA methylation on grapevine (Vitis vinifera L.). Environ. Exp. Bot. 2017, 139, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Deluc, L.G.; Quilici, D.R.; Decendit, A.; Grimplet, J.; Wheatley, M.D.; Schlauch, K.A.; Mérillon, J.-M.; Cushman, J.C.; Cramer, G.R. Water deficit alters differentially metabolic pathways affecting important flavor and quality traits in grape berries of Cabernet Sauvignon and Chardonnay. BMC Genom. 2009, 10, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conde, C.; Agasse, A.; Glissant, D.; Tavares, R.; Gerós, H.; Delrot, S. Pathways of glucose regulation of monosaccharide transport in grape cells. Plant Physiol. 2006, 141, 1563–1578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vos, R.C.H.; Moco, S.; Lommen, A.; Keurentjes, J.J.B.; Bino, R.J.; Hall, R.D. Untargeted large-scale plant metabolomics using liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2007, 2, 778. [Google Scholar] [CrossRef] [PubMed]
- Carreno-Quintero, N.; Acharjee, A.; Maliepaard, C.; Bachem, C.W.B.; Mumm, R.; Bouwmeester, H.; Visser, R.G.F.; Keurentjes, J.J.B. Untargeted metabolic quantitative trait loci analyses reveal a relationship between primary metabolism and potato tuber quality. Plant Physiol. 2012, 158, 1306–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef]
- Lommen, A. Metalign: Interface-driven, versatile metabolomics tool for hyphenated full-scan mass spectrometry data preprocessing. Anal. Chem. 2009, 81, 3079–3086. [Google Scholar] [CrossRef]
- Houshyani, B.; Kabouw, P.; Muth, D.; de Vos, R.C.H.; Bino, R.J.; Bouwmeester, H.J. Characterization of the natural variation in Arabidopsis thaliana metabolome by the analysis of metabolic distance. Metabolomics 2012, 8, 131–145. [Google Scholar] [CrossRef] [Green Version]
- Tikunov, Y.M.; Laptenok, S.; Hall, R.D.; Bovy, A.; de Vos, R.C.H. MSClust: A tool for unsupervised mass spectra extraction of chromatography-mass spectrometry ion-wise aligned data. Metabolomics 2012, 8, 714–718. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.; Sinelnikov, I.V.; Han, B.; Wishart, D.S. MetaboAnalyst 3.0-making metabolomics more meaningful. Nucleic Acids Res. 2015, 43, W251–W257. [Google Scholar] [CrossRef] [Green Version]
- Smilde, A.K.; Jansen, J.J.; Hoefsloot, H.C.J.; Lamers, R.J.A.N.; van der Greef, J.; Timmerman, M.E. ANOVA-simultaneous component analysis (ASCA): A new tool for analyzing designed metabolomics data. Bioinformatics 2005, 21, 3043–3048. [Google Scholar] [CrossRef] [PubMed]
- Engel, J.; Blanchet, L.; Bloemen, B.; van den Heuvel, L.P.; Engelke, U.H.F.; Wevers, R.A.; Buydens, L.M.C. Regularized MANOVA (rMANOVA) in untargeted metabolomics. Anal. Chim. Acta 2015, 899, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hummel, J.; Strehmel, N.; Selbig, J.; Walther, D.; Kopka, J. Decision tree supported substructure prediction of metabolites from GC-MS profiles. Metabolomics 2010, 6, 322–333. [Google Scholar] [CrossRef] [Green Version]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waterhouse, A.L. Determination of Total Phenolics. Curr. Protoc. Food Anal. Chem. 2002, 6, I1.1.1–I1.1.8. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]











| LCMS Data | GCMS Data | ||||||
|---|---|---|---|---|---|---|---|
| Exocarp | Seed | Exocarp | |||||
| Growth Conditions | G | V | M | G | V | M | M |
| Soil irrigation | - | 0.001 (78) | 0.002 (48) | - | 0.016 (30) | 0.264 | 0.147 |
| Kaolin | 0.472 | 0.112 | 0.165 | 0.262 | 0.197 | 0.145 | 0.036 (0) |
| Berry microclimate (HL/LL) | 0.001 (95) | 0.001 (88) | 0.001 (154) | 0.012 (26) | 0.115 | 0.006 (31) | 0.003 (3) |
| Irrigation × Kaolin | - | 0.044 (0) | 0.043 (0) | - | 0.084 | 0.194 | 0.001 (10) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garrido, A.; Engel, J.; Mumm, R.; Conde, A.; Cunha, A.; De Vos, R.C.H. Metabolomics of Photosynthetically Active Tissues in White Grapes: Effects of Light Microclimate and Stress Mitigation Strategies. Metabolites 2021, 11, 205. https://doi.org/10.3390/metabo11040205
Garrido A, Engel J, Mumm R, Conde A, Cunha A, De Vos RCH. Metabolomics of Photosynthetically Active Tissues in White Grapes: Effects of Light Microclimate and Stress Mitigation Strategies. Metabolites. 2021; 11(4):205. https://doi.org/10.3390/metabo11040205
Chicago/Turabian StyleGarrido, Andreia, Jasper Engel, Roland Mumm, Artur Conde, Ana Cunha, and Ric C. H. De Vos. 2021. "Metabolomics of Photosynthetically Active Tissues in White Grapes: Effects of Light Microclimate and Stress Mitigation Strategies" Metabolites 11, no. 4: 205. https://doi.org/10.3390/metabo11040205