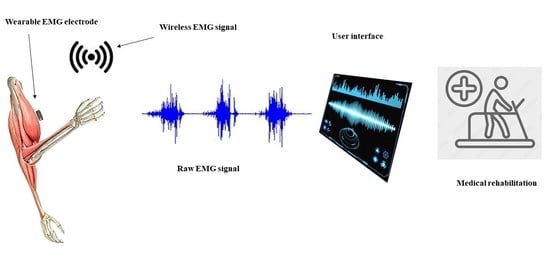
Electromyography Monitoring Systems in Rehabilitation: A Review of Clinical Applications, Wearable Devices and Signal Acquisition Methodologies
Abstract
:1. Introduction
- A complete overview of methods and systems to acquire and analyze EMG signals for tele-rehabilitation applications. In detail, the discussion considers the main applications involving the EMG signals, such as neuromuscular rehabilitation, post-stroke rehabilitation, and sports rehabilitation.
- A detailed discussion about the signal processing techniques for EMG signals, as well as fundamentals about the structure and characteristics of the EMG signals, are provided, along with details on the architectures of EMG acquisition systems.
- A comprehensive and updated scientific literature survey about portable and wearable systems to monitor EMG signals applied to rehabilitation purposes. Comparative analyses are provided for determining the desirable features for the next generation of wearable EMG detectors.
- An up-to-date review of commercial wearable EMG detectors used for rehabilitation applications, and reporting comparative analysis to bring out their main requirements.
2. EMG Methods and Systems Applied to Tele-Rehabilitation Applications
2.1. EMG Applied to Neuromuscular Rehabilitation
- (i)
- sEMG’s present applications and therapeutic effects;
- (ii)
- professionals mainly concerned with sEMG;
- (iii)
- academic aspects;
- (iv)
- potential impediments and explanations for its seeming limited utilization in neurorehabilitation.
2.2. EMG Applied to Post-Stroke Rehabilitation
2.3. EMG Applied to Sports Rehabilitation
3. EMG Signal Processing: General Considerations
3.1. EMG Signal Characteristics
3.2. EMG Instrumentation
- Accuracy: many electronic elements, such as differential amplifiers, ADC converters, and others, are subject to intrinsic noise. The target is to minimize the noise in each element so that accuracy may be achieved.
- Sensitivity: pertains to the analog to digital resolution and, therefore, the total resolution of the device. This helps the medical staff control readings.
- CMRR: which stands for Common-Mode Rejection Ratio, and indicates the ability of a differential amplifier to reject signals common to both inputs. A high CMRR is essential in preventing 50–60 Hz power line interference.
- Input impedance: its compatibility is important in the selection of differential amplifiers and applications relative to the skin type and electrode interface.
- Input range: this specification applies to circuitry and the analog to digital converter, defining the range of EMG signal that can be amplified without saturating the amplifier. To acquire the complete signal, a greater input range is desired, but this necessitates an increase in signal resolution.
- SNR: signal-to-noise ratio measures the power of the desired signal relative to background noise.
4. EMG Portable Devices for Rehabilitation
5. Commercial Wearable Devices
6. Discussion
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mills, K.R. The basics of electromyography. J. Neurol. Neurosurg. Psychiatry 2005, 76, ii32–ii35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizrahi, J. Advances in Applied Electromyography; IntechOpen: London, UK, 2011; ISBN 978-953-307-382-8. Available online: https://www.intechopen.com/books/359 (accessed on 20 December 2022). [CrossRef]
- Flaxman, T.E.; Alkjaer, T.; Smale, K.B.; Simonsen, E.B.; Krogsgaard, M.R.; Benoit, D.L. Differences in EMG–moment relationships between ACL-injured and uninjured adults during a weight-bearing multidirectional force control task. J. Orthop. Res. 2019, 37, 113–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.W.; Liu, X.; Barczyński, M.; Kim, H.Y.; Dionigi, G.; Sun, H.; Chiang, F.Y.; Kamani, D.; Randolph, G.W. Optimal stimulation during monitored thyroid surgery: EMG response characteristics in a porcine model. Laryngoscope 2017, 127, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Preston, D.C.; Shapiro, B.E. Electromyography and Neuromuscular Disorders, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780323661805. [Google Scholar]
- Papagiannis, G.I.; Triantafyllou, A.I.; Roumpelakis, I.M.; Zampeli, F.; Garyfallia Eleni, P.; Koulouvaris, P.; Papadopoulos, E.C.; Papagelopoulos, P.J.; Babis, G.C. Methodology of surface electromyography in gait analysis: Review of the literature. J. Med. Eng. Technol. 2019, 43, 59–65. [Google Scholar] [CrossRef]
- Hellig, T.; Johnen, L.; Mertens, A.; Nitsch, V.; Brandl, C. Investigation of observational methods assessing workload of static working postures based on surface electromyography. Work 2019, 62, 185–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinali, G.; Kara, S.; Yıldırım, M.S. Electromyographic analysis of an ergonomic risk factor: Overhead work. J. Phys. Ther. Sci. 2016, 28, 1924–1927. [Google Scholar] [CrossRef] [Green Version]
- Motamedzade, M.; Afshari, D.; Soltanian, A.R. The Impact of Ergonomically Designed Workstations on Shoulder EMG Activity during Carpet Weaving. Health Promot. Perspect. 2014, 4, 144–150. [Google Scholar] [CrossRef]
- Roggio, F.; Vitale, E.; Filetti, V.; Rapisarda, V.; Musumeci, G.; Romano, E. Ergonomic Evaluation of Young Agricultural Operators Using Handle Equipment Through Electromyography and Vibrations Analysis Between the Fingers. Saf. Health Work. 2022, 13, 440–447. [Google Scholar] [CrossRef]
- Ranavolo, A.; Serrao, M.; Draicchio, F. Critical Issues and Imminent Challenges in the Use of sEMG in Return-To-Work Rehabilitation of Patients Affected by Neurological Disorders in the Epoch of Human–Robot Collaborative Technologies. Front. Neurol. 2020, 22, 572069. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Adams, D.P.; González-Bernal, J.J.; Araque, A.F.; Cano García, A.; Fernández-Lázaro, C.I. Electromyography: A Simple and Accessible Tool to Assess Physical Performance and Health during Hypoxia Training. A Systematic Review. Sustainability 2020, 12, 9137. [Google Scholar] [CrossRef]
- Fukuhara, S.; Kawashima, T.; Oka, H. Indices reflecting muscle contraction performance during exercise based on a combined electromyography and mechanomyography approach. Sci. Rep. 2021, 11, 21208. [Google Scholar] [CrossRef] [PubMed]
- Ficek, K.; Gołas, A.; Pietraszewski, P.; Strózik, M.; Krzysztofik, M. The Effects of a Combined Pre- and Post-Operative Anterior Cruciate Ligament Reconstruction Rehabilitation Program on Lower Extremity Muscle Imbalance. Appl. Sci. 2022, 12, 7411. [Google Scholar] [CrossRef]
- Lee, K. EMG-Triggered Pedaling Training on Muscle Activation, Gait, and Motor Function for Stroke Patients. Brain Sci. 2022, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Osborne, J.A.; Botkin, R.; Colon-Semenza, C.; DeAngelis, T.R.; Gallardo, O.G.; Kosakowski, H.; Martello, J.; Pradhan, S.; Rafferty, M.; Readinger, J.L.; et al. Physical therapist management of parkinson disease: A clinical practice guideline from the American Physical Therapy Association. Phys. Ther. 2021, 102, pzab302. [Google Scholar] [CrossRef] [PubMed]
- Pilkar, R.; Momeni, K.; Ramanujam, A.; Ravi, M.; Garbarini, E.; Gail, F. Forrest; Use of Surface EMG in Clinical Rehabilitation of Individuals With SCI: Barriers and Future Considerations. Front. Neurol. 2020, 11, 578559. [Google Scholar] [CrossRef] [PubMed]
- Musselman, K.E.; Shah, M.; Zariffa, J. Rehabilitation technologies and interventions for individuals with spinal cord injury: Translational potential of current trends. J. Neuroeng. Rehabil. 2018, 15, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campanini, I.; Disselhorst-Klug, C.; Rymer, W.Z.; Merletti, R. Surface EMG in Clinical Assessment and Neurorehabilitation: Barriers Limiting Its Use. Front. Neurol. 2020, 11, 934. [Google Scholar] [CrossRef]
- Cavalcanti Garciaa, M.A.; Vieira, T.M.M. Surface electromyography: Why, when and how to use it. Rev. Andal. Med. Deporte 2011, 4, 17–28. [Google Scholar]
- Ancillao, A. Analysis and Measurement of Human Motion: Modern Protocols and Clinical Considerations. J. Robot. Mech. Eng. Res. 2016, 1, 30–37. [Google Scholar] [CrossRef]
- Rimington, R.P.; Fleming, J.W.; Capel, A.J.; Wheeler, P.C.; Lewis, M.P. Bioengineered model of the human motor unit with physiologically functional neuromuscular junctions. Sci. Rep. 2021, 11, 11695. [Google Scholar] [CrossRef]
- Song, S.; Lee, K.; Jung, S.; Park, S.; Cho, H.; Lee, G. Effect of Horizontal Whole-Body Vibration Training on Trunk and Lower-Extremity Muscle Tone and Activation, Balance, and Gait in a Child with Cerebral Palsy. Am. J. Case Rep. 2018, 19, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Leng, Y.; Zhi, Y.; Jiang, C.; Tian, N.; Luo, Z.; Yu, H.; Song, R. Increased Muscle Activity Accompanying With Decreased Complexity as Spasticity Appears: High-Density EMG-Based Case Studies on Stroke Patients. Front. Bioeng. Biotechnol. 2020, 16, 589321. [Google Scholar] [CrossRef] [PubMed]
- del Olmo, M.; Domingo, R. EMG Characterization and Processing in Production Engineering. Materials 2020, 13, 5815. [Google Scholar] [CrossRef] [PubMed]
- Hakonen, M.; Piitulainen, H.; Visala, A. Current state of digital signal processing in myoelectric interfaces and related applications. Biomed. Signal Process. Control 2015, 18, 334–359. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Liu, C.; Zemiti, N.; Yang, C. Optimal Feature Selection for EMG-Based Finger Force Estimation Using LightGBM Model. In Proceeding of the 2019 28th IEEE International Conference on Robot and Human Interactive Communication (RO-MAN), New Delhi, India, 14–18 October 2019; pp. 1–7. [Google Scholar] [CrossRef] [Green Version]
- Alazrai, R.; Khalifeh, A.; Alnuman, N.; Alabed, D.; Mowafi, Y. An Ensemble-Based Regression Approach for Continuous Estimation of Wrist and Fingers Movements from Surface Electromyography. In Proceeding of the 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016. [Google Scholar] [CrossRef]
- Nayak, J.; Naik, B.; Byomakesha Dash, P.; Souri, A.; Shanmuganathan, V. Hyper-parameter tuned light gradient boosting machine using memetic firefly algorithm for hand gesture recognition. Appl. Soft Comput. 2021, 107, 107478. [Google Scholar] [CrossRef]
- Yang, K.; Xu, M.; Yang, X.; Yang, R.; Chen, Y. A Novel EMG-Based Hand Gesture Recognition Framework Based on Multivariate Variational Mode Decomposition. Sensors 2021, 21, 7002. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lin, S.; Wang, N.; Dai, G. TSE-CNN: A Two-Stage End-to-End CNN for Human Activity Recognition. IEEE J. Biomed. Health Inform. 2019, 24, 292–299. [Google Scholar] [CrossRef]
- Gaetani, F.; Primiceri, P.; Zappatore, G.A.; Visconti, P. Design of an Arduino-based platform interfaced by Bluetooth Low Energy with MYO armband for controlling an under-actuated transradial prosthesis. In Proceeding of the IEEE 2018 International Conference on IC Design & Technology (ICICDT), Otranto, Italy, 4–6 June 2018; pp. 185–188. [Google Scholar] [CrossRef]
- Gatewood, C.T.; Tran, A.A.; Dragoo, J.L. The efficacy of post-operative devices following knee arthroscopic surgery: A systematic review. Knee Surg. Sport. Traumatol. Arthrosc. 2017, 25, 501–516. [Google Scholar] [CrossRef]
- Peretti, A.; Amenta, F.; Tayebati, S.K.; Nittari, G.; Mahdi, S.S. Telerehabilitation: Review of the State-of-the-Art and Areas of Application. JMIR Rehabil. Assist. Technol. 2017, 4, e7. [Google Scholar] [CrossRef]
- Mani, S.; Sharma, S.; Omar, B.; Paungmali, A.; Joseph, L. Validity and reliability of Internet-based physiotherapy assessment for musculoskeletal disorders: A systematic review. J. Telemed. Telecare 2017, 23, 379–391. [Google Scholar] [CrossRef]
- Busch, C.; Baumbach, C.; Willemsen, D.; Nee, O.; Gorath, T.; Hein, A.; Scheffold, T. Supervised training with wireless monitoring of ECG, blood pressure and oxygen-saturation in cardiac patients. J. Telemed. Telecare 2009, 15, 112–114. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, M.A.; Hill, A.J.; O’Leary, S.P.; Raymer, M.E.; Russell, T.G. Service provider perceptions of telerehabilitation as an additional service delivery option within an Australian neurosurgical and orthopaedic physiotherapy screening clinic: A Qualitative Study. Musculoskelet Sci. Pract. 2017, 32, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, M.A.; Hill, A.J.; O’Leary, S.P.; Raymer, M.E.; Russell, T.G. Clinicians’ perspectives of a novel home-based multidisciplinary telehealth service for patients with chronic spinal pain. Int. J. Telerehabilit. 2018, 10, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Howard, I.M.; Kaufman, M.S. Telehealth applications for outpatients with neuromuscular or musculoskeletal disorders. Muscle Nerve 2018, 58, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.; Lindström, B.; Ekenberg, L. Patients’ experiences of telerehabilitation at home after shoulder joint replacement. J. Telemed. Telecare 2011, 17, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Jansen-Kosterink, S.; Huisin’t Veld, R.; Hermens, H.; Vollenbroek-Hutten, M. A telemedicine service as partial replacement of face-to-face physical rehabilitation: The relevance of use. Telemed. J. E-Health 2015, 21, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Russell, T.G.; Buttrum, P.; Wootton, R.; Jull, G.A.; Jull, G.A. Internet-based outpatient telerehabilitation for patients following total knee arthroplasty: A randomized controlled trial. J. Bone Jt. Surg. Am. 2011, 93, 113–120. [Google Scholar] [CrossRef]
- Kairy, D.; Tousignant, M.; Leclerc, N.; Côté, A.M.; Levasseur, M.; Researchers, T.T. The patient’s perspective of in-home telerehabilitation physiotherapy services following total knee arthroplasty. Int. J. Environ. Res. Public Health. 2013, 10, 3998–4011. [Google Scholar] [CrossRef]
- Ben-Pazi, H.; Browne, P.; Chan, P.; Cubo, E.; Guttman, M.; Hassan, A.; Hatcher-Martin, J.; Mari, Z.; Moukheiber, E.; Okubadejo, N.U.; et al. The Promise of Telemedicine for Movement Disorders: An Interdisciplinary Approach. Curr. Neurol. Neurosci. Rep. 2018, 18, 26. [Google Scholar] [CrossRef]
- Lewis, A.; Knight, E.; Bland, M.; Middleton, J.; Mitchell, E.; McCrum, K.; Conway, J.; Bevan-Smith, E. Feasibility of an online platform delivery of pulmonary rehabilitation for individuals with chronic respiratory disease. BMJ Open Respir. Res. 2021, 8, e000880. [Google Scholar] [CrossRef]
- Milani, G.; Dematte, G.; Ferioli, M.; Dallaga, G.; Lavezzi, S.; Basaglia, N.; Straudi, S. Telerehabilitation in Italy during the COVID-19 lockdown: A Feasibility and Acceptability Study. Int. J. Telerehabil. 2021, 13, e6334. [Google Scholar] [CrossRef] [PubMed]
- Eannucci, E.F.; Hazel, K.; Grundstein, M.J.; Nguyen, J.T.; Gallegro, J. Patient satisfaction for telehealth physical therapy services was comparable to that of in-person services during the COVID-19 pandemic. HSS J. Musculoskelet. J. Hosp. Spec. Surg. 2020, 16, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Hongqian, X.; Fang, L.; Jingwen, N. Motion Research of Power-Assisted Lower Extremity Exoskeleton under Walking Process. Mech. Des. Manuf. 2019, 341, 107–110. [Google Scholar] [CrossRef]
- Jiang, N.; Falla, D.; d’Avella, A.; Graimann, B.; Farina, D. Myoelectric control in neurorehabilitation. Crit. Rev. Biomed. Eng. 2010, 38, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Barroso, F.; Bueno, D.R.; Gallego, J.A.; Jaramillo, P.; Kilicarslan, A. Surface emg in neurorehabilitation and ergonomics: State of the art and future perspectives. Emerg. Ther. Neurorehabilit. 2014, 4, 267–284. [Google Scholar]
- Manca, A.; Cereatti, A.; Bar-On, L.; Botter, A.; Della Croce, U.; Knaflitz, M.; Maffiuletti, N.A.; Mazzoli, D.; Merlo, A.; Roatta, S.; et al. A survey on the use and barriers of surface electromyography in neurorehabilitation. Front. Neurol. 2020, 11, 1137. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dai, Y.; Si, X. Analysis and Recognition of Human Lower Limb Motions Based on Electromyography (EMG) Signals. Electronics 2021, 10, 2473. [Google Scholar] [CrossRef]
- Visconti, P.; Gaetani, F.; Zappatore, G.A.; Primiceri, P. Technical features and functionalities of MYO armband: An overview on related literature and advanced applications of myoelectric bracelets mainly focused on arm prostheses. Int. J. Smart Sens. Intell. Syst. 2018, 11, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Gaetani, F.; Primiceri, P.; Zappatore, G.A.; Visconti, P. Hardware design and software development of a motion control and driving system for transradial prosthesis based on a wireless myo-electric armband. IET Sci. Meas. Technol. 2019, 13, 354–362. [Google Scholar] [CrossRef]
- Gordleeva, S.Y.; Lobov, S.A.; Grigorev, N.A.; Savosenkov, A.O.; Shamshin, M.O.; Lukoyanov, M.V.; Khoruzhko, M.A.; Kazantsev, V.B. Real-Time EEG–EMG Human–Machine Interface-Based Control System for a Lower-Limb Exoskeleton. IEEE Access 2020, 8, 84070–84081. [Google Scholar] [CrossRef]
- Stephenson, A.; Stephens, J. An exploration of physiotherapists’ experiences of robotic therapy in upper limb rehabilitation within a stroke rehabilitation centre. Disabil. Rehabil. Assist. Technol. 2018, 13, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.H.; Dionne, T.P. Interventions to improve movement and functional outcomes in adult stroke rehabilitation: Review and evidence summary. J. Particip. Med. 2018, 10, e3. [Google Scholar] [CrossRef] [PubMed]
- Aceves-Fernandez, M.A. Artificial Intelligence: Applications in Medicine and Biology; Intech Open: London, UK, 2019; ISBN 978-1-78984-018-6. Available online: https://www.intechopen.com/books/7723 (accessed on 11 April 2022). [CrossRef]
- Al-Ayyad, M.; Nazeeh, B.; Qasem, N.; Al-Takruri, M. Controlling a Lower-Leg Exoskeleton Using Voltage and Current Variation Signals of a DC Motor Mounted at the Knee Joint. J. Med. Syst. 2019, 43, 229. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Nam, C.; Rong, W.; Li, W.; Guo, Z.; Huang, Y.; Hu, X.; Zheng, Y.; Poon, W. Robotic and neuromuscular electrical stimulation (NMES) hybrid system. In Intelligent Biomechatronics in Neurorehabilitation; Academic Press: Cambridge, MA, USA, 2020; pp. 147–166. [Google Scholar]
- Monte-Silva, K.; Piscitelli, D.; Norouzi-Gheidari, N.; Batalla, M.A.P.; Archambault, P.; Levin, M.F. Electromyogram-related neuromuscular electrical stimulation for restoring wrist and hand movement in poststroke hemiplegia: A systematic review and meta-analysis. Neurorehabilit. Neural Repair 2019, 33, 96–111. [Google Scholar] [CrossRef]
- Hameed, H.K.; Hassan, W.Z.W.; Shafie, S.; Ahmad, S.A.; Jaafar, H. A review on surface electromyography-controlled hand robotic devices used for rehabilitation and assistance in activities of daily living. J. Prosthet. Orthot. 2020, 32, 3–13. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Garcia-Cossio, E.; Birbaumer, N.; Burdet, E.; Ramos-Murguialday, A. Is EMG a viable alternative to BCI for detecting movement intention in severe stroke? IEEE Trans. Biomed. Eng. 2018, 65, 2790–2797. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, S. EMG in sports rehabilitation. Br. J. Sport. Med. 2010, 44, i10. [Google Scholar] [CrossRef] [Green Version]
- Felici, F.; Vecchio, A.D. Surface Electromyography: What limits its use in exercise and sport physiology? Front. Neurol. 2020, 11, 578504. [Google Scholar] [CrossRef]
- de Sire, A.; Demeco, A.; Marotta, N.; Moggio, L.; Palumbo, A.; Iona, T.; Ammendolia, A. Anterior Cruciate Ligament Injury Prevention Exercises: Could a Neuromuscular Warm-Up Improve Muscle Pre-Activation before a Soccer Game? A Proof-of-Principle Study on Professional Football Players. Appl. Sci. 2021, 11, 4958. [Google Scholar] [CrossRef]
- Paiva, A.; Catarino, A.; Carvalho, H.; Postolache, O.; Postolache, G.; Ferreira, F. Design of a Long Sleeve T-Shirt with ECG and EMG for Athletes and Rehabilitation Patients. In Innovation, Engineering and Entrepreneurship. HELIX 2018. Lecture Notes in Electrical Engineering; Machado, J., Soares, F., Veiga, G., Eds.; Springer: Cham, Switzerland, 2018; Volume 505, ISBN 978-3-319-91333-9. [Google Scholar] [CrossRef]
- Smeets, A.; Verschueren, S.; Staes, F.; Vandenneucker, H.; Claes, S.; Vanrenterghem, J. Athletes with an ACL reconstruction show a different neuromuscular response to environmental challenges compared to uninjured athletes. Gait Posture 2021, 83, 44–51. [Google Scholar] [CrossRef]
- Zebis, M.K.; Andersen, L.L.; Brandt, M.; Myklebust, G.; Bencke, J.; Bloch Lauridsen, H.; Bandholm, T.; Thorborg, K.; Hölmich, P.; Aagaard, P. Effects of evidence-based prevention training on neuromuscular and biomechanical risk factors for ACL injury in adolescent female athletes: A randomised controlled trial. Br. J. Sport. Med. 2016, 50, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, G.; Sun, Y.; Lin, K.; Zhou, Z.; Cai, J. Application of Surface Electromyography in Exercise Fatigue: A Review. Front. Syst. Neurosci. 2022, 16, 893275. [Google Scholar] [CrossRef] [PubMed]
- Rampichini, S.; Martins Vieira, T.; Castiglioni, P.; Merati, G. Complexity Analysis of Surface Electromyography for Assessing the Myoelectric Manifestation of Muscle Fatigue: A Review. Entropy 2020, 22, 529. [Google Scholar] [CrossRef] [PubMed]
- Massó, N.; Rey, F.; Romero, D.; Gual, G.; Costa, L.; Germán, A. Surface electromyography applications in the sport. Apunt. Med. L’esport 2010, 45, 121–130. [Google Scholar]
- Hussain, J.; Sundaraj, K.; Low, Y.F.; Kiang, L.C.; Sundaraj, S.; Ali, M.A. A systematic review on fatigue analysis in triceps brachii using surface electromyography. Biomed. Signal Process. Control 2018, 40, 396–414. [Google Scholar] [CrossRef]
- Sgroi, T.A.; Cilenti, M. Rotator cuff repair: Post-operative rehabilitation concepts. Curr. Rev. Musculoskelet. Med. 2018, 11, 86–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, P.K.; Ebert, J.R.; Littlewood, C.; Ackland, T.; Wang, A. A systematic review of electromyography studies in normal shoulders to inform post-operative rehabilitation following rotator cuff repair. J. Orthop. Sport. Phys. Ther. 2017, 47, 931–944. [Google Scholar] [CrossRef]
- Scott, B.R.; Slattery, K.M.; Sculley, D.V.; Lockhart, C.; Dascombe, B.J. Acute Physiological Responses to Moderate-Load Resistance Exercise in Hypoxia. J. Strength Cond. Res. 2017, 31, 1973–1981. [Google Scholar] [CrossRef]
- Taborri, J.; Keogh, J.; Kos, A.; Santuz, A.; Umek, A.; Urbanczyk, C.; van der Kruk, E.; Rossi, S. Sport Biomechanics Applications Using Inertial, Force, and EMG Sensors: A Literature Overview. Appl. Bionics Biomech. 2020, 2020, 2041549. [Google Scholar] [CrossRef]
- Kos, A.; Umek, A. Wearable sensor devices for prevention and rehabilitation in healthcare: Swimming exercise with real-time therapist feedback. IEEE Internet Things J. 2019, 6, 1331–1341. [Google Scholar] [CrossRef]
- Jiang, Y. Combination of wearable sensors and internet of things and its application in sports rehabilitation. Comput. Commun. 2020, 150, 167–176. [Google Scholar] [CrossRef]
- Lynn, S.K.; Watkins, C.; Wong, M.A.; Balfany, K.; Feeney, D.F. Validity and reliability of surface electromyography measurements from a wearable athlete performance system. J. Sport. Sci. Med. 2018, 17, 205. [Google Scholar]
- Rajeswari, J.; Jagannath, M. Advances in biomedical signal and image processing—A systematic review. Inform. Med. Unlocked 2017, 8, 13–19. [Google Scholar] [CrossRef]
- Gohel, V.; Mehendale, N. Review on electromyography signal acquisition and processing. Biophys. Rev. 2020, 12, 1361–1367. [Google Scholar] [CrossRef]
- Houssein, E.H.; Kilany, M.; Hassanien, A.E. ECG signals classification: A review. Int. J. Intell. Eng. Inform. 2017, 5, 376–396. [Google Scholar] [CrossRef]
- Faust, O.; Hagiwara, Y.; Hong, T.J.; Lih, O.S.; Acharya, U.R. Deep learning for healthcare applications based on physiological signals: A review. Comput. Methods Programs Biomed. 2018, 161, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jull, G.; Moore, A.; Falla, D.; Lewis, J.; McCarthy, C.; Sterling, M. Grieve’s Modern Musculoskeletal Physiotherapy, 4th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015; ISBN 9780702051524. [Google Scholar]
- Rahul, J.; Sora, M.; Sharma, L.D. An overview on biomedical signal analysis. Int. J. Recent Technol. Eng. 2019, 7, 206–209. [Google Scholar]
- Tankisi, H.; Burke, D.; Cui, L.; de Carvalho, M.; Kuwabara, S.; Nandedkar, S.D.; Rutkove, S.; Stalberg, E.; van Putten, M.J.A.M.; Fuglsang-Frederiksen, A. Standards of instrumentation of EMG. Clin. Neurophysiol. 2020, 131, 243–258. [Google Scholar] [CrossRef]
- Kimura, J. Electrodiagnosis in Diseases of Nerve and Muscle: Principles and Practice, 4th ed.; Oxford University Press: New York, NY, USA, 2013; ISBN 9780199969296. [Google Scholar]
- Cuesta-Vargas, A. EMG Analysis of the Neuromuscular Activity during Sit-to-Stand from Different Height Chairs in Water. Int. J. Aquat. Res. Educ. 2019, 12, 6. [Google Scholar] [CrossRef] [Green Version]
- Chan, A.Y.K. Biomedical Device Technology: Principles and Design, 2nd ed.; Charles, C., Ed.; Thomas Publisher, Limited: Springfield, IL, USA, 2016; ISBN 9780398090838. [Google Scholar]
- Tun, N.N.; Sanuki, F.; Iramina, K. Electroencephalogram Electromyogram Functional Coupling and Delay Time Change Based on Motor Task Performance. Sensors 2021, 21, 4380. [Google Scholar] [CrossRef]
- Caldwell, R.R.; Lovely, D.F. Commercial Hardware for the Implementation of Myoelectric Control. In Powered Upper Limb Prostheses; Muzumdar, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 55–71. [Google Scholar] [CrossRef]
- Huang, Z.-X.; Zhang, X.-D.; Li, Y.-N. Design of a grasp force adaptive control system with tactile and slip perception. In Proceedings of the 2012 IEEE International Conference on Automation Science and Engineering (CASE), Seoul, Republic of Korea, 20–24 August 2012; pp. 1101–1105. [Google Scholar] [CrossRef]
- Merletti, R.; Farina, D. Surface Electromyography: Physiology, Engineering, and Application, 1st ed.; Wiley-IEEE Press Series on Biomedical Engineering: Hoboken, NJ, USA, 2016; ISBN 9781118987025. [Google Scholar] [CrossRef]
- McManus, L.; De Vito, G.; Lowery, M.M. Analysis and biophysics of surface EMG for physiotherapists and kinesiologists: Toward a common language with rehabilitation engineers. Front. Neurol. 2020, 11, 576729. [Google Scholar] [CrossRef] [PubMed]
- Gaetani, F.; De Fazio, R.; Zappatore, G.A.; Visconti, P. A Prosthetic Limb Managed by Sensors-Based Electronic System: Experimental Results on Amputees. Bull. Electr. Eng. Inform. BEEI 2020, 9, 314–324. [Google Scholar] [CrossRef]
- Campanini, I.; Merlo, A.; Disselhorst-Klug, C.; Mesin, L.; Muceli, S.; Merletti, R. Fundamental Concepts of Bipolar and High-Density Surface EMG Understanding and Teaching for Clinical, Occupational, and Sport Applications: Origin, Detection, and Main Errors. Sensors 2022, 22, 4150. [Google Scholar] [CrossRef] [PubMed]
- Merlo, A.; Bò, M.C.; Campanini, I. Electrode Size and Placement for Surface EMG Bipolar Detection from the Brachioradialis Muscle: A Scoping Review. Sensors 2021, 21, 7322. [Google Scholar] [CrossRef]
- Péter, A.; Andersson, E.; Hegyi, A.; Finni, T.; Tarassova, O.; Cronin, N.; Grundström, H.; Arndt, A. Comparing Surface and Fine-Wire Electromyography Activity of Lower Leg Muscles at Different Walking Speeds. Front. Physiol. 2019, 10, 1283. [Google Scholar] [CrossRef] [Green Version]
- Farina, D.; Merletti, R.; Indino, B.; Nazzaro, M.; Pozzo, M. Surface EMG Crosstalk between Knee Extensor Muscles: Experimental and Model Results. Muscle Nerve 2002, 26, 681–695. [Google Scholar] [CrossRef]
- Meng, Q.; Zhang, J.; Yang, X. Virtual rehabilitation training system based on surface EMG feature extraction and analysis. J. Med. Syst. 2019, 43, 48. [Google Scholar] [CrossRef]
- Moore, J.; Zouridakis, G. Biomedical Technology and Devices Handbook; The Mechanical Engineering Handbook Series; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Song, H.; Park, Y.; Kim, H.; Ko, H. Fully integrated biopotential acquisition analog front-end IC. Sensors 2015, 15, 25139–25156. [Google Scholar] [CrossRef] [Green Version]
- Maceira-Elvira, P.; Popa, T.; Schmid, A.C.; Hummel, F.C. Wearable technology in stroke rehabilitation: Towards improved diagnosis and treatment of upper-limb motor impairment. J. Neuroeng. Rehabil. 2019, 16, 142. [Google Scholar] [CrossRef]
- Porciuncula, F.; Roto, A.V.; Kumar, D.; Davis, I.; Roy, S.; Walsh, C.J.; Awad, L.N. Wearable movement sensors for rehabilitation: A focused review of technological and clinical advances. J. Inj. Funct. Rehabil. 2018, 10 (Suppl. S2), S220–S232. [Google Scholar] [CrossRef] [Green Version]
- Rodgers, M.M.; Alon, G.; Pai, V.M.; Conroy, R.S. Wearable technologies for active living and rehabilitation: Current research challenges and future opportunities. J. Rehabil. Assist. Technol. Eng. 2019, 6, 2055668319839607. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Un, K.F.; Mak, P.I.; Chen, Y.; Muñoz-Ferreras, J.M.; Yang, Z.; Gómez-García, R. Overview of recent development on wireless sensing circuits and systems for healthcare and biomedical applications. IEEE J. Emerg. Sel. Top. Circuits Syst. 2018, 8, 165–177. [Google Scholar] [CrossRef]
- Baig, M.M.; Gholam Hosseini, H.; Moqeem, A.A.; Mirza, F.; Lindén, M. A systematic review of wearable patient monitoring systems—Current challenges and opportunities for clinical adoption. J. Med. Syst. 2017, 41, 115. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Mathew, L.; Syal, P. Increasing trend of wearables and multi-modal interface for human activity monitoring: A review. Biosens. Bioelectron. 2017, 90, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Liu, J.; Gong, Z.; Lei, Y.; OuYang, X.; Chan, C.C.; Ruan, S. Wearable Physiological Monitoring System Based on Electrocardiography and Electromyography for Upper Limb Rehabilitation Training. Sensors 2020, 20, 4861. [Google Scholar] [CrossRef]
- Erickson, J.C.; Stepanyan, E.; Hassid, E. Comparison of Dry and Wet Electrodes for Detecting Gastrointestinal Activity Patterns from Body Surface Electrical Recordings. Ann. Biomed. Eng. 2023, 1–12. [Google Scholar] [CrossRef]
- Liu, S.-H.; Wang, J.-J.; Tan, T.-H. A Portable and Wireless Multi-Channel Acquisition System for Physiological Signal Measurements. Sensors 2019, 19, 5314. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, M.; Gia, T.N.; Westerlund, T. EMG-Based IoT System Using Hand Gestures for Remote Control Applications. In Proceedings of the 2021 IEEE 7th World Forum on Internet of Things (WF-IoT), New Orleans, LA, USA, 14 June–31 July 2021; pp. 911–912. [Google Scholar]
- Park, S.Y.; Cho, J.; Na, K.; Yoon, E. Modular 128-Channel ∆-∆Σ Analog Front-End Architecture Using Spectrum Equalization Scheme for 1024-Channel 3-D Neural Recording Microsystems. IEEE J.-Solid-State Circuits 2017, 53, 501–514. [Google Scholar] [CrossRef]
- Kast, C.; Krenn, M.; Aramphianlert, W.; Hofer, C.; Aszmann, O.C.; Mayr, W. Modular Multi-channel Real-time Bio-signal Acquisition System. In Proceedings of the International Conference on Advancements of Medicine and Health Care through Technology, Cluj-Napoca, Romania, 12–15 October 2016; pp. 95–98. [Google Scholar]
- Tran, L.; Cha, H.K. An ultra-low-power neural signal acquisition analog front-end IC. Microelectron. J. 2021, 107, 104950. [Google Scholar] [CrossRef]
- Piccinini, D.J.; Andino, N.B.; Ponce, S.D.; Roberti, M.A. Wearable system for acquisition and monitoring of biological signals. J. Phys. Conf. Ser. 2016, 705, 012009. [Google Scholar] [CrossRef]
- Sarker, V.K.; Jiang, M.; Gia, T.N.; Anzanpour, A.; Rahmani, A.M.; Liljeberg, P. Portable multipurpose bio-signal acquisition and wireless streaming device for wearables. In Proceedings of the 2017 IEEE Sensors Applications Symposium (SAS), Glassboro, NJ, USA, 13–15 March 2017; pp. 1–6. [Google Scholar]
- Mazzetta, I.; Gentile, P.; Pessione, M.; Suppa, A.; Zampogna, A.; Bianchini, E.; Irrera, F. Stand-alone wearable system for ubiquitous real-time monitoring of muscle activation potentials. Sensors 2018, 18, 1748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Amft, O. Bite Glasses: Measuring Chewing Using Emg and Bone Vibration in Smart Eyeglasses. In Proceedings of the 2016 ACM International Symposium on Wearable Computers, Heidelberg, Germany, 12–16 September 2016; pp. 50–52. [Google Scholar]
- Kim, I.; Bhagat, Y.A.; Homer, J.; Lobo, R. Multimodal analog front end for wearable bio-sensors. IEEE Sens. J. 2016, 16, 8784–8791. [Google Scholar] [CrossRef]
- Nakamura, H.; Sakajiri, Y.; Ishigami, H.; Ueno, A. A Novel Analog Front End with Voltage-Dependent Input Impedance and Bandpass Amplification for Capacitive Biopotential Measurements. Sensors 2020, 20, 2476. [Google Scholar] [CrossRef] [PubMed]
- Biagetti, G.; Crippa, P.; Falaschetti, L.; Orcioni, S.; Turchetti, C. Human activity monitoring system based on wearable sEMG and accelerometer wireless sensor nodes. Biomed. Eng. Online 2018, 17, 132. [Google Scholar] [CrossRef] [Green Version]
- Biagetti, G.; Crippa, P.; Falaschetti, L.; Turchetti, C. A multi-channel electromyography, electrocardiography and inertial wireless sensor module using Bluetooth low-energy. Electronics 2020, 9, 934. [Google Scholar] [CrossRef]
- Li, X.; Sun, Y. NCMB-button: A wearable non-contact system for long-term multiple biopotential monitoring. In Proceedings of the 2017 IEEE/ACM International Conference on Connected Health: Applications, Systems and Engineering Technologies (CHASE), Philadelphia, PA, USA, 17–19 July 2017; pp. 348–355. [Google Scholar]
- Velumani, D.; Nancy Subha, G.; Harishma, S.; Kruthika Reshmi, C.; Meghana, P.; Gokul, M. IoT Based Tele-Sleep Bruxism Diagnosis Tool. In Proceedings of the 2022 International Conference on Augmented Intelligence and Sustainable Systems (ICAISS), Trichy, India, 24–26 November 2022; pp. 1156–1160. [Google Scholar]
- Senapati, B.; Kumar, M.G.L.; Ray, K.B. High resolution reconfigurable bio-potential processor for portable biomedical application. In Devices for Integrated Circuit (DevIC); IEEE: New York, NY, USA, 2017; pp. 517–521. [Google Scholar]
- Lee, S.C.; Lin, Y.S.; Chen, Y.J.; Chiueh, H. A wireless multi-channel physiological signal acquisition system-on-chip for wearable devices. In Proceedings of the 2016 IEEE Sensors Conference, Orlando, FL, USA, 30 October–3 November 2016. [Google Scholar] [CrossRef]
- Augustyniak, P. Remotely programmable architecture of a multi-purpose physiological recorder. Microprocess. Microsyst. 2016, 46, 55–66. [Google Scholar] [CrossRef]
- Bhamra, H.; Lynch, J.; Ward, M.; Irazoqui, P. A noise-power-area optimized biosensing front end for wireless body sensor nodes and medical implantable devices. IEEE Trans. Very Large Scale Integr. (VLSI) Syst. 2017, 25, 2917–2928. [Google Scholar] [CrossRef]
- Dai, Y.; Jin Wu, J.; Niu, J.; Gu, F.; Shen, S. MSEva: A Musculoskeletal Rehabilitation Evaluation System Based on EMG Signals. ACM Trans. Sens. Netw. 2022, 19, 6. [Google Scholar] [CrossRef]
- De Fazio, R.; De Vittorio, M.; Visconti, P. Innovative IoT Solutions and Wearable Sensing Systems for Monitoring Human Biophysical Parameters: A Review. Electronics 2021, 10, 1660. [Google Scholar] [CrossRef]
- De Fazio, R.; Stabile, M.; De Vittorio, M.; Velázquez, R.; Visconti, P. An Overview of Wearable Piezoresistive and Inertial Sensors for Respiration Rate Monitoring. Electronics 2021, 10, 2178. [Google Scholar] [CrossRef]
- Data Acquisition Systems-Biometrics Ltd. Available online: https://www.biometricsltd.com/systems-wireless.htm (accessed on 1 September 2022).
- Surface EMG Sensors-Biometrics Ltd. Available online: https://www.biometricsltd.com/surface-emg-sensor.htm (accessed on 15 August 2022).
- Wearable Sensor Products-Individual Sensors, Shimmer Co. Available online: http://www.shimmersensing.com/products/individual-sensors/ (accessed on 4 April 2022).
- Shimmer3 EMG Unit, Shimmer Co. Available online: http://www.shimmersensing.com/products/shimmer3-emg-sensor#related-tab (accessed on 4 April 2022).
- Shimmer3 ECG Unit, Shimmer Co. Available online: http://www.shimmersensing.com/products/shimmer3-ecg-sensor (accessed on 10 July 2022).
- González-Mendoza, A.; Pérez-SanPablo, A.I.; López-Gutiérrez, R.; Quiñones-Urióstegui, I. Validation of an EMG Sensor for Internet of Things and Robotics. In Proceedings of the 2018 15th International Conference on Electrical Engineering, Computing Science and Automatic Control (CCE), Mexico City, Mexico, 5–7 September 2018; pp. 1–5. [Google Scholar]
- Sakamoto, A.; Khan, M.T.I.; Kurita, T. EMG Signals in Co-Activations of Lower Limb Muscles for Knee Joint Analysis. In Proceedings of the 2015 International Conference on Informatics, Electronics & Vision (ICIEV), Fukuoka, Japan, 15–18 June 2015; pp. 1–5. [Google Scholar]
- BioSemi Products Website, Active High-Density EMG Electrode Array—BioSemi Co. Available online: https://www.biosemi.com/HD_electrode.htm (accessed on 18 July 2022).
- Angeli, T.R.; O’Grady, G.; Erickson, J.C.; Du, P.; Paskaranandavadivel, N.; Bissett, I.P.; Cheng, L.K.; Pullan, A.J. Mapping Small Intestine Bioelectrical Activity Using High-Resolution Printed-Circuit-Board Electrodes. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 4951–4954. [Google Scholar]
- BTSbioengineering FreeEMG|Wireless Surface EMG|BTS Bioengineering. Available online: https://www.btsbioengineering.com/products/freeemg/ (accessed on 12 December 2022).
- Tesio, L.; Malloggi, C.; Malfitano, C.; Coccetta, C.A.; Catino, L.; Rota, V. Limping on Split-Belt Treadmills Implies Opposite Kinematic and Dynamic Lower Limb Asymmetries. Int. J. Rehabil. Res. 2018, 41, 304–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plux Biosignals. Available online: https://www.pluxbiosignals.com/products/ (accessed on 1 July 2022).
- Electromyography (EMG) Sensor—BITalino. Available online: https://www.pluxbiosignals.com/collections/myology/products/electromyography-emg-sensor (accessed on 4 August 2022).
- Plux Biosignalsplux. Available online: https://www.pluxbiosignals.com/collections/biosignalsplux (accessed on 4 August 2022).
- Santos, P.; Quaresma, C.; Garcia, I.; Quintão, C. Neuromotor Evaluation of the Upper Limb during Activities of Daily Living: A Pilot Study. In Proceedings of the Technological Innovation for Digitalization and Virtualization, Cham, Switzerland, 29 June–1 July 2022; pp. 112–121. [Google Scholar]
- Delsys Trigno Research+. Available online: https://delsys.com/trigno/research/ (accessed on 4 April 2022).
- Hu, Z.; Kim, Y.; Zhang, Y.; Zhang, Y.; Li, J.; Tang, X.; Sohn, J.; Kim, S. Correlation of Lower Limb Muscle Activity with Knee Joint Kinematics and Kinetics during Badminton Landing Tasks. Int. J. Environ. Res. Public Health 2022, 19, 16587. [Google Scholar] [CrossRef] [PubMed]













| Reference | Year | Number of Channels | Signal Acquisition | Sampling Frequency | Wireless Connection | Technical Features and Strengths |
|---|---|---|---|---|---|---|
| S. Zhao et al. [110] | 2020 | 2 channels | STM32L15 (MCU) + Precision instrumentation amplifiers + BMD 101 (16-bit ADC) | N.A. a | BLE |
|
| S. H. Liu et al. [112] | 2019 | 8 channels | MSP430 MCU (12-bit ADC) | 100 Hz | Bluetooth 3.0 (BTM-204B) |
|
| M. Nguyen et al. [113] | 2021 | 8 channels | Myo Armband | 200 Hz | Bluetooth |
|
| S.-Y. Park et al. [114] | 2018 | 128 channels | 128-channel Custom AFE (10.9-bit ∆-∆Σ ADC) | 800 kHz | No |
|
| L. Tran et al. [116] | 2021 | 4 channels | 4-channel Neural recording AFE IC (10-bit SAR ADC) | 1–10 kHz | No |
|
| D. J. Piccinini et al. [117] | 2016 | N.A. a | CC3200 MCU + ADS1294 AFE (24-bit ∆Σ) | 32 kHz | WiFi (CC3200) |
|
| V.K. Sarker et al. [118] | 2017 | 8 channels | ATmega328p + ADS1299 AFE (24-bit ADC) | 250–1000 Hz | Bluetooth 2.0 (HC-05) |
|
| I. Mazzetta et al. [119] | 2018 | Differential 1 channel | Bio2Bit | ≥4 kHz | Bluetooth 4.0 |
|
| R. Zhang et al. [120] | 2016 | 1 channel | Bitalino EMG | 1 kHz | No |
|
| I. Kim et al. [121] | 2016 | N.A. a | Cyclone IV FPGA + Custom AFE (10-bit SAR ADC) | 1.10 MHz | No |
|
| H. Nakamura et al. [122] | 2020 | N.A. a | Custom AFE to capacitive biopotential measurements (CBMs) (16-bit ADC) | 1 kHz | No |
|
| G. Biagetti et al. [123] | 2018 | 1 channel | sEMG sensing nodes (12-bit ADC) | 2 kHz | 2.4 GHz radio link |
|
| G. Biagetti et al. [124] | 2020 | 3 channels | nRF52840 MCU + ADS1293 AFE (24-bit ADC) | 3.2 kHz | BLE |
|
| X. Li et al. [125] | 2017 | N.A. a | ATmega328p (10-bit ADC) | N.A. a | 2.4 GHz radio link (nRF24L01) |
|
| D. Velumani et al. [126] | 2022 | 1 channel | ESP32 (12-bit SAR ADC) | N.A. a | WiFi (ESP32) |
|
| B. Senapati et al. [127] | 2017 | N.A. a | Spartan-3E FPGA + Custom AFE (16-bit SAR ADC) | N.A. a | No |
|
| S.C. Lee et al. [128] | 2016 | 8 channels | Open RISC 1200 MCU (12-bit ADC) + Custom AFE | N.A. a | Bluetooth 4.0 |
|
| Augustyniak et al. [129] | 2016 | Single-ended 5 channels | PXA-270 CPU + ADAS1000 (24-bit ADC) | 500 Hz | WiFi |
|
| H. Bhamra et al. [130] | 2017 | N.A. a | ASIC 9.1-bit SAR ADC | 4 kHz | No |
|
| Device | Other Detected Signals | N° of EMG Channels | Dimension/ Weight | Sampling Frequency [Hz] | Electrode Typology | Wireless Connectivity | Suggested Applications |
|---|---|---|---|---|---|---|---|
| Biometrics wireless sensors [134] | Joint angle (electro- goniometers- optionally) | 2, 4, 8, 16 | N.A. a | 500, 1000, 2000 | Disposable sEMG electrodes with 4 mm snap | Yes (WiFi) |
|
| Shimmer3 EMG units [137] | ECG | 2 | 65 mm × 32 mm × 12 mm/31 g | 125–8000 | Patented disposable EMG electrodes | Yes (Bluetooth) |
|
| ActiveTwo EMG unit [141] | EEG, ECG | 280 | N.A. a | 200, 400, 8000, 16,000 | Special silver-plated electrode-tip | No |
|
| FreeEMG sensors [143] | Joint angle (electrogoniometers- optionally) | 1 | 27 mm × 37 mm × 15 mm/14 g | ≤4000 | Standard with a clip connection | Yes (WiFi) |
|
| Trigo Wireless EMG sensors [149] | Inertial data | 4, 8, 16 | N.A. a | 2000 | Silver electrodes | Yes (WiFi and BLE) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ayyad, M.; Owida, H.A.; De Fazio, R.; Al-Naami, B.; Visconti, P. Electromyography Monitoring Systems in Rehabilitation: A Review of Clinical Applications, Wearable Devices and Signal Acquisition Methodologies. Electronics 2023, 12, 1520. https://doi.org/10.3390/electronics12071520
Al-Ayyad M, Owida HA, De Fazio R, Al-Naami B, Visconti P. Electromyography Monitoring Systems in Rehabilitation: A Review of Clinical Applications, Wearable Devices and Signal Acquisition Methodologies. Electronics. 2023; 12(7):1520. https://doi.org/10.3390/electronics12071520
Chicago/Turabian StyleAl-Ayyad, Muhammad, Hamza Abu Owida, Roberto De Fazio, Bassam Al-Naami, and Paolo Visconti. 2023. "Electromyography Monitoring Systems in Rehabilitation: A Review of Clinical Applications, Wearable Devices and Signal Acquisition Methodologies" Electronics 12, no. 7: 1520. https://doi.org/10.3390/electronics12071520