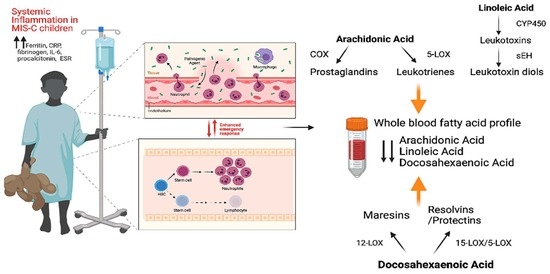
Blood Fatty Acids Profile in MIS-C Children
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Anthropometric and Blood Measurements
4.3. Fatty Acid Analysis
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adeyinka, A.; Bailey, K.; Pierre, L.; Kondamudi, N. COVID 19 infection: Pediatric perspectives. J. Am. Coll. Emerg. Physicians Open 2021, 2, e12375. [Google Scholar] [CrossRef]
- Syangtan, G.; Bista, S.; Dawadi, P.; Rayamajhee, B.; Shrestha, L.B.; Tuladhar, R.; Joshi, D.R. Asymptomatic SARS-CoV-2 Carriers: A Systematic Review and Meta-Analysis. Front. Public Health 2021, 8, 587374. [Google Scholar] [CrossRef]
- Bernardino, F.B.S.; Alencastro, L.C.d.S.; da Silva, R.A.; Ribeiro, A.D.d.N.; Castilho, G.R.d.C.; Gaíva, M.A.M. Epidemiological profile of children and adolescents with COVID-19: A scoping review. Rev. Bras. Enferm. 2021, 74 (Suppl. 1), e20200624. [Google Scholar] [CrossRef] [PubMed]
- Riphagen, S.; Gomez, X.; Gonzalez-Martinez, C.; Wilkinson, N.; Theocharis, P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020, 395, 1607–1608. [Google Scholar] [CrossRef]
- Verdoni, L.; Mazza, A.; Gervasoni, A.; Martelli, L.; Ruggeri, M.; Ciuffreda, M.; Bonanomi, E.; D’Antiga, L. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: An observational cohort study. Lancet 2020, 395, 1771–1778. [Google Scholar] [CrossRef]
- Cabrero-Hernández, M.; García-Salido, A.; Leoz-Gordillo, I.; Alonso-Cadenas, J.A.; Gochi-Valdovinos, A.; González Brabin, A.; de Lama Caro-Patón, G.; Nieto-Moro, M.; De-Azagra-Garde, A.M.-; Serrano-González, A. Severe SARS-CoV-2 infection in children with suspected acute abdomen: A case series from a tertiary hospital in Spain. Pediatr. Infect. Dis. J. 2020, 39, E195–E198. [Google Scholar] [CrossRef]
- Belhadjer, Z.; Méot, M.; Bajolle, F.; Khraiche, D.; Legendre, A.; Abakka, S.; Auriau, J.; Grimaud, M.; Oualha, M.; Beghetti, M.; et al. Acute Heart Failure in Multisystem Inflammatory Syndrome in Children in the Context of Global SARS-CoV-2 Pandemic. Circulation 2020, 142, 429–436. [Google Scholar] [CrossRef]
- Chiotos, K.; Bassiri, H.; Behrens, E.M.; Blatz, A.M.; Chang, J.; Diorio, C.; Fitzgerald, J.C.; Topjian, A.; John, A.R.O. Multisystem inflammatory syndrome in children during the coronavirus 2019 pandemic: A case series. J. Pediatr. Infect. Dis. Soc. 2020, 9, 393–398. [Google Scholar] [CrossRef]
- CDC. Information for Healthcare Providers about Multisystem Inflammatory Syndrome in Children (MIS-C). Available online: https://www.cdc.gov/mis-c/hcp/.html (accessed on 1 June 2021).
- Xu, S.; Chen, M.; Weng, J. COVID-19 and Kawasaki disease in children. Pharmacol. Res. 2020, 159, 104951. [Google Scholar] [CrossRef]
- Shahin, W.; Rabie, W.; Alyossof, O.; Alasiri, M.; Alfaki, M.; Mahmoud, E.; Hijazi, M.; El Faraidi, H.; Alahmari, H. COVID-19 in children ranging from asymptomatic to a multi-system inflammatory disease A single-center study. Saudi Med. J. 2021, 42, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Vogel, T.P.; Top, K.A.; Karatzios, C.; Hilmers, D.C.; Tapia, L.I.; Moceri, P.; Giovannini-Chami, L.; Wood, N.; Chandler, R.E.; Klein, N.P.; et al. Multisystem inflammatory syndrome in children and adults (MIS-C/A): Case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2021, 39, 3037–3049. [Google Scholar]
- Hoste, L.; Van Paemel, R.; Haerynck, F. Multisystem inflammatory syndrome in children related to COVID-19: A systematic review. Eur. J. Pediatr. 2021, 180, 2019–2034. [Google Scholar] [CrossRef]
- Cattalini, M.; Taddio, A.; Bracaglia, C.; Cimaz, R.; Paolera, S.D.; Filocamo, G.; La Torre, F.; Lattanzi, B.; Marchesi, A.; Simonini, G.; et al. Childhood multisystem inflammatory syndrome associated with COVID-19 (MIS-C): A diagnostic and treatment guidance from the Rheumatology Study Group of the Italian Society of Pediatrics. Ital. J. Pediatr. 2021, 47, 24. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Levi, M.; Thachil, J. Coagulopathy in COVID-19. J. Thromb. Haemost. 2020, 18, 2103–2109. [Google Scholar] [CrossRef] [PubMed]
- Mahjoub, Y.; Rodenstein, D.O.; Jounieaux, V. Severe Covid-19 disease: Rather AVDS than ARDS? Crit. Care 2020, 24, 327. [Google Scholar] [CrossRef] [PubMed]
- Arjmand, B.; Alavi-Moghadam, S.; Roudsari, P.P.; Rezaei-Tavirani, M.; Rahim, F.; Gilany, K.; Mohamadi-Jahani, F.; Adibi, H.; Larijani, B. COVID-19 Pathology on Various Organs and Regenerative Medicine and Stem Cell-Based Interventions. Front. Cell Dev. Biol. 2021, 9, 675310. [Google Scholar] [CrossRef]
- Bunyavanich, S.; Do, A.; Vicencio, A. Nasal Gene Expression of Angiotensin-Converting Enzyme 2 in Children and Adults. JAMA J. Am. Med. Assoc. 2020, 323, 2427–2429. [Google Scholar] [CrossRef]
- Nakra, N.; Blumberg, D.; Herrera-Guerra, A.; Lakshminrusimha, S. Multi-System Inflammatory Syndrome in Children (MIS-C) Following SARS-CoV-2 Infection: Review of Clinical Presentation, Hypothetical Pathogenesis, and Proposed Management. Children 2020, 7, 69. [Google Scholar] [CrossRef]
- Sala, A.; Zarini, S.; Bolla, M. Leukotrienes: Lipid bioeffectors of inflammatory reactions. Biochemistry (Moscow) 1998, 63, 84–92. [Google Scholar]
- Calder, P.C. Eicosanoids. Essays Biochem. 2020, 64, 423–441. [Google Scholar]
- Serhan, C.N.; Yacoubian, S.; Yang, R. Anti-inflammatory and proresolving lipid mediators. Annu. Rev. Pathol. 2008, 3, 279–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serhan, C.N.; Gupta, S.K.; Perretti, M.; Godson, C.; Brennan, E.; Li, Y.; Soehnlein, O.; Shimizu, T.; Werz, O.; Chiurchiù, V.; et al. The Atlas of Inflammation Resolution (AIR). Mol. Asp. Med. 2020, 74, 100894. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef]
- Feehan, K.T.; Gilroy, D.W. Is Resolution the End of Inflammation? Trends Mol. Med. 2019, 25, 198–214. [Google Scholar] [CrossRef]
- Pal, A.; Gowdy, K.M.; Oestreich, K.J.; Beck, M.; Shaikh, S.R. Obesity-Driven Deficiencies of Specialized Pro-resolving Mediators May Drive Adverse Outcomes during SARS-CoV-2 Infection. Front. Immunol. 2020, 11, 1997. [Google Scholar] [CrossRef]
- Funk, C.D.; Ardakani, A. A Novel Strategy to Mitigate the Hyperinflammatory Response to COVID-19 by Targeting Leukotrienes. Front. Pharmacol. 2020, 11, 1214. [Google Scholar] [CrossRef]
- Sala, A.; Murphy, R.C.; Voelkel, N.F. Direct airway injury results in elevated levels of sulfidopeptide leukotrienes, detectable in airway secretions. Prostaglandins 1991, 42, 1–7. [Google Scholar] [CrossRef]
- Khodir, A.E.; Ghoneim, H.A.; Rahim, M.A.; Suddek, G.M. Montelukast reduces sepsis-induced lung and renal injury in rats. Can. J. Physiol. Pharmacol. 2014, 92, 839–847. [Google Scholar] [CrossRef]
- Shen, B.; Yi, X.; Sun, Y.; Bi, X.; Du, J.; Zhang, C.; Quan, S.; Zhang, F.; Sun, R.; Qian, L.; et al. Proteomic and Metabolomic Characterization of COVID-19 Patient Sera. Cell 2020, 182, 59–72.e15. [Google Scholar] [CrossRef]
- CDC. Growth Charts. Available online: https://www.cdc.gov/growthcharts/index.htm (accessed on 1 June 2021).
- CDC. National Health and Nutrition Examination Survey. Available online: https://www.cdc.gov/nchs/nhanes/ (accessed on 1 June 2021).
- Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. Pediatrics 2011, 128 (Suppl. 5), S213–S256. [Google Scholar] [CrossRef] [Green Version]
- Vieira-Ribeiro, S.A.; Fonseca, P.C.A.; Andreoli, C.S.; Ribeiro, A.Q.; Hermsdorff, H.H.M.; Pereira, P.F.; Priore, S.E.; Franceschini, S.C.C. The TyG index cutoff point and its association with body adiposity and lifestyle in children. J. Pediatr. 2019, 95, 217–223. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care 2015, 38 (Suppl. 1), S8–S16. [Google Scholar] [CrossRef] [Green Version]
- Stark, K.D.; Henao, J.J.A.; Metherel, A.H.; Pilote, L. Translating plasma and whole blood fatty acid compositional data into the sum of eicosapentaenoic and docosahexaenoic acid in erythrocytes. Prostaglandins Leukot. Essent. Fat. Acids 2016, 104, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Risé, P.; Tragni, E.; Ghezzi, S.; Agostoni, C.; Marangoni, F.; Poli, A.; Catapano, A.L.; Siani, A.; Iacoviello, L.; Galli, C. Different patterns characterize Omega 6 and Omega 3 long chain polyunsaturated fatty acid levels in blood from Italian infants, children, adults and elderly. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 215–220. [Google Scholar] [CrossRef]
- Crippa, A.; Agostoni, C.; Mauri, M.; Molteni, M.; Nobile, M. Polyunsaturated Fatty Acids Are Associated With Behavior But Not With Cognition in Children With and Without ADHD: An Italian study. J. Atten. Disord. 2018, 22, 971–983. [Google Scholar] [CrossRef]
- Bonafini, S.; Giontella, A.; Tagetti, A.; Bresadola, I.; Gaudino, R.; Cavarzere, P.; Ramaroli, D.A.; Branz, L.; Marcon, D.; Pietrobelli, A.; et al. Fatty acid profile and desaturase activities in 7–10-year-old children attending primary school in Verona South District: Association between palmitoleic acid, SCD-16, indices of adiposity, and blood pressure. Int. J. Mol. Sci. 2020, 21, 3899. [Google Scholar] [CrossRef]
- Van der Wurff, I.S.M.; von Schacky, C.; Bergeland, T.; Leontjevas, R.; Zeegers, M.P.; Jolles, J.; Kirschner, P.A.; de Groot, R.H.M. Effect of 1 Year Krill Oil Supplementation on Cognitive Achievement of Dutch Adolescents: A Double-Blind Randomized Controlled Trial. Nutrients 2019, 11, 1230. [Google Scholar] [CrossRef] [Green Version]
- Ryan, A.S.; Nelson, E.B. Assessing the effect of docosahexaenoic acid on cognitive functions in healthy, preschool children: A randomized, placebo-controlled, double-blind study. Clin. Pediatr. 2008, 47, 355–362. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Malik, P.; Patel, U.; Mehta, D.; Patel, N.; Kelkar, R.; Akrmah, M.; Gabrilove, J.L.; Sacks, H. Biomarkers and outcomes of COVID-19 hospitalisations: Systematic review and meta-analysis. BMJ Evid. Based Med. 2021, 26, 107–108. [Google Scholar] [CrossRef]
- Mazzocchi, A.; De Cosmi, V.; Risé, P.; Milani, G.P.; Turolo, S.; Syrén, M.-L.; Sala, A.; Agostoni, C. Bioactive Compounds in Edible Oils and Their Role in Oxidative Stress and Inflammation. Front. Physiol. 2021, 12, 659551. [Google Scholar] [CrossRef] [PubMed]
- McReynolds, C.B.; Cortes-Puch, I.; Ravindran, R.; Khan, I.H.; Hammock, B.G.; Shih, P.-A.B.; Hammock, B.D.; Yang, J. Plasma Linoleate Diols Are Potential Biomarkers for Severe COVID-19 Infections. Front. Physiol. 2021, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Skarke, C.; Alamuddin, N.; Lawson, J.A.; Li, X.; Ferguson, J.F.; Reilly, M.P.; FitzGerald, G.A. Bioactive products formed in humans from fish oils. J. Lipid Res. 2015, 56, 1808–1820. [Google Scholar] [CrossRef] [Green Version]
- Balas, L.; Risé, P.; Gandrath, D.; Rovati, G.; Bolego, C.; Stellari, F.; Trenti, A.; Buccellati, C.; Durand, T.; Sala, A. Rapid Metabolization of Protectin D1 by β-Oxidation of Its Polar Head Chain. J. Med. Chem. 2019, 62, 9961–9975. [Google Scholar] [CrossRef]
- Regidor, P.A.; Santos, F.G.; Rizo, J.M.; Egea, F.M. Pro resolving inflammatory effects of the lipid mediators of omega 3 fatty acids and its implication in SARS COVID-19. Med. Hypotheses 2020, 145, 110340. [Google Scholar] [CrossRef]
- Calcaterra, V.; Bosoni, P.; Dilillo, D.; Mannarino, S.; Fiori, L.; Fabiano, V.; Carlucci, P.; Di Profio, E.; Verduci, E.; Mameli, C.; et al. Impaired Glucose-Insulin Metabolism in Multisystem Inflammatory Syndrome Related to SARS-CoV-2 in Children. Children 2021, 8, 384. [Google Scholar] [CrossRef] [PubMed]
- Saito, E.; Okada, T.; Abe, Y.; Kuromori, Y.; Miyashita, M.; Iwata, F.; Hara, M.; Ayusawa, M.; Mugishima, H.; Kitamura, Y. Docosahexaenoic Acid Content in Plasma Phospholipids and Desaturase Indices in Obese Children. J. Atheroscler. Thromb. 2011, 18, 345–350. [Google Scholar] [CrossRef] [Green Version]
- Burrows, T.; Collins, C.E.; Garg, M.L. Omega-3 index, obesity and insulin resistance in children. Int. J. Pediatr. Obes. 2011, 6, e532–e539. [Google Scholar] [CrossRef]
- Wolters, M.; Schlenz, H.; Foraita, R.; Galli, C.; Risé, P.; Moreno, L.A.; Molnár, D.; Russo, P.; Veidebaum, T.; Tornaritis, M.; et al. Reference values of whole-blood fatty acids by age and sex from European children aged 3–8 years. Int. J. Obes. 2014, 38, S86–S98. [Google Scholar] [CrossRef] [Green Version]
- Song, J.-W.; Lam, S.M.; Fan, X.; Cao, W.-J.; Wang, S.-Y.; Tian, H.; Chua, G.H.; Zhang, C.; Meng, F.-P.; Xu, Z.; et al. Omics-Driven Systems Interrogation of Metabolic Dysregulation in COVID-19 Pathogenesis. Cell Metab. 2020, 32, 188–202.e5. [Google Scholar] [CrossRef]
- Marangoni, F.; Agostoni, C.; Borghi, C.; Catapano, A.L.; Cena, H.; Ghiselli, A.; La Vecchia, C.; Lercker, G.; Manzato, E.; Pirillo, A.; et al. Dietary linoleic acid and human health: Focus on cardiovascular and cardiometabolic effects. Atherosclerosis 2020, 292, 90–98. [Google Scholar] [CrossRef] [Green Version]
- Whelan, J.; Fritsche, K. Linoleic acid. Adv. Nutr. 2013, 4, 311–312. [Google Scholar] [CrossRef]
- Hildreth, K.; Kodani, S.D.; Hammock, B.D.; Zhao, L. Cytochrome P450-derived linoleic acid metabolites EpOMEs and DiHOMEs: A review of recent studies. J. Nutr. Biochem. 2020, 86, 108484. [Google Scholar] [CrossRef]
- Zheng, J.; Plopper, C.G.; Lakritz, J.; Storms, D.H.; Hammock, B.D. Leukotoxin-diol: A putative toxic mediator involved in acute respiratory distress syndrome. Am. J. Respir. Cell Mol. Biol. 2001, 25, 434–438. [Google Scholar] [CrossRef]
- Gerster, H. Can adults adequately convert alpha-linolenic acid (18:3n-3) to eicosapentaenoic acid (20:5n-3) and docosahexaenoic acid (22:6n-3)? Int. J. Vitam. Nutr. Res. 1998, 68, 159–173. [Google Scholar]
- González-Gil, E.M.; Santabárbara, J.; Siani, A.; Ahrens, W.; Sioen, I.; Eiben, G.; Günther, K.; Iacoviello, L.; Molnar, D.; Risé, P.; et al. Whole-blood fatty acids and inflammation in European children: The IDEFICS Study. Eur. J. Clin. Nutr. 2016, 70, 819–823. [Google Scholar] [CrossRef]
- Fontes, J.D.; Rahman, F.; Lacey, S.; Larson, M.G.; Vasan, R.S.; Benjamin, E.J.; Harris, W.S.; Robins, S.J. Red blood cell fatty acids and biomarkers of inflammation: A cross-sectional study in a community-based cohort. Atherosclerosis 2015, 240, 431–436. [Google Scholar] [CrossRef] [Green Version]
- Asher, A.; Tintle, N.L.; Myers, M.; Lockshon, L.; Bacareza, H.; Harris, W.S. Blood omega-3 fatty acids and death from COVID-19: A pilot study. Prostaglandins Leukot. Essent. Fat. Acids 2021, 166, 102250. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Genetic Variation, Diet, Inflammation, and the Risk for COVID-19. Lifestyle Genom. 2021, 14, 37–42. [Google Scholar] [CrossRef]
- Darwesh, A.M.; Bassiouni, W.; Sosnowski, D.K.; Seubert, J.M. Can N-3 polyunsaturated fatty acids be considered a potential adjuvant therapy for COVID-19-associated cardiovascular complications? Pharmacol. Ther. 2021, 219, 107703. [Google Scholar] [CrossRef]
- Torrinhas, R.S.; Calder, P.C.; Lemos, G.O.; Waitzberg, D.L. Parenteral fish oil: An adjuvant pharmacotherapy for coronavirus disease 2019? Nutrition 2021, 81, 110900. [Google Scholar] [CrossRef]
- Doaei, S.; Gholami, S.; Rastgoo, S.; Gholamalizadeh, M.; Bourbour, F.; Bagheri, S.E.; Samipoor, F.; Akbari, M.E.; Shadnoush, M.; Ghorat, F.; et al. The effect of omega-3 fatty acid supplementation on clinical and biochemical parameters of critically ill patients with COVID-19: A randomized clinical trial. J. Transl. Med. 2021, 19, 128. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Rodríguez-Morán, M.; Guerrero-Romero, F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab. Syndr. Relat. Disord. 2008, 6, 299–304. [Google Scholar] [CrossRef]
- Navarro-González, D.; Sánchez-Íñigo, L.; Pastrana-Delgado, J.; Fernández-Montero, A.; Martinez, J.A. Triglyceride-glucose index (TyG index) in comparison with fasting plasma glucose improved diabetes prediction in patients with normal fasting glucose: The Vascular-Metabolic CUN cohort. Prev. Med. 2016, 86, 99–105. [Google Scholar] [CrossRef]
- Calcaterra, V.; Montalbano, C.; de Silvestri, A.; Pelizzo, G.; Regalbuto, C.; Paganelli, V.; Albertini, R.; Cave, F.D.; Larizza, D.; Cena, H. Triglyceride Glucose Index as a Surrogate Measure of Insulin Sensitivity in a Caucasian Pediatric Population. J. Clin. Res. Pediatr. Endocrinol. 2019. online ahead of print. [Google Scholar] [CrossRef]
- Marangoni, F.; Colombo, C.; Galli, C. A method for the direct evaluation of the fatty acid status in a drop of blood from a fingertip in humans: Applicability to nutritional and epidemiological studies. Anal. Biochem. 2004, 326, 267–272. [Google Scholar] [CrossRef]
- Risé, P.; Eligini, S.; Ghezzi, S.; Colli, S.; Galli, C. Fatty acid composition of plasma, blood cells and whole blood: Relevance for the assessment of the fatty acid status in humans. Prostaglandins Leukot. Essent. Fat. Acids 2007, 76, 363–369. [Google Scholar] [CrossRef]
| Mean ± SD | Reference Values | |
|---|---|---|
| N of subjects | 26 | |
| M/F | 21/5 | |
| Age (mo) | 110.79 ± 50.29 | |
| Height (cm) | 135.86 ± 24.24 | |
| Weight (kg) | 36.35 ± 16.26 | |
| BMI (kg/m2) | 18.58 ± 3.19 | |
| BMI z score [31] | 0.48 ± 1.00 | ±2 z score obesity and malnutrition (CDC) |
| Arm circ (cm) [32] | 21.16 ± 4.27 | <5° or >90° pc (NANHES III) |
| Waist circ (cm) [32] | 66.55 ± 10.48 | <5° or >90° pc (NANHES III) |
| Triceps skinfold (mm) [32] | 15.05 ± 7.80 | <5° or >90° pc (NANHES III) |
| Laboratory Markers of Inflammation | Median (25th–75th Percentile) | Reference Values | % Subjects Outside of the Ref Values |
|---|---|---|---|
| Fibrinogen [33] | 7 (6.4–7) | <4 g/L | 100 |
| D-dimer [33] | 2734.5 (1956–4432.5) | <500 µg/L | 100 |
| CRP | 236.5 (109–291.4) | ≤10 mg/L | 100 |
| ESR | 38 (23–69) | ≤30 mm | 61 |
| Procalcitonine [33] | 6.9 (2.3–24) | <0.5 µg/L | 96 |
| IL-6 [33] | 75 (10–253) | ≤7 ng/L | 89 |
| LDH [33] | 255 (221.2–287) | 180–360 U/L | |
| Ferritin [33] | 875.5 (414.8–1960.2) | <300 µg/L | 100 |
| Albumin | 25.5 (22.5–29) | 35–50 g/L | 100 |
| Biochemistry | |||
| Hb [33] | 11 (9.2–11.6) | 11.5–15.5 g/dL | 72 |
| Leukocytes | 8.4 (5.1–14.2) | 4.5–10 × 109/L | 40 |
| Platelets | 156 (111.5–210.5) | M 155–320, F 169–359 × 109/L | 44 |
| INR | 1.3 (1.2–1.4) | <1.2 | 76 |
| Ratio aPTT | 1.4 (1.2–1.4) | 0.84–1.16 | 86 |
| TnT | 47.5 (16–81.5) | ≤15 ng/L | 81 |
| NT-proBNP | 6619 (2621–14,594) | <450 ng/L | 100 |
| Creatinine | 0.5 (0.4–0.7) | 0.15–0.75 mg/dL | |
| Urea | 29 (16.5–44) | 19–50 mg/dL | |
| CK | 68 (35.8–147.2) | M 47–322 U/L; F 29–201 U/L | |
| AST | 57 (43–88.5) | 11–34 U/L | 100 |
| ALT | 31 (16.5–63.2) | M ≤ 49U/L, F ≤ 33 U/L | 32 |
| GGT | 26.5 (20–56.2) | M 12–68 U/L, F 6–40 U/L | 20 |
| Na+ [33] | 132 (130–135) | 135–145 mmol/L | 16 |
| K+ [33] | 3.5 (3–4) | 3.5–5 mmol/L | 52 |
| TSH [33] | 2.3 (1.3–3) | 0.5–4.2 mIU/L | |
| fT3 [33] | 2.7 (2–3.6) | 3.5–6.3 pmol/L | 80 |
| fT4 [33] | 12.2 (11.1–14.3) | 9–19.3 pmol/L | |
| Blood lipid and glucose metabolism | |||
| Total cholesterol [33] | 120 (85–164) | <170 mg/dL | 12 |
| HDL-cholesterol [33] | 16 (7–25.2) | >45 mg/dL | 100 |
| Triglycerides [33] | 190 (124–303.2) | <75 mg/dL 0–9 yo <90 mg/dL age 10–19 yo | 52 |
| TyG Index [34] | 9.2 (8.8–9.7) | <7.88 | 100 |
| Glucose [35] | 110.5 (95.5–125.2) | 70–110 mg/dL | 42 |
| HbA1c [35] | 33 (32–34.8) | ≤39 mmol/mol |
| FA | % w/w ± SD | (Min–Max) |
|---|---|---|
| 16:0 | 27.99 ± 1.68 | (24.65–30.90) |
| 18:0 | 10.16 ± 1.26 | (7.61–13.03) |
| 20:0 | 0.41 ± 0.10 | (0.26–0.62) |
| 22:0 | 1.05 ± 0.22 | (0.67–1.45) |
| 24:0 | 1.75 ± 0.57 | (1.13–3.05) |
| 16:1 | 3.32 ± 1.09 | (1.58–6.44) |
| 18:1 n − 9 | 26.87 ± 3.18 | (20.30–32.72) |
| 18:1 n − 7 | 1.82 ± 0.52 | (1.18–3.94) |
| 20:1 | 0.19 ± 0.10 | (0.05–0.63) |
| 22:1 | 0.09 ± 0.04 | (0.02–0.18) |
| 24:1 | 2.21 ± 0.64 | (1.55–3.64) |
| 20:3 n − 9 | 0.15 ± 0.10 | (0.04–0.44) |
| 18:2 n − 6 | 12.39 ± 2.28 | (8.29–16.99) |
| 18:3 n − 6 | 0.64 ± 0.36 | (0.09–1.78) |
| 20:3 n − 6 | 1.09 ± 0.26 | (067–1.74) |
| 20:4 n − 6 | 6.38 ± 1.05 | (4.86–8.54) |
| 22:4 n − 6 | 0.81 ± 0.21 | (0.54–1.35) |
| 22:5 n − 6 | 0.42 ± 0.18 | (0.27–1.14) |
| 18:3 n − 3 | 0.22 ± 0.10 | (0.09–0.48) |
| 20:5 n − 3 | 0.34 ± 0.09 | (0.17–0.49) |
| 22:5 n − 3 | 0.48 ± 0.09 | (0.28–0.69) |
| 22:6 n − 3 | 1.21 ± 0.43 | (0.39–2.89) |
| SAT | 41.38 ± 2.39 | (37.42–46.37) |
| MONO | 34.50 ± 3.19 | (25.88–40.09) |
| POLY | 24.13 ± 2.75 | (18.36–31.33) |
| Author (Reference) | Present Study | Risé [37] | Crippa [38] | Bonafini [39] | Van der Wurff [40] | Ryan [41] |
|---|---|---|---|---|---|---|
| Age (yo) | 3–18 | <9 | 7–14 | 7–9 | 13–15 | 4 |
| % LA | 12.39 ± 2.28 | 22.54 ± 2.45 | ||||
| <9 yo | 12.72 ± 2.43 | 17.67 ± 1.92 | 19.9 ± 2.32 | |||
| >9 yo | 11.99 ± 2.13 | |||||
| % AA | 6.38 ± 1.05 | 10.10 ± 0.92 | ||||
| <9 yo | 6.14 ± 1.09 | 8.33 ± 1.04 | 12.21 ± 1.67 | 7.50 ± 1.89 | ||
| >9 yo | 6.66 ± 0.97 | 11.01–11.33 | ||||
| % ALA | 0.22 ± 0.10 | |||||
| <9 yo | 0.18 ± 0.10 | 0.15 ± 0.05 | 0.16 ± 0.08 | |||
| >9 yo | 0.27 ± 0.09 | |||||
| % EPA | 0.34 ± 0.09 | 1.13 ± 0.45 | ||||
| <9 yo | 0.32 ± 0.09 | 0.23 ± 0.08 | 0.30 ± 0.17 | 0.30 ± 0.39 | ||
| >9 yo | 0.36 ± 0.08 | 0.34–0.42 | ||||
| % DHA | 1.20 ± 0.43 | 1.93 ± 0.53 | ||||
| <9 yo | 1.08 ± 0.28 | 1.40 ± 0.37 | 2.92 ± 0.76 | 1.00 ± 0.34 | ||
| >9 yo | 1.35 ± 0.53 | 2.49–2.63 |
| LA | AA | ALA | EPA | DHA | O3I | |
|---|---|---|---|---|---|---|
| CRP | −0.023 | −0.036 | 0.508 * | 0.048 | 0.149 | 0.246 |
| IL-6 | −0.236 | −0.378 | −0.092 | −0.097 | −0.174 | −0.117 |
| ESR | 0.466 | 0.054 | −0.161 | −0.140 | 0 | −0.046 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verduci, E.; Risé, P.; Di Profio, E.; Fiori, L.; Vizzuso, S.; Dilillo, D.; Mannarino, S.; Zoia, E.; Calcaterra, V.; Pinna, C.; et al. Blood Fatty Acids Profile in MIS-C Children. Metabolites 2021, 11, 721. https://doi.org/10.3390/metabo11110721
Verduci E, Risé P, Di Profio E, Fiori L, Vizzuso S, Dilillo D, Mannarino S, Zoia E, Calcaterra V, Pinna C, et al. Blood Fatty Acids Profile in MIS-C Children. Metabolites. 2021; 11(11):721. https://doi.org/10.3390/metabo11110721
Chicago/Turabian StyleVerduci, Elvira, Patrizia Risé, Elisabetta Di Profio, Laura Fiori, Sara Vizzuso, Dario Dilillo, Savina Mannarino, Elena Zoia, Valeria Calcaterra, Christian Pinna, and et al. 2021. "Blood Fatty Acids Profile in MIS-C Children" Metabolites 11, no. 11: 721. https://doi.org/10.3390/metabo11110721