Metabolic Profiling of PGPR-Treated Tomato Plants Reveal Priming-Related Adaptations of Secondary Metabolites and Aromatic Amino Acids
Abstract
:1. Introduction
2. Results
2.1. Aromatic Amino Acid Quantification in Tomato Plant Tissues
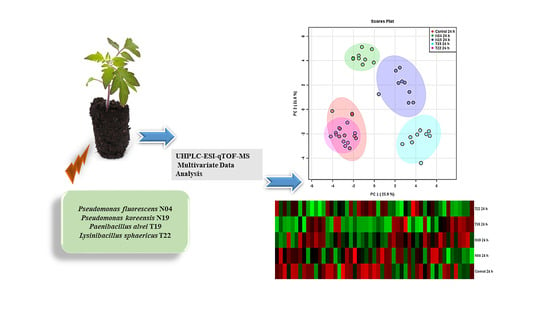
2.2. Metabolic Profiling of PGPR-Induced Changes in Tomato Tissues
2.2.1. Annotation of Signatory Biomarkers of PGPR Priming
2.2.2. Heat Map Analysis
3. Discussion
3.1. Roles of Aromatic Amino Acids in PGPR Priming
3.2. Metabolic Profiling of PGPR-Inoculated Tomato Plants
3.2.1. Tryptophan and Its N-Acetyl Derivative as Markers of PGPR Priming
3.2.2. Guanosine as a Marker of PGPR Priming
3.2.3. Phenylpropanoid Pathway Metabolites as Markers of PGPR Priming
3.2.4. Fatty Acids as Markers of PGPR Priming
3.2.5. Glycoalkaloids as Markers of PGPR Priming
4. Experimental Procedures
4.1. Bacterial Cultures and Growth Conditions
4.2. Plant Material and PGPR Treatment
4.3. Aromatic Amino Acid Quantification
4.3.1. Standard Curve Generation
4.3.2. Plant Extracts
4.3.3. Recovery
4.3.4. MRM Optimization, Selectivity/Specificity, Limit of Detection, and Quantification
4.3.5. Statistical Analysis
4.4. UHPLC-QqQ-MS Analysis
4.5. Untargeted Metabolic Profiling
4.5.1. Plant Tissue Extract Preparation for Metabolite Profiling
4.5.2. UHPLC-ESI-qTOF-MS Conditions
4.6. Multivariate Data Analysis (MVDA)
4.7. Metabolite Annotation
5. Concluding Remarks and Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
References
- Iriti, M.; Faoro, F. Chemical diversity and defence metabolism: How plants cope with pathogens and ozone pollution. Int. J. Mol. Sci. 2009, 10, 3371–3399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gust, A.A.; Brunner, F.; Nürnberger, T. Biotechnological concepts for improving plant innate immunity. Curr. Opin. Biotechnol. 2010, 21, 204–210. [Google Scholar] [CrossRef]
- Thakur, M.; Sohal, B.S. Role of elicitors in inducing resistance in plants against pathogen infection: A Review. ISRN Biochem. 2013, 2013, 762412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreia, J.J.; del Castillo, R.R.; Perez-Vega, E.; Plans, M.; Simo, J.; Casanas, F. Sensory changes related to breeding for plant architecture and resistance to viruses and anthracnose in bean market class Fabada (Phaseolus vulgaris L.). Euphytica 2012, 186, 687–696. [Google Scholar] [CrossRef] [Green Version]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhandari, G. An overview of agrochemicals and their effects on environment in Nepal. Appl. Ecol. Environ. Sci. 2014, 2, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S.; Podile, A.R. Plant growth promoting rhizobacteria (PGPR): The bugs to debug the root zone. Crit. Rev. Microbiol. 2010, 36, 232–244. [Google Scholar] [CrossRef]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M.P. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 4, 1044–1051. [Google Scholar] [CrossRef] [Green Version]
- Gupta, G.; Parihar, S.S.; Ahirwar, N.K.; Snehi, S.K.; Singh, V. Plant growth promoting rhizobacteria (PGPR): Current and future prospects for development of sustainable agriculture. J. Microb. Biochem. Technol. 2015, 7, 96–102. [Google Scholar]
- Mhlongo, M.I.; Piater, L.A.; Madala, N.E.; Labuschagne, N.; Dubery, I.A. The chemistry of plant-microbe interactions in the rhizosphere and the potential for metabolomics to reveal signaling related to defense priming and induced systemic resistance. Front. Plant Sci. 2018, 9, 112. [Google Scholar] [CrossRef] [Green Version]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.-L.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Plant growth-promoting rhizobacteria and root system functioning. Front. Plant Sci. 2013, 4, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; van Themaat, E.V.L.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef] [Green Version]
- Hacquard, S.; Spaepen, S.; Garrido-Oter, R.; Schulze-Lefert, P. Interplay between innate immunity and the plant microbiota. Annu. Rev. Phytopathol. 2017, 55, 565–589. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, N.; Guo, X.; Zhang, Y.; Ye, B. Comparative analysis of bacterial community structure in the rhizosphere of maize by highthroughput pyrosequencing. PLoS ONE 2017, 12, e178425. [Google Scholar]
- Saraf, M.; Pandya, U.; Thakkar, A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar]
- Pineda, A.; Zheng, S.-J.; van Loon, J.J.A.; Pieterse, C.M.J.; Dicke, M. Helping plants to deal with insects: The role of beneficial soil-borne microbes. Trends Plant Sci. 2010, 15, 507–514. [Google Scholar] [CrossRef]
- Saraf, M.; Pandya, U.; Thakkar, A. Role of allelochemicals in plant growth promoting rhizobacteria for biocontrol of phytopathogens. Microbiol. Res. 2014, 169, 18–29. [Google Scholar] [CrossRef]
- Conrath, P.G.U.; Beckers, G.J.M.; Flors, V.; García-agustín, P.; Jakab, G.; Mauch, F.; Newman, M.; Pieterse, C.M.J.; Poinssot, B.; Pozo, M.J.; et al. Priming: Getting ready for battle. Mol. Plant-Microbe Interact. 2009, 19, 1062–1071. [Google Scholar] [CrossRef] [Green Version]
- Conrath, U.; Beckers, G.J.M.; Langenbach, C.J.G.; Jaskiewicz, M.R. Priming for enhanced defense. Annu. Rev. Phytopathol. 2015, 53, 97–119. [Google Scholar] [CrossRef]
- Tanou, G.; Fotopoulos, V.; Molassiotis, A. Priming against environmental challenges and proteomics in plants: Update and agricultural perspectives. Front. Plant Sci. 2012, 3, 216. [Google Scholar] [CrossRef] [Green Version]
- Hilker, M.; Schwachtje, J.; Baier, M.; Balazadeh, S.; Bäurle, I.; Geiselhardt, S.; Hincha, D.K.; Kunze, R.; Mueller-Roeber, B.; Rillig, M.C.; et al. Priming and memory of stress responses in organisms lacking a nervous system. Biol. Rev. Camb. Philos. Soc. 2016, 91, 1118–1133. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Medina, A.; Flors, V.; Heil, M.; Mauch-Mani, B.; Pieterse, C.M.J.; Pozo, M.J.; Ton, J.; Van Dam, N.M.; Conrath, U. Recognizing plant defense priming. Trends Plant Sci. 2016, 21, 818–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frost, C.J.; Mescher, M.C.; Carlson, J.E.; de Moraes, C.M. Plant defense priming against herbivores: Getting ready for a different battle. Plant Physiol. 2008, 146, 818–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crisp, P.A.; Ganguly, D.; Eichten, S.R.; Borevitz, J.O.; Pogson, B.J. Reconsidering plant memory: Intersections between stress recovery, RNA turnover, and epigenetics. Sci. Adv. 2016, 2, e1501340. [Google Scholar] [CrossRef] [Green Version]
- Bruce, T.J.A.; Matthes, M.C.; Napier, J.A.; Pickett, J.A. Stressful “memories” of plants: Evidence and possible mechanisms. Plant Sci. 2007, 173, 603–608. [Google Scholar] [CrossRef]
- Wiklund, S.; Johansson, E.; Sjo, L.; Shockcor, J.P.; Gottfries, J.; Moritz, T.; Trygg, J. Visualization of GC / TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Anal. Chem. 2008, 80, 115–122. [Google Scholar] [CrossRef]
- Eriksson, L.; Trygg, J.; Wold, S. CV-ANOVA for significance testing of PLS and OPLS models. J. Chemom. 2008, 22, 594–600. [Google Scholar] [CrossRef]
- Eriksson, L.; Trygg, J.; Wold, S. A chemometrics toolbox based on projections and latent variables. J. Chemom. 2014, 28, 332–346. [Google Scholar] [CrossRef]
- Hossain, M.B.; Rai, D.K.; Brunton, N.B.; Martin-Diana, A.B.; Barry-Ryan, C. Characterization of phenolic composition in Lamiaceae spices by LC-ESI-MS/MS. J. Agric. Food Chem. 2010, 58, 10576–10581. [Google Scholar] [CrossRef]
- Clifford, M.; Johnston, K.; Knigh, S.; Kuhnert, N. A hierarchical scheme for LC-MSn identification of chlorogenic acid. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef]
- Madala, N.E.; Tugizimana, F.; Steenkamp, P.A. Development and optimization of an UPLC-QTOF-MS/MS method based on an in-source collision induced dissociation approach for comprehensive discrimination of chlorogenic acids isomers from Momordica plant species. J. Anal. Methods Chem. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ncube, E.N.; Mhlongo, M.I.; Piater, L.A.; Steenkamp, P.A.; Dubery, I.A.; Madala, N.E. Analyses of chlorogenic acids and related cinnamic acid derivatives from Nicotiana tabacum tissues with the aid of UPLC-QTOF-MS / MS based on the in-source collision-induced dissociation method. Chem. Cent. J. 2014, 8, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mhlongo, M.I.; Piater, L.A.; Steenkamp, P.A.; Madala, N.E.; Dubery, I.A. Priming agents of plant defence stimulate the accumulation of mono- and di-acylated quinic acids in cultured tobacco cells. Physiol. Mol. Plant Pathol. 2014, 88, 61–66. [Google Scholar] [CrossRef]
- Zeiss, D.R.; Mhlongo, M.I.; Tugizimana, F.; Steenkamp, P.A.; Dubery, I.A. Metabolomic profiling of the host response of tomato (Solanum lycopersicum) following infection by Ralstonia solanacearum. Int. J. Mol. Sci. 2019, 20, 3945. [Google Scholar] [CrossRef] [Green Version]
- Zeiss, D.R.; Mhlongo, M.I.; Tugizimana, F.; Steenkamp, P.A.; Dubery, I.A. Comparative metabolic phenotyping of tomato (Solanum lycopersicum) for the identification of metabolic signatures in cultivars differing in resistance to Ralstonia solanacearum. Int. J. Mol. Sci. 2018, 19, 2558. [Google Scholar] [CrossRef] [Green Version]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Mhlongo, M.I.; Steenkamp, P.A.; Piater, L.A.; Madala, N.E.; Dubery, I.A. Profiling of altered metabolomic states in Nicotiana tabacum cells induced by priming agents. Front. Plant Sci. 2016, 7, 1527. [Google Scholar] [CrossRef] [Green Version]
- Mhlongo, M.I.; Piater, L.A.; Steenkamp, P.A.; Madala, N.E.; Dubery, I. Phenylpropanoid defences in Nicotiana tabacum cells: Overlapping metabolomes indicate common aspects to priming responses induced by lipopolysaccharides, chitosan and flagellin-22. PLoS ONE 2016, 11, e0151350. [Google Scholar] [CrossRef]
- Gowda, H.; Ivanisevic, J.; Johnson, C.H.; Kurczy, M.E.; Benton, H.P.; Rinehart, D.; Nguyen, T.; Ray, J.; Kuehl, J.; Arevalo, B.; et al. Interactive XCMS online: Simplifying advanced metabolomic processing and subsequent statistical analyses. Anal. Chem. 2014, 84, 6931–6939. [Google Scholar] [CrossRef]
- MetaboAnalyst - Statistical, Functional and Integrative Analysis of Metabolomics Data. Available online: www.metaboanalyst.ca (accessed on 13 May 2020).
- Weston, D.J.; Pelletier, D.A.; Morrell-Falvey, J.L.; Tschaplinski, T.J.; Jawdy, S.S.; Lu, T.-Y.; Allen, S.M.; Melton, S.J.; Martin, M.Z.; Schadt, C.W.; et al. Pseudomonas fluorescens induces strain-dependent and strain-independent host plant responses in defense networks, primary metabolism, photosynthesis, and fitness. Mol. Plant-Microbe Interact. 2012, 25, 765–778. [Google Scholar] [CrossRef] [Green Version]
- Mareya, C.R.; Tugizimana, F.; Piater, L.A.; Madala, N.E.; Steenkamp, P.A.; Dubery, I.A. Untargeted metabolomics reveal defensome-related metabolic reprogramming in Sorghum bicolor against infection by Burkholderia andropogonis. Metabolites 2019, 9, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camañes, G.; Scalschi, L.; Vicedo, B.; González-Bosch, C.; García-Agustín, P. An untargeted global metabolomic analysis reveals the biochemical changes underlying basal resistance and priming in Solanum lycopersicum, and identifies 1-methyltryptophan as a metabolite involved in plant responses to Botrytis cinerea and Pseudomonas sy. Plant J. 2015, 84, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Tugizimana, F.; Steenkamp, P.A.; Piater, L.A.; Labuschagne, N.; Dubery, I.A. Unravelling the metabolic reconfiguration of the post-challenge primed state in Sorghum bicolor responding to Colletotrichum sublineolum infection. Metabolites 2019, 9, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzin, V.; Galili, G. The Biosynthetic Pathways for Shikimate and Aromatic Amino Acids in Arabidopsis thaliana. In The Arabidopsis Book; American Society of Plant Biologists: Rockville, MD, USA, 2010; p. 0132. ISBN 1543-8120. [Google Scholar]
- Parthasarathy, A.; Cross, P.J.; Dobson, R.C.J.; Adams, L.E.; Savka, M.A.; Hudson, A.O. A Three-ring circus: Metabolism of the three proteogenic aromatic amino acids and their role in the health of plants and animals. Front. Mol. Biosci. 2018, 5, 1–30. [Google Scholar] [CrossRef]
- Finnegan, T.; Steenkamp, P.A.; Piater, L.A.; Dubery, I.A. The lipopolysaccharide-induced metabolome signature in Arabidopsis thaliana Reveals dynamic reprogramming of phytoalexin and phytoanticipin pathways. PLoS ONE 2016, 11, e0163572. [Google Scholar] [CrossRef]
- Walker, V.; Bertrand, C.; Bellvert, F.; Moënne-Loccoz, Y.; Bally, R.; Comte, G. Host plant secondary metabolite profiling shows a complex, strain-dependent response of maize to plant growth-promoting rhizobacteria of the genus Azospirillum. New Phytol. 2011, 189, 494–506. [Google Scholar] [CrossRef]
- Chamam, A.; Wisniewski-Dyé, F.; Comte, G.; Bertrand, C.; Prigent-Combaret, C. Differential responses of Oryza sativa secondary metabolism to biotic interactions with cooperative, commensal and phytopathogenic bacteria. Planta 2015, 242, 1439–1452. [Google Scholar] [CrossRef]
- Jansen, J.J.; Allwood, J.W.; Marsden-Edwards, E.; Putten, W.H.; Goodacre, R.; Dam, N.M. Metabolomic analysis of the interaction between plants and herbivores. Metabolomics 2008, 5, 150–161. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, A.; Hashimoto, Y.; Tanaka, C.; Dubouzet, J.G.; Nakao, T.; Matsuda, F.; Nishioka, T.; Miyagawa, H.; Wakasa, K. The tryptophan pathway is involved in the defense responses of rice against pathogenic infection via serotonin production. Plant J. 2008, 54, 481–495. [Google Scholar] [CrossRef]
- Zeier, J. New insights into the regulation of plant immunity by amino acid metabolic pathways. Plant. Cell Environ. 2013, 36, 2085–2103. [Google Scholar] [CrossRef]
- Gross, I.; Durner, J. In search of enzymes with a role in 3’,5’-Cyclic guanosine monophosphate metabolism in plants. Front. Plant Sci. 2016, 7, 576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelkefi, H.; Sugliani, M.; Ke, H.; Harchouni, S.; Soubigou-Taconnat, L.; Citerne, S.; Mouille, G.; Fakhfakh, H.; Robaglia, C.; Field, B. Guanosine tetraphosphate modulates salicylic acid signalling and the resistance of Arabidopsis thaliana to Turnip mosaic virus. Mol. Plant Pathol. 2018, 19, 634–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [Green Version]
- Widhalm, J.R.; Dudareva, N. A familiar ring to it: Biosynthesis of plant benzoic acids. Mol. Plant 2015, 8, 83–97. [Google Scholar] [CrossRef] [Green Version]
- Qualley, A.V.; Widhalm, J.R.; Adebesin, F.; Kish, C.M.; Dudareva, N. Completion of the core β-oxidative pathway of benzoic acid biosynthesis in plants. PNAS 2012, 109, 16383–16388. [Google Scholar] [CrossRef] [Green Version]
- Madala, N.E.; Steenkamp, P.A.; Piater, L.A.; Dubery, I.A. Metabolomic insights into the bioconversion of isonitrosoacetophenone in Arabidopsis thaliana and its effects on defense-related pathways. Plant Physiol. Biochem. 2014, 84, 87–95. [Google Scholar] [CrossRef]
- Rossouw, L.T.; Madala, N.E.; Tugizimana, F.; Steenkamp, P.A.; Esterhuizen, L.L.; Dubery, I.A. Deciphering the resistance mechanism of tomato plants against whitefly-mediated tomato curly stunt virus infection through ultra-high- performance liquid chromatography coupled to mass spectrometry (UHPLC-MS)-based metabolomics approaches. Metabolites 2019, 9, 60. [Google Scholar] [CrossRef] [Green Version]
- Sharaf, E.F.; Farrag, A.A. Induced resistance in tomato plants by IAA against Fusarium oxysporum lycopersici. Polish J. Microbiol. 2004, 53, 111–116. [Google Scholar]
- Blount, J.W.; Dixon, R.A.; Paiva, N.L. Stress responses in alfalfa (Medicago sativa L.) XVI. Antifungal activity of medicarpin and its biosynthetic precursors; implications for the genetic manipulation of stress metabolites. Physiol. Mol. Plant Pathol. 1992, 41, 333–349. [Google Scholar] [CrossRef]
- Dai, G.H.; Nicole, M.; Andary, C.; Martinez, C.; Bresson, E.; Boher, B.; Daniel, J.F.; Geiger, J.P. Flavonoids accumulate in cell walls, middle lamellae and callose-rich papillae during an incompatible interaction between Xanthomonas campestris pv. malvacearum and cotton. Physiol. Mol. Plant Pathol. 1996, 9, 225–227. [Google Scholar]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef] [PubMed]
- Beckman, C.H. Phenolic-storing cells: Keys to programmed cell death and periderm formation in wilt disease resistance and in general defence responses in plants? Physiol. Mol. Plant Pathol. 2000, 57, 101–110. [Google Scholar] [CrossRef]
- Mishra, A.K.; Mishra, A.; Kehri, H.K.; Sharma, B.; Pandey, A.K. Inhibitory activity of Indian spice plant Cinnamomum zeylanicum extracts against Alternaria solani and Curvularia lunata, the pathogenic dematiaceous moulds. Ann. Clin. Microbiol. Antimicrob. 2009, 8, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Zang, X.; He, M.; Pan, S.; Xu, X. Structure-activity relationship of flavonoids on their anti- Escherichia coli activity and inhibition of DNA gyrase. J. Agric. Food Chem. 2013, 61, 8185–8190. [Google Scholar] [CrossRef]
- Hammerschmidt, R. Chlorogenic acid: A versatile defense compound. Physiol. Mol. Plant Pathol. 2014, 88, 3–4. [Google Scholar] [CrossRef]
- Liang, Y.S.; Kim, H.K.; Lefeber, A.W.M.; Erkelens, C.; Choi, Y.H.; Verpoorte, R. Identification of phenylpropanoids in methyl jasmonate treated Brassica rapa leaves using two-dimensional nuclear magnetic resonance spectroscopy. J. Chromatogr. 2006, 1112, 148–155. [Google Scholar] [CrossRef]
- López-Gresa, M.P.; Torres, C.; Campos, L.; Lisón, P.; Rodrigo, I.; Bellés, J.M.; Conejero, V. Identification of defence metabolites in tomato plants infected by the bacterial pathogen Pseudomonas syringae. Environ. Exp. Bot. 2011, 74, 216–228. [Google Scholar] [CrossRef]
- Bostock, R.M.; Wilcox, S.M.; Wang, G.; Adaskaveg, J.E. Suppression of Monilinia fructicola cutinase production by peach fruit surface phenolic acids. Physiol. Mol. Plant Pathol. 1999, 54, 37–50. [Google Scholar] [CrossRef]
- Lee, M.-H.; Bostock, R.M. Fruit exocarp phenols in relation to quiescence and development of Monilinia fructicola infections in Prunus spp.: A role for cellular redox? Phytopathology 2007, 97, 269–277. [Google Scholar] [CrossRef]
- Newman, M.; Von Roepenack-lahaye, E.; Parr, A.; Daniels, M.J.; Dow, J.M. Prior exposure to lipopolysaccharide potentiates expression of plant defenses in response to bacteria. Plant J. 2002, 29, 487–495. [Google Scholar] [CrossRef]
- Tugizimana, F.; Steenkamp, P.A.; Piater, L.A.; Dubery, I.A. Multi-platform metabolomic analyses of ergosterol-induced dynamic changes in Nicotiana tabacum cells. PLoS ONE 2014, 9, e87846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tugizimana, F.; Djami-Tchatchou, A.T.; Steenkamp, P.A.; Piater, L.A.; Dubery, I.A. Metabolomic analysis of defense-related reprogramming in Sorghum bicolor in response to Colletotrichum sublineolum infection reveals a functional metabolic web of phenylpropanoid and flavonoid pathways. Front. Plant Sci. 2019, 9, 1840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siebers, M.; Brands, M.; Wewer, V.; Duan, Y.; Hölzl, G.; Dörmann, P. Lipids in plant–microbe interactions. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2016, 1861, 1379–1395. [Google Scholar] [CrossRef] [PubMed]
- Pushpa, D.; Yogendra, K.N.; Gunnaiah, R.; Kushalappa, A.C.; Murphy, A. Identification of late blight resistance-related metabolites and genes in potato through nontargeted metabolomics. Plant Mol. Biol. Rep. 2013, 32, 584–595. [Google Scholar] [CrossRef]
- Raffaele, S.; Leger, A.; Roby, D. Very long chain fatty acid and lipid signaling in the response of plants to pathogens. Plant Signal. Behav. 2009, 4, 94–99. [Google Scholar] [CrossRef] [Green Version]
- June, P.M.C.; Augustin, M.M.; Ruzicka, D.R.; Shukla, A.K.; Augustin, J.M.; Starks, C.M.; Neil-johnson, M.O.; Mckain, M.R.; Evans, B.S.; Barrett, D.; et al. Elucidating steroid alkaloid biosynthesis in Veratrum californicum: Production of verazine in Sf9 cells. Plant J. 2016, 82, 991–1003. [Google Scholar]
- Milner, S.E.; Brunton, N.P.; Jones, P.W.; O Brien, N.M.; Collins, S.G.; Maguire, A.R. Bioactivities of glycoalkaloids and their aglycones from solanum species. J. Agric. Food Chem. 2011, 59, 3454–3484. [Google Scholar] [CrossRef]
- Altesor, P.; García, Á.; Font, E.; Rodríguez-Haralambides, A.; Vilaró, F.; Oesterheld, M.; Soler, R.; González, A. Glycoalkaloids of wild and cultivated Solanum: Effects on specialist and generalist insect herbivores. J. Chem. Ecol. 2014, 40, 599–608. [Google Scholar] [CrossRef]
- Ito, S.I.; Ihara, T.; Tamura, H.; Tanaka, S.; Ikeda, T.; Kajihara, H.; Dissanayake, C.; Abdel-Motaal, F.F.; El-Sayed, M.A. α-Tomatine, the major saponin in tomato, induces programmed cell death mediated by reactive oxygen species in the fungal pathogen Fusarium oxysporum. FEBS Lett. 2007, 581, 3217–3222. [Google Scholar] [CrossRef] [Green Version]
- Friedman, M. Tomato glycoalkaloids: Role in the plant and in the diet. J. Agric. Food Chem. 2002, 50, 5751–5780. [Google Scholar] [CrossRef]
- Judelson, H.S.; Ah-Fong, A.M.V. Exchanges at the plant-oomycete interface that influence disease. Plant Physiol. 2019, 179, 1198–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, S.I.; Eto, T.; Tanaka, S.; Yamauchi, N.; Takahara, H.; Ikeda, T. Tomatidine and lycotetraose, hydrolysis products of α-tomatine by Fusarium oxysporum tomatinase, suppress induced defense responses in tomato cells. FEBS Lett. 2004, 571, 31–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augustin, J.M.; Kuzina, V.; Andersen, S.B.; Bak, S. Molecular activities, biosynthesis and evolution of triterpenoid saponins. Phytochemistry 2011, 72, 435–457. [Google Scholar] [CrossRef] [PubMed]
- Sandrock, R.W.; VanEtten, H.D. Fungal sensitivity to and enzymatic degradation of the phytoanticipin α-tomatine. Phytopathology 1998, 88, 137–143. [Google Scholar] [CrossRef]
- Garcia, P.G.; dos Santos, F.N.; Zanotta, S.; Eberlin, M.N.; Carazzone, C. Metabolomics of solanum lycopersicum infected with Phytophthora infestans leads to early detection of late blight in asymptomatic plants. Molecules 2018, 23, 3330. [Google Scholar] [CrossRef] [Green Version]
- Hassen, A.I.; Labuschagne, N. Root colonization and growth enhancement in wheat and tomato by rhizobacteria isolated from the rhizoplane of grasses. World J. Microbiol. Biotechnol. 2010, 26, 1837–1846. [Google Scholar] [CrossRef]
- Rudolph, N.; Labuschagne, N.; Aveling, T.A.S. The effect of plant growth promoting rhizobacteria on seed germination and seedling growth of maize. Seed Sci. Technol. 2015, 43, 507–518. [Google Scholar] [CrossRef]
- Carlson, R.; Tugizimana, F.; Steenkamp, P.A.; Dubery, I.A.; Labuschagne, N. Differential metabolic reprogramming in Paenibacillus Alvei-primed Sorghum bicolor seedlings in response to Fusarium pseudograminearum infection. Metabolites 2019, 9, 150. [Google Scholar] [CrossRef] [Green Version]
- Riet, K.B.; Ndlovu, N.; Piater, L.A.; Dubery, I.A. Simultaneous analysis of defense-related phytohormones in Arabidopsis thaliana responding to fungal infection. Appl. Plant Sci. 2016, 4, 1600013. [Google Scholar] [CrossRef]
- Ghosh, D.; Gupta, A.; Mohapatra, S. Dynamics of endogenous hormone regulation in plants by phytohormone secreting rhizobacteria under water-stress. Symbiosis 2019, 77, 265–278. [Google Scholar] [CrossRef]
- Sangster, T.; Major, H.; Plumb, R. A pragmatic and readily implemented quality control strategy for HPLC-MS and GC-MS-based metabonomic analysis. Analyst 2006, 131, 1075–1078. [Google Scholar] [CrossRef] [PubMed]
- Godzien, J.; Alonso-Herranz, V.; Barbas, C.; Armitage, E.G. Controlling the quality of metabolomics data: New strategies to get the best out of the QC sample. Metabolomics 2015, 11, 518–528. [Google Scholar] [CrossRef]
- Sadeghi-bazargani, H.; Bangdiwala, S.I.; Mohammad, K. Compared application of the new OPLS-DA statistical model versus partial least squares regression to manage large numbers of variables in an injury case-control study. Sci. Res. Essays 2011, 6, 4369–4377. [Google Scholar]
- Dictionary of Natural Products. Available online: www.dnp.chemnetbase.com (accessed on 13 May 2020).
- Chemspider – Search and share chemistry. Available online: www.chemspider.com (accessed on 13 May 2020).
- Sumner, L.W.; Samuel, T.; Noble, R.; Gmbh, S.D.; Barrett, D.; Beale, M.H.; Hardy, N. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2013, 3, 211–221. [Google Scholar] [CrossRef] [Green Version]






| No. | Rt | m/z | Ionization | MS/MS Fragments | Compound Name | Abbreviation |
|---|---|---|---|---|---|---|
| 1 | 2.56 | 371.058 | [M − H]− | 209, 191, 147,135 | Caffeoylglucaric acid I | CGA I |
| 2 | 2.56 | 353.075 | [M − H]− | 191, 179, 135 | 3-Caffeoylquinic acid | 3-CQA |
| 3 | 2.71 | 203.079 | [M − H]− | 142, 116 | Tryptophan | Trp |
| 4 | 2.72 | 371.054 | [M − H]− | 209, 191, 147,135 | Caffeoylglucaric acid II | CGA II |
| 5 | 3.92 | 385.087 | [M − H]− | 223, 208, 179 | Sinapoyl glycoside | S-glyc I |
| 6 | 3.93 | 367.157 | [M − H]− | 191,179, 173, 135 | trans-4-Feruloylquinic acid | t-4-FQA |
| 8 | 3.97 | 707.182 | [M − H]− | 353, 191, 135 | 5-Caffeoylquinic acid | 5-CQA |
| 9 | 4.08 | 367.157 | [M − H]− | 191, 135 | trans-5-Feruloylquinic acid | t-5-FQA |
| 10 | 4.16 | 367.157 | [M − H]− | 191, 135 | trans-5-Feruloylquinic acid | c-5-FQA |
| 11 | 4.3 | 385.106 | [M − H]− | 223, 208, 179 | Sinapoyl glycoside | S-glyc II |
| 12 | 4.35 | 353.085 | [M − H]− | 191, 135 | 5-Caffeoylquinic acid | 5-CQA |
| 14 | 4.7 | 355.101 | [M − H]− | 193 | Feruloylglycoside I | F-glyc I |
| 15 | 4.7 | 401.141 | [M − H]− | 293, 269, 233, 191, 161, 149, 131, 125, 101 | Benzyl alcohol hexose-pentose | Be-acl hex-pent |
| 16 | 4.87 | 385.107 | [M − H]− | 223, 208, 179 | Sinapoylglycoside | S-glyc III |
| 17 | 4.97 | 353.085 | [M − H]− | 191, 179, 173, 135 | 4-Caffeoylquinic acid | 4-CQA |
| 18 | 5.07 | 411.172 | [M − H]− | 321, 249, 135 | Caffeoylputrescine glycoside | C-putr glyc I |
| 19 | 5.15 | 355.110 | [M − H]− | 193 | Feruloylglycoside isomer | F-glyc II |
| 20 | 5.71 | 385.184 | [M − H]− | 223, 208, 179 | Sinapoylglycoside | S-glyc IV |
| 21 | 5.75 | 441.124 431.155 | [M − H]− | 269, 223, 161, 113, 101 | Benzyl alcohol dihexose | Be-alc dihex I |
| 22 | 5.77 | 411.184 | [M − H]− | 321, 249, 179, 135 | Caffeoylputrescine glycoside | C-put glyc II |
| 23 | 6.5 | 337.148 | [M − H]− | 191, 163, 119 | 5-p-Coumaroylquinic acid | 5-p-CoQA |
| 24 | 6.92 | 245.009 | [M − H]− | 203, 142, 116 | Acetyl tryptophan | Acetyl Trp |
| 25 | 7.42 | 609.142 | [M − H]− | 300 | Rutin | Rutin |
| 26 | 7.55 | 355.120 | [M − H]− | 193 | Feruloylglycoside II | F-glyc II |
| 27 | 7.72 | 449.195 | [M − H]− | 287 | Eriodictyol glycoside I | Eri-glyc I |
| 28 | 8.2 | 335.051 | [M − H]− | 179 | 3-Caffeoylshikimic acid | 3-CSA |
| 29 | 8.28 | 441.191 431.156 | [M − H]− | 269, 223, 161, 113, 101 | Benzyl alcohol dihexose | B-alc-dihex II |
| 30 | 8.3 | 449.164 | [M − H]− | 287 | Eriodictyol glycoside II | Eir-glyc II |
| 31 | 8.84 | 449.191 | [M − H]− | 287 | Eriodictyol glycoside III | Eri-glyc III |
| 32 | 9.03 | 949.251 | [M + H]+ | 787, 479, 317 | Petunidin-3-(caffeoyl) -rutinoside-5-O-glucoside | Pe-3-(C)-rut-5-O-gluc |
| 33 | 9.19 | 1032.184 | [M + H]+ | 576, 414 | Dehydrotomatine | De-tom I |
| 34 | 9.93 | 282.112 | [M − H]− | 164 | Guanosine | Gua |
| 35 | 10.01 | 1032.184 | [M + H]+ | 576, 414 | Dehydrotomatine | De-tom II |
| 36 | 11.04 | 1032.185 | [M + H]+ | 576, 527, 414 | Dehydrotomatine | De-tom III |
| 37 | 11.19 | 1034.546 | [M + H]+ | 578, 528, 416 | α-Tomatine I | α-Tom I |
| 38 | 11.48 | 1034.547 | [M + H]+ | 578, 528, 416 | α-Tomatine II | α-Tom II |
| 39 | 11.71 | 447.221 | [M − H]− | 315 | Isorhamnetin pentoside | Iso-pent |
| 40 | 11.75 | 1034.188 | [M + H]+ | 578, 528, 416 | α-Tomatine II | α-Tom III |
| 41 | 11.88 | 474.181 | [M − H]− | 312, 178 | Feruloyltyramine glycoside | F-tyr glyc I |
| 42 | 12.59 | 597.239 | [M − H]− | 273 | Phloritin di-glycoside | Ph-diglyc I |
| 43 | 13.35 | 597.239 | [M − H]− | 273 | Phloritin dihexoside | Ph-diglyc II |
| 44 | 17.99 | 593.281 | [M − H]− | 285 | Kaempferol 3-rutinoside | Ka-3-rut |
| 45 | 20.66 | 474.181 | [M − H]− | 312, 178 | Feruloyltyramine glycoside | F-tyr glyc II |
| 46 | 20.95 | 474.181 | [M − H]− | 312, 178 | Feruloyltyramine glycoside | F-tyr glyc III |
| 47 | 21.13 | 474.181 | [M − H]− | 312, 178 | Feruloyltyramine glycoside | F-tyr glyc IV |
| 48 | 22.01 | 546.194 | [M − H]− | 342, 178 | Feruloyl-3-methoxytyramine conjugate | F-3-me-tyr glyc I |
| 49 | 22.23 | 546.194 | [M − H]− | 342, 178 | Feruloyl-3-methoxytyramine conjugate | F-3-me-tyr glyc II |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mhlongo, M.I.; Piater, L.A.; Steenkamp, P.A.; Labuschagne, N.; Dubery, I.A. Metabolic Profiling of PGPR-Treated Tomato Plants Reveal Priming-Related Adaptations of Secondary Metabolites and Aromatic Amino Acids. Metabolites 2020, 10, 210. https://doi.org/10.3390/metabo10050210
Mhlongo MI, Piater LA, Steenkamp PA, Labuschagne N, Dubery IA. Metabolic Profiling of PGPR-Treated Tomato Plants Reveal Priming-Related Adaptations of Secondary Metabolites and Aromatic Amino Acids. Metabolites. 2020; 10(5):210. https://doi.org/10.3390/metabo10050210
Chicago/Turabian StyleMhlongo, Msizi I., Lizelle A. Piater, Paul A. Steenkamp, Nico Labuschagne, and Ian A. Dubery. 2020. "Metabolic Profiling of PGPR-Treated Tomato Plants Reveal Priming-Related Adaptations of Secondary Metabolites and Aromatic Amino Acids" Metabolites 10, no. 5: 210. https://doi.org/10.3390/metabo10050210