How Ceramides Orchestrate Cardiometabolic Health—An Ode to Physically Active Living
Abstract
:1. Introduction
2. Ceramide Metabolism
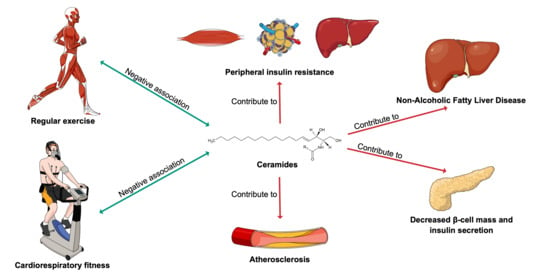
3. Ceramides in Cardiometabolic Health and Diseases
4. Ceramide Measurement in Clinical Research and Practice
5. Exercise-A Modulator of Ceramide Levels
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the global burden of disease study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Murray, C.J.B.R.; Foreman, K.J.; GBD 2019 DALYs and HALE Collaborator. Global, regional, and national disability-adjusted life years (dalys) for diseases and injuries and healthy life expectancy (hale), 1990 to 2019: Quantifying the epidemiological transition. In GBD Compare Data Visualization; Institute for Health Metrics and Evaluation, University of Washington: Seattle, WA, USA, 2019. [Google Scholar]
- Hunter, D.J.; Reddy, K.S. Noncommunicable diseases. N. Engl. J. Med. 2013, 369, 1336–1343. [Google Scholar] [CrossRef] [Green Version]
- Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the global burden of disease study 2013. Lancet 2015, 385, 117–171. [CrossRef]
- Benziger, C.P.; Roth, G.A.; Moran, A.E. The global burden of disease study and the preventable burden of ncd. Glob. Heart 2016, 11, 393–397. [Google Scholar] [CrossRef]
- Morrison, L.M.; Hall, L.; Chaney, A.L. Cholesterol metabolism and its relationship to atherosclerosis, coronary artery disease, and arteriosclerosis. Am. J. Med. 1948, 4, 616. [Google Scholar] [CrossRef]
- Kuijpers, P. History in medicine: The story of cholesterol, lipids and cardiology. J. Cradiol. Pract. 2021, 19, 1–5. [Google Scholar]
- Wang, M.; Wang, C.; Han, R.H.; Han, X. Novel advances in shotgun lipidomics for biology and medicine. Prog. Lipid Res. 2016, 61, 83–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laaksonen, R.; Ekroos, K.; Sysi-Aho, M.; Hilvo, M.; Vihervaara, T.; Kauhanen, D.; Suoniemi, M.; Hurme, R.; Marz, W.; Scharnagl, H.; et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond ldl-cholesterol. Eur. Heart J. 2016, 37, 1967–1976. [Google Scholar] [CrossRef]
- Havulinna, A.S.; Sysi-Aho, M.; Hilvo, M.; Kauhanen, D.; Hurme, R.; Ekroos, K.; Salomaa, V.; Laaksonen, R. Circulating ceramides predict cardiovascular outcomes in the population-based finrisk 2002 cohort. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2424–2430. [Google Scholar] [CrossRef] [Green Version]
- Meeusen, J.W.; Donato, L.J.; Bryant, S.C.; Baudhuin, L.M.; Berger, P.B.; Jaffe, A.S. Plasma ceramides. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1933–1939. [Google Scholar] [CrossRef] [Green Version]
- Hilvo, M.; Meikle, P.J.; Pedersen, E.R.; Tell, G.S.; Dhar, I.; Brenner, H.; Schöttker, B.; Lääperi, M.; Kauhanen, D.; Koistinen, K.M.; et al. Development and validation of a ceramide- and phospholipid-based cardiovascular risk estimation score for coronary artery disease patients. Eur. Heart J. 2019, 41, 371–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Summers, S.A. Could ceramides become the new cholesterol? Cell Metab. 2018, 27, 276–280. [Google Scholar] [CrossRef] [Green Version]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World health organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.M.; Thompson, A.M.; Blair, S.N.; Sallis, J.F.; Powell, K.E.; Bull, F.C.; Bauman, A.E. Sport and exercise as contributors to the health of nations. Lancet 2012, 380, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Kohl, H.W., III; Craig, C.L.; Lambert, E.V.; Inoue, S.; Alkandari, J.R.; Leetongin, G.; Kahlmeier, S. The pandemic of physical inactivity: Global action for public health. Lancet 2012, 380, 294–305. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef] [Green Version]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 esc guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef]
- Fiuza-Luces, C.; Santos-Lozano, A.; Joyner, M.; Carrera-Bastos, P.; Picazo, O.; Zugaza, J.L.; Izquierdo, M.; Ruilope, L.M.; Lucia, A. Exercise benefits in cardiovascular disease: Beyond attenuation of traditional risk factors. Nat. Rev. Cardiol. 2018, 15, 731–743. [Google Scholar] [CrossRef]
- Lavie, C.J.; Ozemek, C.; Carbone, S.; Katzmarzyk, P.T.; Blair, S.N. Sedentary behavior, exercise, and cardiovascular health. Circ. Res. 2019, 124, 799–815. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25, 1–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: The Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [Green Version]
- Magkos, F.; Hjorth, M.F.; Astrup, A. Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020, 16, 545–555. [Google Scholar] [CrossRef]
- Slentz, C.A.; Bateman, L.A.; Willis, L.H.; Granville, E.O.; Piner, L.W.; Samsa, G.P.; Setji, T.L.; Muehlbauer, M.J.; Huffman, K.M.; Bales, C.W.; et al. Effects of exercise training alone vs a combined exercise and nutritional lifestyle intervention on glucose homeostasis in prediabetic individuals: A randomised controlled trial. Diabetologia 2016, 59, 2088–2098. [Google Scholar] [CrossRef]
- Farzanegi, P.; Dana, A.; Ebrahimpoor, Z.; Asadi, M.; Azarbayjani, M.A. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (nafld): Roles of oxidative stress and inflammation. Eur. J. Sport Sci. 2019, 19, 994–1003. [Google Scholar] [CrossRef]
- Lear, S.A.; Hu, W.; Rangarajan, S.; Gasevic, D.; Leong, D.; Iqbal, R.; Casanova, A.; Swaminathan, S.; Anjana, R.M.; Kumar, R.; et al. The effect of physical activity on mortality and cardiovascular disease in 130,000 people from 17 high-income, middle-income, and low-income countries: The pure study. Lancet 2017, 390, 2643–2654. [Google Scholar] [CrossRef]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar] [PubMed]
- Tan-Chen, S.; Guitton, J.; Bourron, O.; Le Stunff, H.; Hajduch, E. Sphingolipid metabolism and signaling in skeletal muscle: From physiology to physiopathology. Front. Endocrinol. 2020, 11, 491. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Senkal, C.E.; Salama, M.F.; Snider, A.J.; Allopenna, J.J.; Rana, N.A.; Koller, A.; Hannun, Y.A.; Obeid, L.M. Ceramide is metabolized to acylceramide and stored in lipid droplets. Cell Metab. 2017, 25, 686–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, C.D.; Maceyka, M.; Cowart, L.A.; Spiegel, S. Sphingolipids in metabolic disease: The good, the bad, and the unknown. Cell Metab. 2021, 33, 1293–1306. [Google Scholar] [CrossRef]
- Chaurasia, B.; Talbot, C.L.; Summers, S.A. Adipocyte ceramides—The nexus of inflammation and metabolic disease. Front. Immunol. 2020, 11, 2282. [Google Scholar] [CrossRef] [PubMed]
- Field, B.C.; Gordillo, R.; Scherer, P.E. The role of ceramides in diabetes and cardiovascular disease regulation of ceramides by adipokines. Front. Endocrinol. 2020, 11, 763. [Google Scholar] [CrossRef]
- Poss, A.M.; Summers, S.A. Too much of a good thing? An evolutionary theory to explain the role of ceramides in nafld. Front. Endocrinol. 2020, 11, 505. [Google Scholar] [CrossRef]
- Yazıcı, D.; Sezer, H. Insulin resistance, obesity and lipotoxicity. Adv. Exp. Med. Biol. 2017, 960, 277–304. [Google Scholar] [CrossRef]
- Heras, V.; Castellano, J.M.; Fernandois, D.; Velasco, I.; Rodríguez-Vazquez, E.; Roa, J.; Vazquez, M.J.; Ruiz-Pino, F.; Rubio, M.; Pineda, R.; et al. Central ceramide signaling mediates obesity-induced precocious puberty. Cell Metab. 2020, 32, 951–966.e958. [Google Scholar] [CrossRef]
- Aburasayn, H.; Al Batran, R.; Ussher, J.R. Targeting ceramide metabolism in obesity. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E423–E435. [Google Scholar] [CrossRef] [PubMed]
- Amati, F.; Dubé, J.J.; Alvarez-Carnero, E.; Edreira, M.M.; Chomentowski, P.; Coen, P.M.; Switzer, G.E.; Bickel, P.E.; Stefanovic-Racic, M.; Toledo, F.G.S.; et al. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: Another paradox in endurance-trained athletes? Diabetes 2011, 60, 2588–2597. [Google Scholar] [CrossRef] [Green Version]
- Holland, W.L.; Brozinick, J.T.; Wang, L.-P.; Hawkins, E.D.; Sargent, K.M.; Liu, Y.; Narra, K.; Hoehn, K.L.; Knotts, T.A.; Siesky, A.; et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007, 5, 167–179. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Yang, X.; Xing, S.; Bian, F.; Yao, W.; Bai, X.; Zheng, T.; Wu, G.; Jin, S. Endogenous ceramide contributes to the transcytosis of oxldl across endothelial cells and promotes its subendothelial retention in vascular wall. Oxid. Med. Cell Longev. 2014, 2014, 823071. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, Y.; Wang, P.; Zhang, S.-Y.; Dong, Y.; Zeng, G.; Yan, Y.; Sun, L.; Wu, Q.; Liu, H.; et al. Adipocyte hypoxia-inducible factor 2α suppresses atherosclerosis by promoting adipose ceramide catabolism. Cell Metab. 2019, 30, 937–951.e5. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, B.; Summers, S.A. Ceramides—Lipotoxic inducers of metabolic disorders. Trends Endocrinol. Metab. 2015, 26, 538–550. [Google Scholar] [CrossRef]
- Chavez, J.A.; Knotts, T.A.; Wang, L.-P.; Li, G.; Dobrowsky, R.T.; Florant, G.L.; Summers, S.A. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J. Biol. Chem. 2003, 278, 10297–10303. [Google Scholar] [CrossRef] [Green Version]
- Lemaitre, R.N.; Yu, C.; Hoofnagle, A.; Hari, N.; Jensen, P.N.; Fretts, A.M.; Umans, J.G.; Howard, B.V.; Sitlani, C.M.; Siscovick, D.S.; et al. Circulating sphingolipids, insulin, homa-ir, and homa-b: The strong heart family study. Diabetes 2018, 67, 1663–1672. [Google Scholar] [CrossRef] [Green Version]
- Haus, J.M.; Kashyap, S.R.; Kasumov, T.; Zhang, R.; Kelly, K.R.; Defronzo, R.A.; Kirwan, J.P. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes 2009, 58, 337–343. [Google Scholar] [CrossRef] [Green Version]
- Boon, J.; Hoy, A.J.; Stark, R.; Brown, R.D.; Meex, R.C.; Henstridge, D.C.; Schenk, S.; Meikle, P.J.; Horowitz, J.F.; Kingwell, B.A.; et al. Ceramides contained in ldl are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes 2013, 62, 401–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zierath, J.R. The path to insulin resistance: Paved with ceramides? Cell Metab. 2007, 5, 161–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reidy, P.T.; Mahmassani, Z.S.; McKenzie, A.I.; Petrocelli, J.J.; Summers, S.A.; Drummond, M.J. Influence of exercise training on skeletal muscle insulin resistance in aging: Spotlight on muscle ceramides. Int. J. Mol. Sci. 2020, 21, 1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaurasia, B.; Tippetts, T.S.; Monibas, R.M.; Liu, J.; Li, Y.; Wang, L.; Wilkerson, J.L.; Sweeney, C.R.; Pereira, R.F.; Sumida, D.H.; et al. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science 2019, 365, 386–392. [Google Scholar] [CrossRef]
- Kowluru, A.; Kowluru, R.A. Racking up ceramide-induced islet β-cell dysfunction. Biochem. Pharmacol. 2018, 154, 161–169. [Google Scholar] [CrossRef]
- Lang, F.; Ullrich, S.; Gulbins, E. Ceramide formation as a target in beta-cell survival and function. Expert Opin. Ther. Targets 2011, 15, 1061–1071. [Google Scholar] [CrossRef]
- Turpin, S.M.; Nicholls, H.T.; Willmes, D.M.; Mourier, A.; Brodesser, S.; Wunderlich, C.M.; Mauer, J.; Xu, E.; Hammerschmidt, P.; Brönneke, H.S.; et al. Obesity-induced cers6-dependent c16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 2014, 20, 678–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergman, B.C.; Brozinick, J.T.; Strauss, A.; Bacon, S.; Kerege, A.; Bui, H.H.; Sanders, P.; Siddall, P.; Wei, T.; Thomas, M.K.; et al. Muscle sphingolipids during rest and exercise: A c18:0 signature for insulin resistance in humans. Diabetologia 2016, 59, 785–798. [Google Scholar] [CrossRef] [Green Version]
- Zobel, E.H.; Wretlind, A.; Ripa, R.S.; Rotbain Curovic, V.; von Scholten, B.J.; Suvitaival, T.; Hansen, T.W.; Kjær, A.; Legido-Quigley, C.; Rossing, P. Ceramides and phospholipids are downregulated with liraglutide treatment: Results from the liraflame randomized controlled trial. BMJ Open Diabetes Res. Care 2021, 9, e002395. [Google Scholar] [CrossRef] [PubMed]
- Akawi, N.; Checa, A.; Antonopoulos, A.S.; Akoumianakis, I.; Daskalaki, E.; Kotanidis, C.P.; Kondo, H.; Lee, K.; Yesilyurt, D.; Badi, I.; et al. Fat-secreted ceramides regulate vascular redox state and influence outcomes in patients with cardiovascular disease. J. Am. Coll. Cardiol. 2021, 77, 2494–2513. [Google Scholar] [CrossRef]
- Wang, D.D.; Toledo, E.; Hruby, A.; Rosner, B.A.; Willett, W.C.; Sun, Q.; Razquin, C.; Zheng, Y.; Ruiz-Canela, M.; Guasch-Ferré, M.; et al. Plasma ceramides, mediterranean diet, and incident cardiovascular disease in the predimed trial (prevención con dieta mediterránea). Circulation 2017, 135, 2028–2040. [Google Scholar] [CrossRef] [Green Version]
- Chapman, M.J.; Orsoni, A.; Tan, R.; Mellett, N.A.; Nguyen, A.; Robillard, P.; Giral, P.; Thérond, P.; Meikle, P.J. Ldl subclass lipidomics in atherogenic dyslipidemia: Effect of statin therapy on bioactive lipids and dense ldl[s]. J. Lipid Res. 2020, 61, 911–932. [Google Scholar] [CrossRef] [Green Version]
- Ng, T.W.K.; Ooi, E.M.M.; Watts, G.F.; Chan, D.C.; Weir, J.M.; Meikle, P.J.; Barrett, P.H.R. Dose-dependent effects of rosuvastatin on the plasma sphingolipidome and phospholipidome in the metabolic syndrome. J. Clin. Endocrinol. Metab. 2014, 99, E2335–E2340. [Google Scholar] [CrossRef] [Green Version]
- Tarasov, K.; Ekroos, K.; Suoniemi, M.; Kauhanen, D.; Sylvänne, T.; Hurme, R.; Gouni-Berthold, I.; Berthold, H.K.; Kleber, M.E.; Laaksonen, R.; et al. Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and pcsk9 deficiency. J. Clin. Endocrinol. Metab. 2014, 99, E45–E52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Q.; Svatikova, A.; Meeusen, J.W.; Kludtke, E.L.; Kopecky, S.L. Effect of proprotein convertase subtilisin/kexin type 9 inhibitors on plasma ceramide levels. Am. J. Cardiol. 2020, 128, 163–167. [Google Scholar] [CrossRef]
- Choi, R.H.; Tatum, S.M.; Symons, J.D.; Summers, S.A.; Holland, W.L. Ceramides and other sphingolipids as drivers of cardiovascular disease. Nat. Rev. Cardiol. 2021, 18, 701–711. [Google Scholar] [CrossRef]
- Burla, B.; Muralidharan, S.; Wenk, M.R.; Torta, F. Sphingolipid analysis in clinical research. In Clinical Metabolomics: Methods and Protocols; Giera, M., Ed.; Springer: New York, NY, USA, 2018; pp. 135–162. [Google Scholar] [CrossRef]
- Wang, J.-R.; Zhang, H.; Yau, L.F.; Mi, J.-N.; Lee, S.; Lee, K.C.; Hu, P.; Liu, L.; Jiang, Z.-H. Improved sphingolipidomic approach based on ultra-high performance liquid chromatography and multiple mass spectrometries with application to cellular neurotoxicity. Anal. Chem. 2014, 86, 5688–5696. [Google Scholar] [CrossRef]
- Burla, B.; Arita, M.; Arita, M.; Bendt, A.K.; Cazenave-Gassiot, A.; Dennis, E.A.; Ekroos, K.; Han, X.; Ikeda, K.; Liebisch, G.; et al. Ms-based lipidomics of human blood plasma: A community-initiated position paper to develop accepted guidelines1. J. Lipid Res. 2018, 59, 2001–2017. [Google Scholar] [CrossRef] [Green Version]
- Carrard, J.; Gallart-Ayala, H.; Infanger, D.; Teav, T.; Wagner, J.; Knaier, R.; Colledge, F.; Streese, L.; Königstein, K.; Hinrichs, T.; et al. Metabolic view on human healthspan: A lipidome-wide association study. Metabolites 2021, 11, 287. [Google Scholar] [CrossRef] [PubMed]
- Ceramides: A Class of Lipids with Links to Heart Disease. Available online: https://www.mayoclinic.org/medical-professionals/cardiovascular-diseases/news/ceramides-a-class-of-lipids-with-links-to-heart-disease/mac-20429577 (accessed on 1 September 2021).
- Mi-Heart Ceramides. Be in the Know. Now. Available online: https://news.mayocliniclabs.com/ceramides-miheart/#form-row-right (accessed on 1 September 2021).
- Peterson, L.R.; Xanthakis, V.; Duncan, M.S.; Gross, S.; Friedrich, N.; Völzke, H.; Felix, S.B.; Jiang, H.; Sidhu, R.; Nauck, M.; et al. Ceramide remodeling and risk of cardiovascular events and mortality. J. Am. Heart Assoc. 2018, 7, e007931. [Google Scholar] [CrossRef]
- Alshehry, Z.H.; Mundra, P.A.; Barlow, C.K.; Mellett, N.A.; Wong, G.; McConville, M.J.; Simes, J.; Tonkin, A.M.; Sullivan, D.R.; Barnes, E.H.; et al. Plasma lipidomic profiles improve on traditional risk factors for the prediction of cardiovascular events in type 2 diabetes mellitus. Circulation 2016, 134, 1637–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anroedh, S.; Hilvo, M.; Akkerhuis, K.M.; Kauhanen, D.; Koistinen, K.; Oemrawsingh, R.; Serruys, P.; van Geuns, R.J.; Boersma, E.; Laaksonen, R.; et al. Plasma concentrations of molecular lipid species predict long-term clinical outcome in coronary artery disease patients. J. Lipid Res. 2018, 59, 1729–1737. [Google Scholar] [CrossRef]
- Lemaitre, R.N.; Jensen, P.N.; Hoofnagle, A.; McKnight, B.; Fretts, A.M.; King, I.B.; Siscovick, D.S.; Psaty, B.M.; Heckbert, S.R.; Mozaffarian, D.; et al. Plasma ceramides and sphingomyelins in relation to heart failure risk. Circ. Heart Fail. 2019, 12, e005708. [Google Scholar] [CrossRef]
- Peterson, L.R.; Jiang, X.; Chen, L.; Goldberg, A.C.; Farmer, M.S.; Ory, D.S.; Schaffer, J.E. Alterations in plasma triglycerides and ceramides: Links with cardiac function in humans with type 2 diabetes. J. Lipid Res. 2020, 61, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Nwabuo, C.C.; Duncan, M.; Xanthakis, V.; Peterson, L.R.; Mitchell, G.F.; McManus, D.; Cheng, S.; Vasan, R.S. Association of circulating ceramides with cardiac structure and function in the community: The framingham heart study. J. Am. Heart Assoc. 2019, 8, e013050. [Google Scholar] [CrossRef]
- Hilvo, M.; Lääperi, M.; Jylhä, A.; Kleber, M.E.; Hurme, R.; Scharnagl, H.; März, W.; Sinisalo, J.; Laaksonen, R. Prior myocardial infarction, coronary artery disease extent, diabetes mellitus, and cert2 score for risk stratification in stable coronary artery disease. Eur. J. Prev. Cardiol. 2021, zwab122. [Google Scholar] [CrossRef]
- Gencer, B.; Morrow, D.A.; Braunwald, E.; Goodrich, E.L.; Hilvo, M.; Kauhanen, D.; Sabatine, M.S.; Laaksonen, R.; O’Donoghue, M.L. Plasma ceramide and phospholipid-based risk score and the risk of cardiovascular death in patients after acute coronary syndrome. Eur J. Prev. Cardiol. 2020, zwaa143. [Google Scholar] [CrossRef] [PubMed]
- Leiherer, A.; Mündlein, A.; Laaksonen, R.; Lääperi, M.; Jylhä, A.; Fraunberger, P.; Drexel, H. Comparison of recent ceramide-based coronary risk prediction scores in cardiovascular disease patients. Eur. J. Prev. Cardiol. 2021, zwab112. [Google Scholar] [CrossRef] [PubMed]
- SCORE2 Working Group; ESC Cardiovascular Risk Collaboration. Score2 risk prediction algorithms: New models to estimate 10-year risk of cardiovascular disease in Europe. Eur. Heart J. 2021, 42, 2439–2454. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Fang, Z.; Li, S.; Xu, M.; Zhang, J.; Han, D.; Hu, W.; Yan, L.; Wang, Y.; Fan, L.; et al. Circulating ceramide: A new cardiometabolic biomarker in patients with comorbid acute coronary syndrome and type 2 diabetes mellitus. Front. Physiol. 2020, 11, 1104. [Google Scholar] [CrossRef] [PubMed]
- Hilvo, M.; Wallentin, L.; Lakic, T.G.; Held, C.; Kauhanen, D.; Jylhä, A.; Lindbäck, J.; Siegbahn, A.; Granger, C.B.; Koenig, W.; et al. Prediction of residual risk by ceramide-phospholipid score in patients with stable coronary heart disease on optimal medical therapy. J. Am. Heart Assoc. 2020, 9, e015258. [Google Scholar] [CrossRef]
- Mantovani, A.; Dugo, C. Ceramides and risk of major adverse cardiovascular events: A meta-analysis of longitudinal studies. J. Clin. Lipidol. 2020, 14, 176–185. [Google Scholar] [CrossRef]
- Poss, A.M.; Holland, W.L.; Summers, S.A. Risky lipids: Refining the ceramide score that measures cardiovascular health. Eur. Heart J. 2019, 41, 381–382. [Google Scholar] [CrossRef]
- Thorens, B.; Rodriguez, A.; Cruciani-Guglielmacci, C.; Wigger, L.; Ibberson, M.; Magnan, C. Use of preclinical models to identify markers of type 2 diabetes susceptibility and novel regulators of insulin secretion—A step towards precision medicine. Mol. Metab. 2019, 27, S147–S154. [Google Scholar] [CrossRef] [PubMed]
- Wigger, L.; Cruciani-Guglielmacci, C.; Nicolas, A.; Denom, J.; Fernandez, N.; Fumeron, F.; Marques-Vidal, P.; Ktorza, A.; Kramer, W.; Schulte, A.; et al. Plasma dihydroceramides are diabetes susceptibility biomarker candidates in mice and humans. Cell Rep. 2017, 18, 2269–2279. [Google Scholar] [CrossRef]
- Hilvo, M.; Vasile, V.C.; Donato, L.J.; Hurme, R.; Laaksonen, R. Ceramides and ceramide scores: Clinical applications for cardiometabolic risk stratification. Front. Endocrinol. 2020, 11, 628. [Google Scholar] [CrossRef]
- Ding, D.; Lawson, K.D.; Kolbe-Alexander, T.L.; Finkelstein, E.A.; Katzmarzyk, P.T.; Van Mechelen, W.; Pratt, M. Lancet Physical Activity Series 2 Executive Committee. The economic burden of physical inactivity: A global analysis of major non-communicable diseases. Lancet 2016, 388, 1311–1324. [Google Scholar] [CrossRef]
- Merkur, S.; Sassi, F.; McDaid, D. Promoting Health, Preventing Disease: Is there an Economic Case? WHO: Copenhagen, Denmark, 2013; p. 72. [Google Scholar]
- Gojanovic, B. Physical activity is an opportunity for the health of nations: What should we do next? Praxis 2018, 107, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Bergman, B.C.; Brozinick, J.T.; Strauss, A.; Bacon, S.; Kerege, A.; Bui, H.H.; Sanders, P.; Siddall, P.; Kuo, M.S.; Perreault, L. Serum sphingolipids: Relationships to insulin sensitivity and changes with exercise in humans. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E398–E408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helge, J.W.; Dobrzyn, A.; Saltin, B.; Gorski, J. Exercise and training effects on ceramide metabolism in human skeletal muscle. Exp. Physiol. 2004, 89, 119–127. [Google Scholar] [CrossRef]
- Kasumov, T.; Solomon, T.P.J.; Hwang, C.; Huang, H.; Haus, J.M.; Zhang, R.; Kirwan, J.P. Improved insulin sensitivity after exercise training is linked to reduced plasma c14:0 ceramide in obesity and type 2 diabetes. Obesity 2015, 23, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, S.O.; Cocks, M.; Meikle, P.J.; Mellett, N.A.; Ranasinghe, A.M.; Barker, T.A.; Wagenmakers, A.J.M.; Shaw, C.S. Lipid droplet remodelling and reduced muscle ceramides following sprint interval and moderate-intensity continuous exercise training in obese males. Int. J. Obes. 2017, 41, 1745–1754. [Google Scholar] [CrossRef]
- Bruce, C.R.; Thrush, A.B.; Mertz, V.A.; Bezaire, V.; Chabowski, A.; Heigenhauser, G.J.; Dyck, D.J. Endurance training in obese humans improves glucose tolerance and mitochondrial fatty acid oxidation and alters muscle lipid content. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E99–E107. [Google Scholar] [CrossRef] [Green Version]
- Dubé, J.J.; Amati, F.; Stefanovic-Racic, M.; Toledo, F.G.; Sauers, S.E.; Goodpaster, B.H. Exercise-induced alterations in intramyocellular lipids and insulin resistance: The athlete’s paradox revisited. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E882–E888. [Google Scholar] [CrossRef] [Green Version]
- Robsahm, T.E.; Falk, R.S.; Heir, T.; Sandvik, L.; Vos, L.; Erikssen, J.E.; Tretli, S. Measured cardiorespiratory fitness and self-reported physical activity: Associations with cancer risk and death in a long-term prospective cohort study. Cancer Med. 2016, 5, 2136–2144. [Google Scholar] [CrossRef] [Green Version]
- Gander, J.C.; Sui, X.; Hébert, J.R.; Hazlett, L.J.; Cai, B.; Lavie, C.J.; Blair, S.N. Association of cardiorespiratory fitness with coronary heart disease in asymptomatic men. Mayo Clin. Proc. 2015, 90, 1372–1379. [Google Scholar] [CrossRef] [Green Version]
- Farrell, S.W.; Finley, C.E.; Radford, N.B.; Haskell, W.L. Cardiorespiratory fitness, body mass index, and heart failure mortality in men. Circ. Heart Fail. 2013, 6, 898–905. [Google Scholar] [CrossRef] [Green Version]
- Ross, R.; Blair, S.N.; Arena, R.; Church, T.S.; Despres, J.P.; Franklin, B.A.; Haskell, W.L.; Kaminsky, L.A.; Levine, B.D.; Lavie, C.J.; et al. Importance of assessing cardiorespiratory fitness in clinical practice: A case for fitness as a clinical vital sign: A scientific statement from the american heart association. Circulation 2016, 134, e653–e699. [Google Scholar] [CrossRef] [PubMed]
- Juraschek, S.P.; Blaha, M.J.; Whelton, S.P.; Blumenthal, R.; Jones, S.R.; Keteyian, S.J.; Schairer, J.; Brawner, C.A.; Al-Mallah, M.H. Physical fitness and hypertension in a population at risk for cardiovascular disease: The henry ford exercise testing (fit) project. J. Am. Heart Assoc. 2014, 3, e001268. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, N.S.; Ruiz, J.R.; Hurtig-Wennlöf, A.; Ortega, F.B.; Sjöström, M. Relationship of physical activity, fitness, and fatness with clustered metabolic risk in children and adolescents: The european youth heart study. J. Pediatr. 2007, 150, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; Kaykha, A.; George, S.; Abella, J.; Zaheer, N.; Lear, S.; Yamazaki, T.; Froelicher, V. Fitness versus physical activity patterns in predicting mortality in men. Am. J. Med. 2004, 117, 912–918. [Google Scholar] [CrossRef]
- Lee, D.-C.; Sui, X.; Ortega, F.B.; Kim, Y.-S.; Church, T.S.; Winett, R.A.; Ekelund, U.; Katzmarzyk, P.T.; Blair, S.N. Comparisons of leisure-time physical activity and cardiorespiratory fitness as predictors of all-cause mortality in men and women. Br. J. Sports Med. 2011, 45, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.T. Physical fitness and activity as separate heart disease risk factors: A meta-analysis. Med. Sci. Sports Exerc. 2001, 33, 754–761. [Google Scholar] [CrossRef]
- Contrepois, K.; Wu, S.; Moneghetti, K.J.; Hornburg, D.; Ahadi, S.; Tsai, M.S.; Metwally, A.A.; Wei, E.; Lee-McMullen, B.; Quijada, J.V.; et al. Molecular choreography of acute exercise. Cell 2020, 181, 1112.e1116–1130.e1116. [Google Scholar] [CrossRef]
- Nayor, M.; Shah, R.V.; Miller, P.E.; Blodgett, J.B.; Tanguay, M.; Pico, A.R.; Murthy, V.L.; Malhotra, R.; Houstis, N.E.; Deik, A.; et al. Metabolic architecture of acute exercise response in middle-aged adults in the community. Circulation 2020, 142, 1905–1924. [Google Scholar] [CrossRef]
- Saleem, M.; Herrmann, N.; Dinoff, A.; Marzolini, S.; Mielke, M.M.; Andreazza, A.; Oh, P.I.; Vattem Venkata, S.L.; Haughey, N.J.; Lanctôt, K.L. Association between sphingolipids and cardiopulmonary fitness in coronary artery disease patients undertaking cardiac rehabilitation. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 75, 671–679. [Google Scholar] [CrossRef] [Green Version]
- Fabbri, E.; Yang, A.; Simonsick, E.M.; Chia, C.W.; Zoli, M.; Haughey, N.J.; Mielke, M.M.; Ferrucci, L.; Coen, P.M. Circulating ceramides are inversely associated with cardiorespiratory fitness in participants aged 54-96 years from the baltimore longitudinal study of aging. Aging Cell 2016, 15, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Contaifer, D.; Buckley, L.F.; Wohlford, G.; Kumar, N.G.; Morriss, J.M.; Ranasinghe, A.D.; Carbone, S.; Canada, J.M.; Trankle, C.; Abbate, A.; et al. Metabolic modulation predicts heart failure tests performance. PLoS ONE 2019, 14, e218153. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrard, J.; Gallart-Ayala, H.; Weber, N.; Colledge, F.; Streese, L.; Hanssen, H.; Schmied, C.; Ivanisevic, J.; Schmidt-Trucksäss, A. How Ceramides Orchestrate Cardiometabolic Health—An Ode to Physically Active Living. Metabolites 2021, 11, 675. https://doi.org/10.3390/metabo11100675
Carrard J, Gallart-Ayala H, Weber N, Colledge F, Streese L, Hanssen H, Schmied C, Ivanisevic J, Schmidt-Trucksäss A. How Ceramides Orchestrate Cardiometabolic Health—An Ode to Physically Active Living. Metabolites. 2021; 11(10):675. https://doi.org/10.3390/metabo11100675
Chicago/Turabian StyleCarrard, Justin, Hector Gallart-Ayala, Nadia Weber, Flora Colledge, Lukas Streese, Henner Hanssen, Christian Schmied, Julijana Ivanisevic, and Arno Schmidt-Trucksäss. 2021. "How Ceramides Orchestrate Cardiometabolic Health—An Ode to Physically Active Living" Metabolites 11, no. 10: 675. https://doi.org/10.3390/metabo11100675