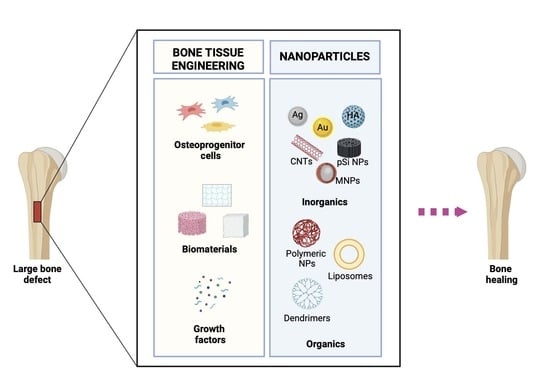
Bone Tissue Engineering and Nanotechnology: A Promising Combination for Bone Regeneration
Abstract
:Simple Summary
Abstract
1. Introduction
2. Conventional and Innovative Bone Graft Approaches
3. Biomaterials for BTE Applications
- (a)
- Ceramics are the most promising biomaterials due to their good mechanical properties and excellent biocompatibility [48]. They include bioglass, alumina, zirconia, and CaP-based materials such as HA, b-TCP, and biphasic calcium phosphate (BCP) [49,50]. They can accurately imitate the ECM composition of natural bone [51], thereby improving osteoblastic proliferation and differentiation [52], and their biodegradability allows the release of ions that can contribute to bone tissue regeneration [53]. Furthermore, ceramic biomaterials provide highly interconnected porous structures that enable neo-vascularization, cell migration, and bone growth [54]. In their study, Mondal et al. demonstrated that fish scale-derived natural HA (FS-HAp) scaffolds successfully mimicked the cancellous/cortical bone system in terms of structure, porosity, and mechanical strength and exhibited excellent bioactive behavior. Furthermore, in vitro and in vivo studies by those authors suggested that these scaffolds could provide osteoconductive support, facilitating new cell growth on their surface [55]. In their in vitro and in vivo study, Jiao et al., investigated the osteogenic and bone-repair properties of β-TCP by developing a 3D-printed b-TCP scaffold. Their findings suggested that β-TCP exhibited good biocompatibility and promoted osteogenic differentiation by inducing the expression of osteogenic factors, such as methyltransferase-like 3 (METTL3) and Runx2 [56]. On the other hand, slow biodegradability and extreme fragility limit their use in clinical applications [57,58]. To overcome this problem, ceramic-based composite scaffolds have been developed.
- (b)
- Natural polymers are the most widely used biomaterials due to the high affinity of their structure with the native ECM made up of nano-/microscale protein fibers with different arrangements. They include collagen (Col), alginate (ALG), chitin, and chitosan (CS). Natural polymers have low toxicity, poor immunogenicity, and good biocompatibility, as they are derived from natural sources such as plants, animals, and microorganisms; they also possess the ability to stimulate cell growth and adhesion, thereby promoting bone tissue regeneration [59,60]. Lin et al. showed that natural collagen derived from marine sponge was able to promote cell adhesion and mineralization in vitro [61]. Similarly, Sukul et al. investigated the effect of chitosan sponges on the adhesion, growth, and differentiation of primary human osteoblasts, suggesting that 3D sponges could contribute to angiogenesis and bone remodeling [62]. Nonetheless, low mechanical stability, poor osteoinductivity, and quick biodegradability limit their application compared with other ceramic or metal biomaterials [63]. To overcome these drawbacks, natural polymers have been combined with other materials.
- (c)
- Synthetic polymers, including polystyrene, PLA, PGA, PCL, and polylactic-co-glycolic acid (PLGA), are often used, due to the possibility of regulating their mechanical properties, biodegradability, morphology, and structure during the fabrication process [64,65]. Recently, some in vitro studies have shown that 3D-printed PLA scaffolds are able to promote the adhesion, proliferation, and differentiation of osteoblast cells [66,67]. In another study, the osteoregenerative capability of a porous PLGA (P) scaffold combined with magnesium hydroxide (MH, M), bone-extracellular matrix (bECM, E), and bioactive polydeoxyribonucleotide (PDRN, P) (PMEP scaffold) was evaluated. The authors showed that the developed PMEP scaffold displayed remarkable biological properties in terms of cell adhesion, proliferation, and osteogenic differentiation in vitro [68]. Despite these advantages, some important limitations, such as poor biocompatibility, high toxicity, and reduced bioactivity and osteoconductivity have restricted their application in BTE [69]. These limitations can be overcome by combining synthetic polymers with natural polymers or ceramics.
- (d)
- Metals, such as iron, chromium, stainless steel, titanium, and cobalt alloys, are particularly attractive biomaterials for bone implants, due to their exceptional mechanical properties, which include high elasticity, resistance and ductility, and structural stability [70,71]. Deng and colleagues discovered that 3D-printed Ti6Al4V scaffolds promoted bone formation in vivo, which is strongly influenced by scaffold porosity [72]. Despite this, there are several limitations associated with the use of metal as a scaffold, including a high Young’s modulus, poor degradability, metal ion toxicity, and particle release [73]. To limit these disadvantages, it is possible to improve their chemical structures (e.g., porosity), combine them with other biomaterials, or use biodegradable metals, such as magnesium, zinc, and calcium [74].
- (e)
- Composite (or hybrid) biomaterials are made by combining two or more biomaterials, such as co-polymers, polymeric/ceramic or metallic/ceramic compounds, and metal implants coated with polymers (PLA/PGA, PLA/HA, PGA/PCA, HA/PGA, HA/CS, or HA/Col) [75]. Calabrese et al. showed that hybrid scaffolds made of collagen and hydroxyapatite are able to induce osteogenic differentiation in hADSCs and stimulate bone augmentation after ectopic transplantation in mice [76,77]. Hence, these biomaterials yield improvements in terms of their biological, chemical, and structural properties, although their manufacturing procedures are laborious [78].
- (f)
- Hydrogels are hydrophilic polymers that have a high absorption capacity for water or biological fluids [79]. They are good candidates for BTE applications due to their elastic nature, which is comparable to that of ECM [80,81]. Hydrogels can be of natural (e.g., hyaluronic acid) or synthetic (e.g., polyethylene oxide (PEO)) origin. In this context, a hyaluronic acid-based hydrogel combined with BMP-2 and human MSCs was found to increase cell survival in vitro and to encourage bone formation and vascularization in vivo [82]. Jo et al. demonstrated that the injection of chitosan-PEO hydrogel, in combination with BMP-2 and MSCs, promoted bone formation in vivo [83]. Despite their limitations in terms of biocompatibility and biodegradability in vivo [84], their flexibility, i.e., the ability to adjust the structural parameters during the manufacturing processes [85], and the possibility of minimally invasive implantation [86], strongly encourage their application in the BTE field.
4. Nanotechnology
5. Organic NPs
5.1. Natural Polymers
5.2. Synthetic Polymers
5.3. Liposomes
5.4. Dendrimers
6. Inorganic NPs
6.1. Metal NPs
6.2. Ceramic NPs
6.3. Magnetic NPs
7. Carbon-Based Nanomaterials
7.1. 0D CNMs
7.2. 1D CNMs
7.3. 2D CNMs
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hadjidakis, D.J.; Androulakis, I.I. Bone remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Keating, J.F.; Simpson, A.H.R.W.; Robinson, C.M. The management of fractures with bone loss. J. Bone Joint Surg. Br. 2005, 87-B, 142–150. [Google Scholar] [CrossRef]
- Vidal, L.; Kampleitner, C.; Brennan, M.A.; Hoornaert, A.; Layrolle, P. Reconstruction of Large Skeletal Defects: Current Clinical Therapeutic Strategies and Future Directions Using 3D Printing. Front. Bioeng. Biotechnol. 2020, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.M. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021, 2, e580–e592. [Google Scholar] [CrossRef]
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Lond. Engl. 2020, 396, 2006–2017. [Google Scholar] [CrossRef]
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef]
- Georgeanu, V.A.; Gingu, O.; Antoniac, I.V.; Manolea, H.O. Current Options and Future Perspectives on Bone Graft and Biomaterials Substitutes for Bone Repair, from Clinical Needs to Advanced Biomaterials Research. Appl. Sci. 2023, 13, 8471. [Google Scholar] [CrossRef]
- Franco, D.; Leonardi, A.A.; Rizzo, M.G.; Palermo, N.; Irrera, A.; Calabrese, G.; Conoci, S. Biological Response Evaluation of Human Fetal Osteoblast Cells and Bacterial Cells on Fractal Silver Dendrites for Bone Tissue Engineering. Nanomaterials 2023, 13, 1107. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef]
- Delpierre, A.; Savard, G.; Renaud, M.; Rochefort, G.Y. Tissue Engineering Strategies Applied in Bone Regeneration and Bone Repair. Bioengineering 2023, 10, 644. [Google Scholar] [CrossRef]
- Pérez, R.A.; Won, J.-E.; Knowles, J.C.; Kim, H.-W. Naturally and synthetic smart composite biomaterials for tissue regeneration. Adv. Drug Deliv. Rev. 2013, 65, 471–496. [Google Scholar] [CrossRef]
- Cheng, R.; Meng, F.; Deng, C.; Klok, H.-A.; Zhong, Z. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials 2013, 34, 3647–3657. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Pant, T.; Rohra, N.; Goyal, A.; Lawrence, M.; Dey, A.; Ganguly, P. Nanobiotechnology in Bone Tissue Engineering Applications: Recent Advances and Future Perspectives. Appl. Biosci. 2023, 2, 617–638. [Google Scholar] [CrossRef]
- Schmidt, A.H. Autologous bone graft: Is it still the gold standard? Injury 2021, 52, S18–S22. [Google Scholar] [CrossRef]
- Flierl, M.A.; Smith, W.R.; Mauffrey, C.; Irgit, K.; Williams, A.E.; Ross, E.; Peacher, G.; Hak, D.G.; Stahel, P.F. Outcomes and complication rates of different bone grafting modalities in long bone fracture nonunions: A retrospective cohort study in 182 patients. J. Orthop. Surg. 2013, 8, 33. [Google Scholar] [CrossRef]
- Brydone, A.S.; Meek, D.; Maclaine, S. Bone grafting, orthopaedic biomaterials, and the clinical need for bone engineering. Proc. Inst. Mech. Eng. H 2010, 224, 1329–1343. [Google Scholar] [CrossRef]
- Holt, G.; Arthur, A.; Frame, D.; Muirhead, A. Human skeletal allograft collection--room for improvement? Scott. Med. J. 2004, 49, 146–148. [Google Scholar] [CrossRef]
- Amid, R.; Kheiri, A.; Kheiri, L.; Kadkhodazadeh, M.; Ekhlasmandkermani, M. Structural and chemical features of xenograft bone substitutes: A systematic review of in vitro studies. Biotechnol. Appl. Biochem. 2020, 68, 1432–1452. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Jupiter, D.C. Bone Graft Substitute. Clin. Podiatr. Med. Surg. 2015, 32, 21–34. [Google Scholar] [CrossRef]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma. 2019, 33, 203–213. [Google Scholar] [CrossRef]
- Yu, X.; Tang, X.; Gohil, S.V.; Laurencin, C.T. Biomaterials for Bone Regenerative Engineering. Adv. Healthc. Mater. 2015, 4, 1268–1285. [Google Scholar] [CrossRef]
- Dolcimascolo, A.; Calabrese, G.; Conoci, S.; Parenti, R. Innovative Biomaterials for Tissue Engineering. In Biomaterial-Supported Tissue Reconstruction or Regeneration; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Pape, H.C.; Evans, A.; Kobbe, P. Autologous bone graft: Properties and techniques. J. Orthop. Trauma. 2010, 24, S36–S40. [Google Scholar] [CrossRef]
- Wang, J.; Blalock, S.K.F.; Levitan, G.S.; Prichard, H.L.; Niklason, L.E.; Kirkton, R.D. Biological mechanisms of infection resistance in tissue engineered blood vessels compared to synthetic expanded polytetrafluoroethylene grafts. JVS-Vasc. Sci. 2023, 4, 100120. [Google Scholar] [CrossRef]
- O’Brien, F.G. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Ruan, Y.C.; Yu, M.K.; O’Laughlin, M.; Wise, H.; Chen, D.; Tian, L.; Shi, D.; Wang, J. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat. Med. 2016, 22, 1160–1169. [Google Scholar] [CrossRef]
- Salgado, A.J.; Coutinho, O.P.; Reis, R.L. Bone tissue engineering: State of the art and future trends. Macromol. Biosci. 2004, 4, 743–765. [Google Scholar] [CrossRef]
- Seong, J.M.; Kim, B.C.; Park, J.H.; Kwon, I.K.; Mantalaris, A.; Hwang, Y.S. Stem cells in bone tissue engineering. Biomed. Mater. 2010, 5, 062001. [Google Scholar] [CrossRef]
- Murphy, W.L.; Peters, M.C.; Kohn, D.H.; Mooney, D.J. Sustained release of vascular endothelial growth factor from mineralized poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials 2000, 21, 2521–2527. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Ishizuya, T.; Kintou, N.; Wada, Y.; Katagiri, T.; Wozney, J.M.; Rosen, V.; Yoshiki, S. Effects of BMP-2, BMP-4, and BMP-6 on osteoblastic differentiation of bone marrow-derived stromal cell lines, ST2 and MC3T3-G2/PA6. Biochem. Biophys. Res. Commun. 1996, 220, 366–371. [Google Scholar] [CrossRef]
- Bolander, M.E. Regulation of Fracture Repair by Growth Factors. Exp. Biol. Med. 1992, 200, 165–170. [Google Scholar] [CrossRef]
- McCarthy, T.L.; Centrella, M.; Canalis, E. Regulatory effects of insulin-like growth factors I and II on bone collagen synthesis in rat calvarial cultures. Endocrinology 1989, 124, 301–309. [Google Scholar] [CrossRef]
- Li, Y. Sustained Release of VEGF to Promote Angiogenesis and Osteointegration of Three-Dimensional Printed Biomimetic Titanium Alloy Implants. Front. Bioeng. Biotechnol. 2021, 9, 757767. [Google Scholar] [CrossRef]
- Itkin, T.; Kaufmann, K.B.; Gur-Cohen, S.; Ludin, A.; Lapidot, T. Fibroblast growth factor signaling promotes physiological bone remodeling and stem cell self-renewal. Curr. Opin. Hematol. 2013, 20, 237–244. [Google Scholar] [CrossRef]
- Canalis, E.; Varghese, S.; McCarthy, T.L.; Centrella, M. Role of platelet derived growth factor in bone cell function. Growth Regul. 1992, 2, 151–155. [Google Scholar]
- Mistry, A.S.; Mikos, A.G. Tissue engineering strategies for bone regeneration. Adv. Biochem. Eng. Biotechnol. 2005, 94, 1–22. [Google Scholar] [CrossRef]
- Lee, S.S.; Du, X.; Kim, I.; Ferguson, S.J. Scaffolds for bone-tissue engineering. Matter 2022, 5, 2722–2759. [Google Scholar] [CrossRef]
- Ravaglioli, A.; Krajewski, A. Bioceramics; Springer: Dordrecht, The Netherlands, 1992. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Mikos, A.G.; McIntire, L.V.; Anderson, J.M.; Babensee, J.E. Host response to tissue engineered devices. Adv. Drug Deliv. Rev. 1998, 33, 111–139. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef]
- Sadowska, J.M.; Genoud, K.J.; Kelly, D.J.; O’Brien, F.J. Bone biomaterials for overcoming antimicrobial resistance: Advances in non-antibiotic antimicrobial approaches for regeneration of infected osseous tissue. Mater. Today 2021, 46, 136–154. [Google Scholar] [CrossRef]
- Stedman, T.L. Stedman’s Medical Dictionary, 25th ed.; Williams & Wilkins: Baltimore, MD, USA, 1990. [Google Scholar]
- Cypher, T.J.; Grossman, J.P. Biological principles of bone graft healing. J. Foot Ankle Surg. 1996, 35, 413–417. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36, S20–S27. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.F.; Cheah, C.M.; Chua, C.K. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials 2003, 24, 2363–2378. [Google Scholar] [CrossRef]
- Hadjipanayi, E.; Mudera, V.; Brown, R.A. Close dependence of fibroblast proliferation on collagen scaffold matrix stiffness. J. Tissue Eng. Regen. Med. 2009, 3, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for bone tissue engineering: State of the art and new perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
- Gao, C.; Deng, Y.; Feng, P.; Mao, Z.; Li, P.; Yang, B.; Deng, J.; Cao, Y.; Shuai, C.; Peng, S. Current Progress in Bioactive Ceramic Scaffolds for Bone Repair and Regeneration. Int. J. Mol. Sci. 2014, 15, 4714–4732. [Google Scholar] [CrossRef]
- Ielo, I.; Calabrese, G.; De Luca, G.; Conoci, S. Recent Advances in Hydroxyapatite-Based Biocomposites for Bone Tissue Regeneration in Orthopedics. Int. J. Mol. Sci. 2022, 23, 9721. [Google Scholar] [CrossRef]
- Rizzi, S.C.; Heath, D.J.; Coombes, A.J.; Bock, N.; Textor, M.; Downes, S. Biodegradable polymer/hydroxyapatite composites: Surface analysis and initial attachment of human osteoblasts. J. Biomed. Mater. Res. 2001, 55, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, A.M.; Sahota, J.S.; Khan, Y.; Laurencin, C.T. A novel amorphous calcium phosphate polymer ceramic for bone repair: I. Synthesis and characterization. J. Biomed. Mater. Res. 2001, 58, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Samavedi, S.; Whittington, A.R.; Goldstein, A.S. Calcium phosphate ceramics in bone tissue engineering: A review of properties and their influence on cell behavior. Acta Biomater. 2013, 9, 8037–8045. [Google Scholar] [CrossRef]
- Baino, F.; Novajra, G.; Vitale-Brovarone, C. Bioceramics and Scaffolds: A Winning Combination for Tissue Engineering. Front. Bioeng. Biotechnol. 2015, 3, 202. [Google Scholar] [CrossRef]
- Mondal, S.; Pal, U.; Dey, A. Natural origin hydroxyapatite scaffold as potential bone tissue engineering substitute. Ceram. Int. 2016, 42, 18338–18346. [Google Scholar] [CrossRef]
- Jiao, X.; Sun, X.; Li, W.; Chu, W.; Zhang, Y.; Li, Y.; Wang, Z.; Zhou, X.; Ma, J.; Xu, C.; et al. 3D-Printed β-Tricalcium Phosphate Scaffolds Promote Osteogenic Differentiation of Bone Marrow-Deprived Mesenchymal Stem Cells in an N6-methyladenosine-Dependent Manner. Int. J. Bioprinting 2022, 8, 544. [Google Scholar] [CrossRef]
- Chen, Y.C.; Hsu, P.Y.; Tuan, W.H.; Chen, C.Y.; Wu, C.J.; Lai, P.L. Long-term in vitro degradation and in vivo evaluation of resorbable bioceramics. J. Mater. Sci. Mater. Med. 2021, 32, 13. [Google Scholar] [CrossRef]
- Wang, M. Developing bioactive composite materials for tissue replacement. Biomaterials 2003, 24, 2133–2151. [Google Scholar] [CrossRef] [PubMed]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of Scaffolds for Bone-Tissue Regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef] [PubMed]
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and Synthetic Polymers for Bone Scaffolds Optimization. Polymers 2020, 12, 905. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Solomon, K.L.; Zhang, X.; Pavlos, N.J.; Abel, T.; Willers, C.; Dai, K.; Xu, J.; Zheng, Q.; Zheng, M. In vitro evaluation of natural marine sponge collagen as a scaffold for bone tissue engineering. Int. J. Biol. Sci. 2011, 7, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Sukul, M.; Sahariah, P.; Lauzon, H.L.; Borges, J.; Másson, M.; Mano, J.F.; Haugen, H.J.; Reseland, J.E. In vitro biological response of human osteoblasts in 3D chitosan sponges with controlled degree of deacetylation and molecular weight. Carbohydr. Polym. 2021, 254, 117434. [Google Scholar] [CrossRef]
- Dorati, R.; DeTrizio, A.; Modena, T.; Conti, B.; Benazzo, F.; Gastaldi, G.; Genta, I. Biodegradable Scaffolds for Bone Regeneration Combined with Drug-Delivery Systems in Osteomyelitis Therapy. Pharmaceuticals 2017, 10, 96. [Google Scholar] [CrossRef]
- Shi, C.; Yuan, Z.; Han, F.; Zhu, C.; Li, B. Polymeric biomaterials for bone regeneration. Ann. Jt. 2016, 1, 27. [Google Scholar] [CrossRef]
- Yu, H.; Liu, H.; Shen, Y.; Ao, Q. Synthetic biodegradable polymer materials in the repair of tumor-associated bone defects. Front. Bioeng. Biotechnol. 2023, 11, 1096525. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.G.; Palermo, N.; Alibrandi, P.; Sciuto, E.L.; Del Gaudio, C.; Filardi, V.; Fazio, B.; Caccamo, A.; Oddo, S.; Calabrese, G.; et al. Physiologic Response Evaluation of Human Foetal Osteoblast Cells within Engineered 3D-Printed Polylactic Acid Scaffolds. Biology 2023, 12, 424. [Google Scholar] [CrossRef] [PubMed]
- Ledda, M.; Merco, M.; Sciortino, A.; Scatena, E.; Convertino, A.; Lisi, A.; Del Gaudio, C. Biological Response to Bioinspired Microporous 3D-Printed Scaffolds for Bone Tissue Engineering. Int. J. Mol. Sci. 2022, 23, 5383. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Lee, J.K.; Jung, J.W.; Baek, S.W.; Kim, J.H.; Heo, Y.; Kim, T.H.; Han, D.K. Promotion of Bone Regeneration Using Bioinspired PLGA/MH/ECM Scaffold Combined with Bioactive PDRN. Materials 2021, 14, 4149. [Google Scholar] [CrossRef] [PubMed]
- Gunatillake, P.A.; Adhikari, R. Biodegradable synthetic polymers for tissue engineering. Eur. Cell. Mater. 2003, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hermawan, H.; Ramdan, D.; Djuansjah, J.R.P.; Hermawan, H.; Ramdan, D.; Djuansjah, J.R.P. Metals for Biomedical Applications. In Biomedical Engineering—From Theory to Applications; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Liu, L.; Li, Z.; Liu, J. 3D printed Ti6Al4V bone scaffolds with different pore structure effects on bone ingrowth. J. Biol. Eng. 2021, 15, 4. [Google Scholar] [CrossRef]
- Badhe, R.V.; Akinfosile, O.; Bijukumar, D.; Barba, M.; Mathew, M.T. Systemic toxicity eliciting metal ion levels from metallic implants and orthopedic devices—A mini review. Toxicol. Lett. 2021, 350, 213–224. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, B.; Liu, G.; Tang, T.; Lu, E.; Xie, K.; Lan, C.; Liu, J.; Qin, Z.; Wang, L. Metal Material, Properties and Design Methods of Porous Biomedical Scaffolds for Additive Manufacturing: A Review. Front. Bioeng. Biotechnol. 2021, 9, 641130. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, S.; Mayer, J.; Wintermantel, E.; Leong, K.W. Biomedical applications of polymer-composite materials: A review. Compos. Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Calabrese, G.; Giuffrida, R.; Fabbi, C.; Figallo, E.; Lo Furno, D.; Gulino, R.; Colarossi, C.; Fullone, F.; Giuffrida, R.; Parenti, R.; et al. Collagen-Hydroxyapatite Scaffolds Induce Human Adipose Derived Stem Cells Osteogenic Differentiation In Vitro. PLoS ONE 2016, 11, e0151181. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, G.; Giuffrida, R.; Forte, S.; Salvatorelli, L.; Fabbi, C.; Figallo, E.; Gulisano, M.; Parenti, R.; Magro, G.; Colarossi, C.; et al. Bone augmentation after ectopic implantation of a cell-free collagen-hydroxyapatite scaffold in the mouse. Sci. Rep. 2016, 6, 36399. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Poot, A.A.; Grijpma, D.W. Advanced polymer-based composites and structures for biomedical applications. Eur. Polym. J. 2021, 149, 110388. [Google Scholar] [CrossRef]
- Pasqui, D.; Torricelli, P.; De Cagna, M.; Fini, M.; Barbucci, R. Carboxymethyl cellulose—Hydroxyapatite hybrid hydrogel as a composite material for bone tissue engineering applications. J. Biomed. Mater. Res. A 2014, 102, 1568–1579. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, L.; Liu, K.; Gao, F. Hydrogel scaffolds in bone regeneration: Their promising roles in angiogenesis. Front. Pharmacol. 2023, 14, 1050954. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert. Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, I.S.; Cho, T.H.; Lee, K.B.; Hwang, S.J.; Tae, G.; Noh, I.; Lee, S.H.; Park, Y.; Sun, K. Bone regeneration using hyaluronic acid-based hydrogel with bone morphogenic protein-2 and human mesenchymal stem cells. Biomaterials 2007, 28, 1830–1837. [Google Scholar] [CrossRef]
- Jo, S.; Kim, S.; Cho, T.H.; Shin, E.; Hwang, S.J.; Noh, I. Effects of recombinant human bone morphogenic protein-2 and human bone marrow-derived stromal cells on in vivo bone regeneration of chitosan-poly(ethylene oxide) hydrogel. J. Biomed. Mater. Res. Part A 2013, 101, 892–901. [Google Scholar] [CrossRef]
- D’Amora, U.; Ronca, A.; Scialla, S.; Soriente, A.; Manini, P.; Phua, J.W.; Ottenheim, C.; Pezzella, A.; Calabrese, G.; Raucci, M.G.; et al. Bioactive Composite Methacrylated Gellan Gum for 3D-Printed Bone Tissue-Engineered Scaffolds. Nanomaterials 2023, 13, 772. [Google Scholar] [CrossRef]
- Song, M.; Wang, J.; He, J.; Kan, D.; Chen, K.; Lu, J. Synthesis of Hydrogels and Their Progress in Environmental Remediation and Antimicrobial Application. Gels 2023, 9, 16. [Google Scholar] [CrossRef]
- Sun, D.; Wang, H.; Liu, J.; Wang, X.; Guo, H.; Xue, L.; Li, L.; Li, J.; Zhang, B.; Xue, Y.; et al. An enzyme cross-linked hydrogel as a minimally invasive arterial tissue sealing and anti-adhesion barrier. Nano Today 2022, 44, 101467. [Google Scholar] [CrossRef]
- Kim, E.S.; Ahn, E.H.; Dvir, T.; Kim, D.H. Emerging nanotechnology approaches in tissue engineering and regenerative medicine. Int. J. Nanomed. 2014, 9, 1–5. [Google Scholar] [CrossRef]
- Kolahalam, L.A.; Viswanath, I.V.K.; Diwakar, B.S.; Govindh, B.; Reddy, V.; Murthy, Y.L.N. Review on nanomaterials: Synthesis and applications. Mater. Today Proc. 2019, 18, 2182–2190. [Google Scholar] [CrossRef]
- Nikzamir, M.; Akbarzadeh, A.; Panahi, Y. An overview on nanoparticles used in biomedicine and their cytotoxicity. J. Drug Deliv. Sci. Technol. 2021, 61, 1773–2247. [Google Scholar] [CrossRef]
- Calabrese, G.; Petralia, S.; Fabbi, C.; Forte, S.; Franco, D.; Guglielmino, S.; Esposito, E.; Cuzzocrea, S.; Traina, F.; Conoci, S. Au, Pd and maghemite nanofunctionalized hydroxyapatite scaffolds for bone regeneration. Regen. Biomater. 2020, 7, 461–469. [Google Scholar] [CrossRef]
- Babuska, V.; Kasi, P.B.; Chocholata, P.; Wiesnerova, L.; Dvorakova, J.; Vrzakova, R.; Nekleionova, A.; Landsmann, L.; Kulda, V. Nanomaterials in Bone Regeneration. Appl. Sci. 2022, 12, 6793. [Google Scholar] [CrossRef]
- Abbasi, R.; Shineh, G.; Mobaraki, M.; Doughty, S.; Tayebi, L. Structural parameters of nanoparticles affecting their toxicity for biomedical applications: A review. J. Nanopart Res. 2023, 25, 43. [Google Scholar] [CrossRef]
- Kumar, A.; Gajraj, V.; Das, A.; Sen, D.; Xu, H.; Mariappan, C.R. Silver, Copper, Magnesium and Zinc Contained Electroactive Mesoporous Bioactive S53P4 Glass–Ceramics Nanoparticle for Bone Regeneration: Bioactivity, Biocompatibility and Antibacterial Activity. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2309–2321. [Google Scholar] [CrossRef]
- Yu, N.; Zhao, L.; Cheng, D.; Ding, M.; Lyu, Y.; Zhao, J.; Li, J. Radioactive Organic Semiconducting Polymer Nanoparticles for Multimodal Cancer Theranostics. J. Colloid. Interface Sci. 2022, 619, 219–228. [Google Scholar] [CrossRef]
- Wang, N.; Qi, D.; Liu, L.; Zhu, Y.; Liu, H.; Zhu, S. Fabrication of In Situ Grown Hydroxyapatite Nanoparticles Modified Porous Polyetheretherketone Matrix Composites to Promote Osteointegration and Enhance Bone Repair. Front. Bioeng. Biotechnol. 2022, 10, 831288. [Google Scholar] [CrossRef]
- Calabrese, G.; Petralia, S.; Franco, D.; Nocito, G.; Fabbi, C.; Forte, L.; Guglielmino, S.; Squarzoni, S.; Traina, F.; Conoci, S. A new Ag-nanostructured hydroxyapatite porous scaffold: Antibacterial effect and cytotoxicity study. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111394. [Google Scholar] [CrossRef]
- Ealia, S.A.M.; Saravanakumar, M.P. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032019. [Google Scholar] [CrossRef]
- Fan, L.; Ren, Y.; Emmert, S.; Vučković, I.; Stojanovic, S.; Najman, S.; Schnettler, R.; Barbeck, M.; Schenke-Layland, S.; Xiong, X. The Use of Collagen-Based Materials in Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 3744. [Google Scholar] [CrossRef]
- Liu, S.; Sun, Y.; Fu, Y.; Chang, D.; Fu, C.; Wang, G.; Liu, Y.; Tay, F.R.; Zhou, Y. Bioinspired Collagen-Apatite Nanocomposites for Bone Regeneration. J. Endod. 2016, 42, 1226–1232. [Google Scholar] [CrossRef]
- Patel, A.; Zaky, S.H.; Schoedel, K.; Li, H.; Sant, V.; Beniash, E.; Sfeir, C.; Stolz, D.B.; Sant, S. Design and Evaluation of Collagen-Inspired Mineral-Hydrogel Nanocomposites for Bone Regeneration. Acta Biomater. 2020, 112, 262–273. [Google Scholar] [CrossRef]
- Gresita, A.; Raja, I.; Petcu, E.; Hadjiargyrou, M. Collagen-Coated Hyperelastic Bone Promotes Osteoblast Adhesion and Proliferation. Materials 2023, 16, 6996. [Google Scholar] [CrossRef]
- Yasmin, R.; Shah, M.; Khan, S.A.; Ali, R. Gelatin Nanoparticles: A Potential Candidate for Medical Applications. Nanotechnol. Rev. 2017, 6, 191–207. [Google Scholar] [CrossRef]
- Loyo, C.; Cordoba, A.; Palza, H.; Canales, D.; Melo, F.; Vivanco, J.F.; Baier, R.V.; Millán, C.; Corrales, T.; Zapata, P.A. Effect of Gelatin Coating and GO Incorporation on the Properties and Degradability of Electrospun PCL Scaffolds for Bone Tissue Regeneration. Polymers 2023, 16, 129. [Google Scholar] [CrossRef]
- Chen, P.; Liu, L.; Pan, J.; Mei, J.; Li, C.; Zheng, Y. Biomimetic composite scaffold of hydroxyapatite/gelatin-chitosan core-shell nanofibers for bone tissue engineering. Mater. Sci. Eng. C 2019, 97, 325–335. [Google Scholar] [CrossRef]
- Li, X.; Xie, J.; Yuan, X.; Xia, Y. Coating electrospun poly(epsilon-caprolactone) fibers with gelatin and calcium phosphate and their use as biomimetic scaffolds for bone tissue engineering. Langmuir 2008, 24, 14145–14150. [Google Scholar] [CrossRef]
- Samadian, H.; Khastar, H.; Ehterami, A.; Salehi, M. Bioengineered 3D Nanocomposite Based on Gold Nanoparticles and Gelatin Nanofibers for Bone Regeneration: In Vitro and in Vivo Study. Sci. Rep. 2021, 11, 13877. [Google Scholar] [CrossRef] [PubMed]
- Breen, A.; O’Brien, T.; Pandit, A. Fibrin as a Delivery System for Therapeutic Drugs and Biomolecules. Tissue Eng. Part B Rev. 2009, 15, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Lee, J. Enhanced bone healing by improved fibrin-clot formation via fibrinogen adsorption on biphasic calcium phosphate granules. Clin. Oral. Implant. Res. 2015, 26, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Lamghari, M.; Almeida, C.R.; Oliveira, M.I.; Neves, N.; Ribeiro, A.C.; Barbosa, J.N.; Barros, R.; Maciel, J.; Martins, M.C.L.; et al. Adsorbed fibrinogen leads to improved bone regeneration and correlates with differences in the systemic immune response. Acta Biomater. 2013, 7, 7209–7217. [Google Scholar] [CrossRef] [PubMed]
- Noori, A.; Ashrafi, S.J.; Vaez-Ghaemi, R.; Hatamian-Zaremi, A.; Webster, T.J. A review of fibrin and fibrin composites for bone tissue engineering. Int. J. Nanomed. 2017, 12, 4937–4961. [Google Scholar] [CrossRef]
- Janmey, P.A.; Winer, J.P.; Weisel, J.W. Fibrin gels and their clinical and bioengineering applications. J. R. Soc. Interface. 2009, 6, 1–10. [Google Scholar] [CrossRef]
- Periyathambi, P.; Vedakumari, W.S.; Kumar Baskar, S.; Bojja, S.; Sastry, T.P. Osteogenic potency of magnetic fibrin nanoparticles—A novel perspective in bone tissue engineering. Mater. Lett. 2015, 139, 108–111. [Google Scholar] [CrossRef]
- George, M.; Abraham, E.T. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—A review. J. Control Rel. 2006, 114, 1–14. [Google Scholar] [CrossRef]
- Li, T.; Shi, X.W.; Du, Y.M.; Tang, Y.M. Quaternized Chitosan/Alginate Nanoparticles for Protein Delivery. J. Biomed. Mat. Res. Part A 2007, 83, 383–390. [Google Scholar] [CrossRef]
- Benedini, L.; Placente, D.; Pieroni, O.; Messina, P. Assessment of Synergistic Interactions on Self-Assembled Sodium Alginate/Nano-Hydroxyapatite Composites: To the Conception of New Bone Tissue Dressings. Colloid. Polym. Sci. 2017, 295, 2109–2121. [Google Scholar] [CrossRef]
- Chae, T.; Yang, H.; Moon, H.; Troczynski, T.; Ko, F.K. Biomimetically Mineralized Alginate Nanocomposite Fibers for Bone Tissue Engineering: Mechanical Properties and in Vitro Cellular Interactions. ACS Appl. Bio Mater. 2020, 3, 6746–6755. [Google Scholar] [CrossRef] [PubMed]
- Barros, J.; Ferraz, M.P.; Azeredo, J.; Fernandes, M.H.; Gomes, P.S.; Monteiro, F.G. Alginate-nanohydroxyapatite hydrogel system: Optimizing the formulation for enhanced bone regeneration. Mater. Sci. Eng. 2019, 105, 109985. [Google Scholar] [CrossRef] [PubMed]
- Benedini, L.; Laiuppa, J.; Santillán, G.; Baldini, M.; Messina, P. Antibacterial alginate/nano-hydroxyapatite composites for bone tissue engineering: Assessment of their bioactivity, biocompatibility, and antibacterial activity. Mater. Sci. Eng. 2020, 115, 11110. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Dargah, M.S.; Azar, M.H.; Alizadeh, R.; Mahdavi, F.S.; Sayedain, S.S.; Kaviani, A.; Asadollahi, M.; Azami, M.; Beheshtizadeh, N. Enhanced Bone Tissue Regeneration Using a 3D-Printed Poly(Lactic Acid)/Ti6Al4V Composite Scaffold with Plasma Treatment Modification. Sci. Rep. 2023, 13, 3139. [Google Scholar] [CrossRef] [PubMed]
- Torres-Giner, S.; Gimeno-Alcañiz, W.J.; Ocio, M.J.; Lagaron, J.M. Optimization of Electrospun Polylactide-Based Ultrathin Fibers for Osteoconductive Bone Scaffolds. J. Appl. Polym. Sci. 2011, 122, 914–925. [Google Scholar] [CrossRef]
- Schofer, M.D.; Fuchs-Winkelmann, S.; Gräbedünkel, C.; Wack, C.; Dersch, R.; Rudisile, M.; Wendorff, J.H.; Greiner, A.; Paletta, J.R.; Boudriot, U. Influence of Poly(L-Lactic Acid) Nanofibers and BMP-2-Containing Poly(L-Lactic Acid) Nanofibers on Growth and Osteogenic Differentiation of Human Mesenchymal Stem Cells. Sci. World J. 2018, 8, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Chiu, J.B.; Liu, C.; Hsiao, B.S.; Chu, B.; Hadjiargyrou, H. Functionalization of Poly(L-Lactide) Nanofibrous Scaffolds with Bioactive Collagen Molecules. J. Biomed. Mater. Res. Part A 2007, 83, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wong, H.M.; Yeung, K.W.K.; Tjong, S.C. Novel Electrospun Polylactic Acid Nanocomposite Fiber Mats with Hybrid Graphene Oxide and Nanohydroxyapatite Reinforcements Having Enhanced Biocompatibility. Polymers 2016, 8, 287. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Cheng, D.; Niu, B.; Wang, X.; Wu, X.; Wang, A. Properties of Poly (Lactic-Co-Glycolic Acid) and Progress of Poly (Lactic-Co-Glycolic Acid)-Based Biodegradable Materials in Biomedical Research. Pharmaceuticals 2023, 16, 454. [Google Scholar] [CrossRef]
- Loureiro, J.A.; Pereira, M.C. PLGA Based Drug Carrier and Pharmaceutical Applications: The Most Recent Advances. Pharmaceutics 2020, 12, 903. [Google Scholar] [CrossRef]
- Rahman, C.V.; Ben-David, D.; Dhillon, A.; Kuhn, G.; Gould, T.W.A.; Müller, R.; Rose, F.R.A.J.; Shakesheff, K.M.; Livne, E. Controlled Release of BMP-2 from a Sintered Polymer Scaffold Enhances Bone Repair in a Mouse Calvarial Defect Model. J. Tissue Eng. Regen. Med. 2014, 8, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Évora, M.; García-Pizarro, E.; del Rosario, C.; Pérez-López, J.; Reyes, R.; Delgado, A.; Rodríguez-Rey, J.C.; Évora, C. Smurf1 Knocked-down, Mesenchymal Stem Cells and BMP-2 in an Electrospun System for Bone Regeneration. Biomacromolecules 2014, 15, 1311–1322. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Selph, S.; McDonagh, M.; Peterson, K.; Tiwari, A.; Chou, R.; Helfand, M. Effectiveness and Harms of Recombinant Human Bone Morphogenetic Protein-2 in Spine Fusion: A Systematic Review and Meta-Analysis. Ann. Intern. Med. 2013, 158, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Zhu, Y.; Qiu, J.; Guan, H.; Li, L.; Zheng, S.; Dong, X.; Xiao, J. Synthesis and characterization of UPPE-PLGA-rhBMP2 scaffolds for bone regeneration. Curr. Med Sci. 2012, 32, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Hamidi, M. Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metab. Pers. Ther. 2019, 34, 20180032. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Wu, Z.-X.; Zhao, Z.; Liu, J.; Sun, K.; Guo, C.; Wang, H.; Wu, Z. Engineering of Bone- and CD44-Dual-Targeting Redox-Sensitive Liposomes for the Treatment of Orthotopic Osteosarcoma. ACS Appl. Mater. Interfaces 2019, 11, 7357–7368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fan, J.; Lee, C.S.; Kim, S.; Chen, C.; Aghaloo, T.; Lee, M. Apatite-binding nanoparticulate agonist of hedgehog signaling for bone repair. Adv. Funct. Mater. 2020, 30, 1909218. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Liu, L.; Xiang, Y.; Lu, Y.; Deng, L.; Zhang, H.; Santos, H.A.; Cui, W. Advanced liposome-loaded scaffolds for therapeutic and tissue engineering applications. Biomaterials 2020, 232, 119706. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Babadağli, M.E.; Uludağ, H. Bisphosphonate-derivatized liposomes to control drug release from collagen/hydroxyapatite scaffolds. Mol. Pharm. 2011, 8, 1025–1034. [Google Scholar] [CrossRef]
- Lee, C.S.; Hsu, G.C.Y.; Sono, T.; Lee, M.; James, A.W. Development of a Biomaterial Scaffold Integrated with Osteoinductive Oxysterol Liposomes to Enhance Hedgehog Signaling and Bone Repair. Mol. Pharm. 2021, 18, 1677–1689. [Google Scholar] [CrossRef]
- Lee, C.S.; Kim, S.; Fan, J.; Hwang, H.S.; Aghaloo, T.; Lee, M. Smoothened Agonist Sterosome Immobilized Hybrid Scaffold for Bone Regeneration. Sci. Adv. 2020, 6, eaaz7822. [Google Scholar] [CrossRef] [PubMed]
- Cottrill, E.; Lazzari, J.; Pennington, Z.; Ehresman, J.; Schilling, A.; Dirckx, N.; Theodore, N.; Sciubba, D.; Witham, T. Oxysterols as promising small molecules for bone tissue engineering: Systematic review. World J. Orthop. 2020, 11, 328–344. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef]
- Oliveira, I.M.; Carvalho, M.R.; Reis, R.L.; Oliveira, J.M. Chapter 18—Dendrimers in tissue engineering. In Dendrimer-Based Nanotherapeutics; Kesharwani, P., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 327–336. [Google Scholar] [CrossRef]
- Kurian, A.M.; Mandakhbayar, N.; Singh, R.K.; Lee, J.W.; Jin, G.; Kim, H.W. Multifunctional dendrimer@nanoceria engineered GelMA hydrogel accelerates bone regeneration through orchestrated cellular responses. Mater. Today Bio 2023, 20, 100664. [Google Scholar] [CrossRef]
- Santos, J.L.; Oramas, E.; Pêgo, A.P.; Granja, P.L.; Tomás, H. Osteogenic Differentiation of Mesenchymal Stem Cells Using PAMAM Dendrimers as Gene Delivery Vectors. J. Control Release 2009, 134, 141–148. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Kotobuki, N.; Marques, A.P.; Pirraco, R.P.; Benesch, J.; Hirose, M.; Costa, S.A.; Mano, J.F.; Ohgushi, H.; Reis, R.L. Surface engineered carboxymethylchitosan/poly(amidoamine) dendrimer nanoparticles for intracellular targeting. Adv. Funct. Mater. 2008, 18, 1840–1853. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Sousa, R.A.; Kotobuki, N.; Tadokoro, M.; Hirose, M.; Mano, J.F.; Reis, R.L.; Ohgushi, H. The osteogenic differentiation of rat bone marrow stromal cells with dexamethasone-loaded carboxymethylchitosan/poly(amidoamine) dendrimer nanoparticles. Biomaterials 2009, 30, 804–813. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Sousa, R.A.; Kotobuki, N.; Malafaya, P.B.; Hirose, M.; Mano, J.F.; Reis, R.L.; Ohgushi, H. Dexamethasone-loaded carboxymethylchitosan/poly(amidoamine) dendrimer nanoparticles enhances bone formation in vivo. Tissue Eng, Part A 2008, 14, 721. [Google Scholar]
- Khalid, K.; Tan, X.; Mohd Zaid, H.F.; Tao, Y.; Chew, C.L.; Chu, D.T.; Lam, M.K.; Ho, Y.C.; Lim, J.W.; Wei, L.C. Advanced in developmental organic and inorganic nanomaterial: A review. Bioengineered 2020, 11, 328–355. [Google Scholar] [CrossRef]
- Huang, Y.W.; Cambre, M.; Lee, H.J. The toxicity of nanoparticles depends on multiple molecular and physicochemical mechanisms. Int. J. Mol. Sci. 2017, 18, 2702. [Google Scholar] [CrossRef]
- Zoroddu, M.A.; Medici, S.; Ledda, A.; Nurchi, V.M.; Lachowicz, J.I.; Peana, M. Toxicity of nanoparticles. Curr. Med. Chem. 2014, 21, 3837–3853. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Hagens, W.I.; Krystek, P.; Burger, M.C.; Sips, A.J.A.M.; Geertsma, R.E. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials 2008, 29, 1912–1919. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Jin, S.; Ma, X.; Xue, X.; Yang, K.; Kumar, A.; Wang, P.C.; Zhang, J.; Hu, Z.; Liang, X.-J. Ultrasmall gold nanoparticles as carriers for nucleus-based gene therapy due to size-dependent nuclear entry. ACS Nano 2014, 8, 5852–5862. [Google Scholar] [CrossRef] [PubMed]
- Champion, J.A.; Mitragotri, S. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. USA 2006, 103, 4930–4934. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ng, S.; Heng, B.C.; Guo, J.; Ma, L.; Tan, T.T.Y. Cytotoxicity of hydroxyapatite nanoparticles is shape and cell dependent. Arch. Toxicol. 2013, 87, 1037–1052. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, C.; Yang, D.; Zhang, H.; Xi, Z. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: The role of particle size, shape, and composition. J. Appl. Toxicol. 2009, 29, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, G.; Franco, D.; Petralia, S.; Monforte, F.; Condorelli, G.G.; Squarzoni, S.; Traina, F.; Conoci, S. Dual-Functional Nano-Functionalized Titanium Scaffolds to Inhibit Bacterial Growth and Enhance Osteointegration. Nanomaterials 2021, 11, 2634. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.; Calabrese, G.; Guglielmino, S.P.P.; Conoci, S. Metal-Based Nanoparticles: Antibacterial Mechanisms and Biomedical Application. Microorganisms 2022, 10, 1778. [Google Scholar] [CrossRef] [PubMed]
- Eivazzadeh-Keihan, R.; Noruzi, E.B.; Chenab, K.K.; Jafari, A.; Radinekiyan, F.; Hashemi, S.M.; Ahmadpour, F.; Behboudi, A.; Mosafer, J.; Mokhtarzadeh, A.; et al. Metal-based nanoparticles for bone tissue engineering. J. Tissue Eng. Regen. Med. 2020, 14, 1687–1714. [Google Scholar] [CrossRef]
- Franco, D.; Calabrese, G.; Petralia, S.; Neri, G.; Corsaro, C.; Forte, L.; Squarzoni, S.; Guglielmino, S.; Traina, F.; Fazio, E.; et al. Antimicrobial Effect and Cytotoxic Evaluation of Mg-Doped Hydroxyapatite Functionalized with Au-Nano Rods. Molecules 2021, 26, 1099. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, S.; García, A.; González-Jiménez, A.; Vallet-Regí, M. Antibacterial effect of 3D printed mesoporous bioactive glass scaffolds doped with metallic silver nanoparticles. Acta Biomater. 2023, 155, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.; Ting, K.; Zhang, X.; Soo, C.; Zheng, Z. Current Development of Silver Nanoparticle Preparation, Investigation, and Application in the Field of Medicine. J. Nanomater. 2015, 2015, 696918. [Google Scholar] [CrossRef]
- Lu, H.; Liu, Y.; Guo, J.; Wu, H.; Wang, J.; Wu, G. Biomaterials with antibacterial and osteoinductive properties to repair infected bone defects. Int. J. Mol. Sci. 2016, 17, 334. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Waibhaw, G.; Saxena, V.; Pandey, L.M. Nano-biocomposite scaffolds of chitosan, carboxymethyl cellulose and silver nanoparticle modified cellulose nanowhiskers for bone tissue engineering applications. Int. J. Biol. Macromol. 2018, 111, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Qing, T.; Mahmood, M.; Zheng, Y.; Biris, A.S.; Shi, L.; Casciano, D.A. A genomic characterization of the influence of silver nanoparticles on bone differentiation in MC3T3-E1 cells. J. Appl. Toxicol. 2018, 38, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Lee, P.; Lui, V.C.H.; Chen, Y.; Liu, X.; Lok, C.N.; To, M.; Yeung, K.W.K.; Wong, K.K.Y. Silver nanoparticles promote osteogenesis of mesenchymal stem cells and improve bone fracture healing in osteogenesis mechanism mouse model. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1949–1959. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.M.; Lu, X.; Wang, K.F.; Meng, F.Z.; Jiang, O.P.; Zhang, H.P.; Zhi, W.; Fang, L.M. Silver nanoparticles and growth factors incorporated hydroxyapatite coatings on metallic implant surfaces for enhancement of osteoinductivity and antibacterial properties. ACS Appl. Mater. Interfaces 2014, 6, 8580–8589. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.C.; Chang, C.H.; Chang, Y.; Hsieh, J.H.; Ueng, S.W. Beneficial Effect of TaON-Ag Nanocomposite Titanium on Antibacterial Capacity in Orthopedic Application. Int. J. Nanomed. 2020, 15, 7889–7900. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, D.; Hefnawy, A.; Al-Wakeel, E.; El-Fallal, A.; El-Sherbiny, I.M. New biodegradable nanoparticles-in-nanofibers based membranes for guided periodontal tissue and bone regeneration with enhanced antibacterial activity. J. Adv. Res. 2020, 10, 51–62. [Google Scholar] [CrossRef]
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef]
- Vial, S.; Reis, R.L.; Oliveira, J.M. Recent advances using gold nanoparticles as a promising multimodal tool for tissue engineering and regenerative medicine. Curr. Opin. Solid. St. M 2017, 21, 92–112. [Google Scholar] [CrossRef]
- Yi, C.; Liu, D.; Fong, C.-C.; Zhang, J.; Yang, M. Gold nanoparticles promote osteogenic differentiation of mesenchymal stem cells through p38 MAPK pathway. ACS Nano 2010, 4, 6439–6448. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Song, M.S.; Ryu, P.D.; Lam, A.T.N.; Joo, S.W.; Lee, S.Y. Gold nanoparticles promote osteogenic differentiation in human adipose-derived mesenchymal stem cells through the Wnt/β-catenin signaling pathway. Int. J. Nanomed. 2015, 10, 4383–4392. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, D.; Zhang, J.; Fong, C.; Yang, M. Gold nanoparticles stimulate differentiation and mineralization of primary osteoblasts through the ERK/MAPK signaling pathway. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 42, 70–77. [Google Scholar] [CrossRef]
- Lee, D.; Heo, D.N.; Kim, H.-J.; Ko, W.K.; Lee, S.J.; Heo, M.; Bang, J.B.; Lee, J.B.; Hwang, D.S.; Do, S.H.; et al. Inhibition of osteoclast differentiation and bone resorption by bisphosphonate-conjugated gold nanoparticles. Sci. Rep. 2016, 6, 27336. [Google Scholar] [CrossRef]
- Heo, D.N.; Ko, W.-K.; Bae, M.S.; Lee, J.B.; Lee, D.W.; Byun, W.; Lee, C.H.; Kim, E.C.; Jung, B.Y.; Kwon, K. Enhanced bone regeneration with a gold nanoparticle–hydrogel complex. J. Mater. Chem. B 2014, 2, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, P.; Mao, H.; Zhang, Y.; Zheng, L.; Yu, P.; Guo, Z.; Li, L.; Jiang, Q. PEGylated gold nanoparticles promote Osteogenic differentiation in in vitro and in vivo systems. Mater. Des. 2020, 197, 109231. [Google Scholar] [CrossRef]
- Pan, Y.; Neuss, S.; Leifer, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-dependent cytotoxicity of gold nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef]
- Soenen, S.J.; Manshian, B.; Montenegro, J.M.; Amin, F.; Meermann, B.; Thiron, T.; Cornelissen, M.; Vanhaecke, F.; Doak, S.; Parak, W.J. Cytotoxic effects of gold nanoparticles: A multiparametric study. ACS Nano 2012, 6, 5767–5783. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, J.J.; Zhang, J.; Wang, X.; Kawazoe, N.; Chen, G. Gold nanoparticle size and shape influence on osteogenesis of mesenchymal stem cells. Nanoscale 2016, 8, 7992–8007. [Google Scholar] [CrossRef]
- Ko, W.K.; Heo, D.N.; Moon, H.J.; Lee, S.J.; Bae, M.S.; Lee, J.B.; Sun, I.C.; Jeon, H.B.; Park, H.K.; Kwon, I.K. The effect of gold nanoparticle size on osteogenic differentiation of adipose-derived stem cells. J. Colloid. Interface Sci. 2015, 438, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.T.V.; Huynh, T.C.; Manivasagan, P.; Mondal, S.; Oh, J. An Up-To-Date Review on Biomedical Applications of Palladium Nanoparticles. Nanomaterials 2019, 10, 66. [Google Scholar] [CrossRef]
- Bharathiraja, S.; Bui, N.Q.; Manivasagan, P.; Moorthy, M.S.; Mondal, S.; Seo, H.; Phuoc, N.T.; Vy Phan, T.T.; Kim, H.; Lee, K.D. Multimodal tumor-homing chitosan oligosaccharide-coated biocompatible palladium nanoparticles for photo-based imaging and therapy. Sci. Rep. 2018, 8, 500. [Google Scholar] [CrossRef]
- Phan, T.T.V.; Hoang, G.; Nguyen, V.T.; Nguyen, T.P.; Kim, H.H.; Mondal, S.; Manivasagan, P.; Moorthy, M.S.; Lee, K.D.; Junghwan, O. Chitosan as a stabilizer and size-control agent for synthesis of porous flower-shaped palladium nanoparticles and their applications on photo-based therapies. Carbohydr. Polym. 2019, 205, 340–352. [Google Scholar] [CrossRef]
- Azizi, S.; Shahri, M.M.; Rahman, H.S.; Rahim, R.A.; Rasedee, A.; Mohamad, R. Green synthesis palladium nanoparticles mediated by white tea (Camellia sinensis) extract with antioxidant, antibacterial, and antiproliferative activities toward the human leukemia (MOLT-4) cell line. Int. J. Nanomed. 2017, 12, 8841–8853. [Google Scholar] [CrossRef]
- Fang, G.; Li, W.; Shen, X.; Perez-Aguilar, J.M.; Chong, Y.; Gao, X.; Chai, Z.; Chen, C.; Ge, C.; Zhou, R. Differential Pd-nanocrystal facets demonstrate distinct antibacterial activity against Gram-positive and Gram-negative bacteria. Nat. Commun. 2018, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Shin, W.; Kang, K.; Choi, M.-H.; Kim, Y.-J.; Kim, Y.-K.; Min, D.-H.; Jang, H. Revisiting of Pd Nanoparticles in Cancer Treatment: All-Round Excellence of Porous Pd Nanoplates in Gene-Thermo Combinational Therapy. ACS Appl. Mater. Interfaces 2018, 10, 13819–13828. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.T.; Dawson, J.C.; Macleod, K.G.; Rybski, W.; Fraser, C.; Torres-Sánchez, C.; Patton, E.E.; Bradley, M.; Carragher, N.O.; Unciti-Broceta, A. Extracellular palladium-catalysed dealkylation of 5-fluoro-1-propargyl-uracil as a bioorthogonally activated prodrug approach. Nat. Commun. 2014, 5, 3277. [Google Scholar] [CrossRef]
- Ismail, E.; Mabrouk, M.; Zeinab, A.; Nermeen, S.; Beherei, A.H. Evaluation of innovative polyvinyl alcohol/alginate/green palladium nanoparticles composite scaffolds: Effect on differentiated human dental pulp stem cells into osteoblasts. J. Mech. Behav. Biomed. Mater. 2023, 140, 105700. [Google Scholar] [CrossRef]
- Balaji, M.; Nithya, P.; Mayakrishnan, A.; Jegatheeswaran, S.; Selvam, S.; Cai, Y.; Yao, J.; Sundrarajan, M. Fabrication of palladium nano-particles anchored polypyrrole functionalized reduced graphene oxide nanocomposite for antibiofilm associated orthopedic tissue engineering. Appl. Surf. Sci. 2020, 510, 145403. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. Trace elements in human physiology and pathology. Copper. Biomed. Pharmacother. 2003, 57, 386–398. [Google Scholar] [CrossRef]
- Usman, M.S.; Zowalaty, M.E.E.; Shameli, K.; Zainuddin, N.; Salama, M.; Ibrahim, N.A. Synthesis, characterization, and antimicrobial properties of copper nanoparticles. Int. J. Nanomed. 2013, 8, 4467–4479. [Google Scholar] [CrossRef]
- Gérard, C.; Bordeleau, L.-J.; Barralet, J.; Doillon, C.J. The stimulation of angiogenesis and collagen deposition by copper. Biomaterials 2010, 31, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.P.; Ríos, S.; González, M. Modulation of the proliferation and differentiation of human mesenchymal stem cells by copper. J. Cell Biochem. 2002, 85, 92–100. [Google Scholar] [CrossRef]
- Tripathi, A.; Saravanan, S.; Pattnaik, S.; Moorthi, A.; Partridge, N.C.; Selvamurugan, N. Bio-composite scaffolds containing chitosan/nano-hydroxyapatite/nano-copper-zinc for bone tissue engineering. Int. J. Biol. Macromol. 2012, 50, 294–299. [Google Scholar] [CrossRef]
- Martín Vilardell, A.; Cantillo Alzamora, V.; Bauso, L.V.; Madrid, C.; Krakhmalev, P.; Albu, M.; Yadroitsava, I.; Yadroitsev, I.; Garcia-Giralt, N. Effect of Heat Treatment on Osteoblast Performance and Bactericidal Behavior of Ti6Al4V(ELI)-3at.%Cu Fabricated by Laser Powder Bed Fusion. J. Funct. Biomater. 2023, 14, 63. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Y.; Xu, M.; Han, P.; Chen, L.; Chang, J.; Xiao, Y. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials 2012, 34, 422. [Google Scholar] [CrossRef]
- Ewald, A.; Kappel, C.; Vorndran, E.; Moseke, C.; Gelinsky, M.; Gbureck, U. The effect of Cu (II)-loaded brushite scaffolds on growth and activity of osteoblastic cells. J. Biomed. Mate. Res. A 2012, 100, 2392. [Google Scholar] [CrossRef] [PubMed]
- Grigore, M.E.; Biscu, E.R.; Holban, A.M.; Gestal, M.C.; Grumezescu, A.M. Methods of synthesis, properties and biomedical applications of CuO nanoparticles. Pharmaceuticals 2016, 9, 75. [Google Scholar] [CrossRef]
- Sahmani, S.; Shahali, M.; Nejad, M.G.; Khandan, A.; Aghdam, M.M.; Saber-Samandari, S. Effect of copper oxide nanoparticles on electrical conductivity and cell viability of calcium phosphate scaffolds with improved mechanical strength for bone tissue engineering. Eur. Phys. J. Plus 2019, 134, 7. [Google Scholar] [CrossRef]
- Huang, T.; Yan, G.; Guan, M. Zinc homeostasis in bone: Zinc transporters and bone diseases. Int. J. Mol. Sci. 2020, 21, 1236. [Google Scholar] [CrossRef]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef] [PubMed]
- Krol, A.; Pomastowski, P.; Rafinska, K.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid. Interface Sci. 2017, 249, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Hanley, C.; Layne, J.; Punnoose, A. Preferential killing of cancer cells and activated human T cells using ZnO nanoparticles. Nanotechnology 2008, 19, 295103. [Google Scholar] [CrossRef]
- Zalama, E.; Karrouf, G.; Rizk, A.; Salama, B.; Samy, A. Does zinc oxide nanoparticles potentiate the regenerative effect of platelet-rich fibrin in healing of critical bone defect in rabbits? BMC Vet. Res. 2022, 18, 130. [Google Scholar] [CrossRef] [PubMed]
- Khader, A.; Arinzeh, T.L. Biodegradable zinc oxide composite scaffolds promote osteochondral differentiation of mesenchymal stem cells. Biotechnol. Bioeng. 2020, 117, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Maimaiti, B.; Zhang, N.; Yan, L. Stable ZnO-doped hydroxyapatite nanocoating for anti-infection and osteogenic on titanium. Colloids Surf. B Biointerfaces 2020, 186, 110731. [Google Scholar] [CrossRef]
- Shen, X.; Hu, Y.; Xu, G.; Chen, W.; Xu, K.; Ran, Q.; Ma, P.; Zhang, Y.; Li, J.; Cai, K. Regulation of the biological functions of osteoblasts and bone formation by zn-incorporated coating on microrough titanium. ACS Appl. Mater. Interfaces 2014, 6, 16426–16440. [Google Scholar] [CrossRef] [PubMed]
- Shitole, A.A.; Raut, P.W.; Sharma, N.; Giram, P.; Khandwekar, A.P.; Garnaik, B. Electrospun polycaprolactone/hydroxyapatite/ZnO nanofibers as potential biomaterials for bone tissue regeneration. J. Mater. Sci. Mater. Med. 2019, 30, 51. [Google Scholar] [CrossRef]
- Calabrese, G.; De Luca, G.; Franco, D.; Morganti, D.; Rizzo, M.G.; Bonavita, A.; Neri, G.; Fazio, E.; Neri, F.; Fazio, B.; et al. Structural and antibacterial studies of novel ZnO and ZnxMn(1−x)O nanostructured titanium scaffolds for biomedical applications. Biomater. Adv. 2023, 145, 213193. [Google Scholar] [CrossRef]
- Att, W.; Hori, N.; Takeuchi, M.; Ouyang, J.; Yang, Y.; Anpo, M.; Ogawa, T. Time-dependent degradation of titanium osteocon- ductivity: An implication of biological aging of implant materials. Biomaterials 2009, 30, 5352–5363. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. Res. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Chen, M.; Yang, L.; Zhang, L.; Han, Y.; Lu, Z.; Qin, G.; Zhang, E. Effect of nano/micro-Ag compound particles on the bio-corrosion, antibacterial properties and cell biocompatibility of Ti-Ag alloys. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 906–917. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.; Hamzah, E.; Low, H.; Kasiri-Asgarani, M.; Farahany, S.; Akbari, E.; Cho, M. Fabrication of biodegradable Zn-Al-Mg alloy: Mechanical properties, corrosion behavior, cytotoxicity, and antibacterial activities. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 73, 215–219. [Google Scholar] [CrossRef]
- Ramires, J.P.A.; Romito, A.; Cosentino, F.; Milella, E. The influence of titania/hydroxyapatite composite coatings on in vitro osteoblasts behaviour. Biomaterials 2001, 22, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Li, Y.; Abdullah, A.O.; Wang, W.; Qi, M.; Liu, Y. Micro/nano-hierarchical structured TiO2 coating on titanium by micro- arc oxidation enhances osteoblast adhesion and differentiation. R. Soc. Open Sci. 2019, 6, 182031. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, N.; Ressler, A.; Hussainova, I. Bioactive Ceramic Scaffolds for Bone Tissue Engineering by Powder Bed Selective Laser Processing: A Review. Materials 2021, 14, 5338. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.S.; Cui, F.Z.; Zhang, W.; Feng, Q.L. Hierarchically Biomimetic Bone Scaffold Materials: Nano-HA/Collagen/PLA Composite. J. Biomed. Mat. Res. Part B Appl. Biomater. 2004, 69, 158–165. [Google Scholar] [CrossRef]
- Niaza, K.V.; Senatov, F.S.; Kaloshkin, S.D.; Maksimkin, A.V.; Chukov, D.I. «3D-printed scaffolds based on PLA/HA nanocomposites for trabecular bone reconstruction. J. Phys. Conf. Ser. 2017, 741, 012068. [Google Scholar] [CrossRef]
- Sattary, M.; Rafienia, M.; Kazemi, M.; Salehi, H.; Mahmoudzadeh, M. Promoting effect of nano hydroxyapatite and vitamin D3 on the osteogenic differentiation of human adipose-derived stem cells in polycaprolactone/gelatin scaffold for bone tissue engineering. Mater. Sci. Eng. 2019, 97, 141–155. [Google Scholar] [CrossRef]
- Ronca, D.; Langella, F.; Chierchia, M.; D’Amora, U.; Russo, T.; Domingos, M.; Gloria, A.; Bartolo, P.; Ambrosio, L. Bone Tissue Engineering: 3D PCL-Based Nanocomposite Scaffolds with Tailored Properties. Procedia CIRP 2016, 49, 51–54. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Miyaji, H.; Nishida, E.; Kawamoto, K.; Miyata, S.; Takita, H.; Akasaka, T.; Fugetsu, B.; Iwanaga, T.; Hongo, H.; et al. Dose Effects of Beta-Tricalcium Phosphate Nanoparticles on Biocompatibility and Bone Conductive Ability of Three-Dimensional Collagen Scaffolds. Dent. Mater. J. 2017, 36, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Shi, J.; Liu, Y.; Si, J.; Yuan, Y.; Liu, C. A Mechanically Robust and Flexible PEGylated Poly(Glycerol Sebacate)/β-TCP Nanoparticle Composite Membrane for Guided Bone Regeneration. J. Mater. Chem. B 2019, 7, 3279–3290. [Google Scholar] [CrossRef]
- Jing, T.; Liu, N.Y.; Xu, L.; Chen, C.; Liu, F. The Incorporation of β-Tricalcium Phosphate Nanoparticles within Silk Fibroin Composite Scaffolds for Enhanced Bone Regeneration: An in Vitro and in Vivo Study. J. Biomater. Appl. 2022, 36, 1567–1578. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Botelho, M.G.; Dorozhkin, S.V. Biphasic calcium phosphates bioceramics (HA/TCP): Concept, physicochemical properties and the impact of standardization of study protocols in biomaterials research. Mater. Sci. Eng. 2017, 71, 1293–1312. [Google Scholar] [CrossRef]
- Nie, L.; Wu, Q.; Long, H.; Hu, K.; Li, P.; Wang, C.; Sun, M.; Dong, J.; Wei, X.; Suo, J.; et al. Development of Chitosan/Gelatin Hydrogels Incorporation of Biphasic Calcium Phosphate Nanoparticles for Bone Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2019, 30, 1636–1657. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kawazoe, N.; Chen, G. Preparation of dexamethasone-loaded biphasic calcium phosphate nanoparticles/collagen porous composite scaffolds for bone tissue engineering. Acta Biomater. 2018, 67, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Touya, N.; Washio, A.; Kitamura, C.; Naveau, A.; Tabata, Y.; Devillard, R.; Kérourédan, O. In Vivo Application of Silica-Derived Inks for Bone Tissue Engineering: A 10-Year Systematic Review. Bioengineering 2022, 9, 388. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Dheen, S.T.; Fuh, J.Y.H.; Kumar, A.S. A Review of Multi-Functional Ceramic Nanoparticles in 3D Printed Bone Tissue Engineering. Bioprinting 2021, 23, e00146. [Google Scholar] [CrossRef]
- Beck, G.R.B.; Ha, S.W.; Camalier, C.E.; Yamaguchi, M.; Li, Y.; Lee, J.K.; Weitzmann, M.N. Bioactive silica-based nanoparticles stimulate bone-forming osteoblasts, suppress bone-resorbing osteoclasts, and enhance bone mineral density in vivo. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 793–803. [Google Scholar] [CrossRef]
- Nandi, S.K.; Fielding, G.; Banerjee, D.; Bandyopadhyay, A.; Bose, S. 3D-Printed β-TCP Bone Tissue Engineering Scaffolds: Effects of Chemistry on in Vivo Biological Properties in a Rabbit Tibia Model. J. Mater. Res. 2018, 33, 1939–1947. [Google Scholar] [CrossRef]
- Bartkowiak, A.; Suchanek, K.; Menaszek, E.; Szaraniec, B.; Lekki, J.; Perzanowski, M.; Marszałek, M. Biological effect of hydrothermally synthesized silica nanoparticles within crystalline hydroxyapatite coatings for titanium implants. Mater. Sci. Eng. 2018, 82, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, R.P.; Cicha, I.; Alexiou, C. Iron Oxide Nanoparticles in Regenerative Medicine and Tissue Engineering. Nanomaterials 2021, 11, 2337. [Google Scholar] [CrossRef]
- Fan, D.; Wang, Q.; Zhu, T.; Wang, H.; Liu, B.; Wang, Y.; Liu, Z.; Liu, X.; Fan, D.; Wang, X. Recent Advances of Magnetic Nanomaterials in Bone Tissue Repair. Front. Chem. 2020, 8, 745. [Google Scholar] [CrossRef]
- Popescu, R.C.; Andronescu, E.; Vasile, B.S. Recent Advances in Magnetite Nanoparticle Functionalization for Nanomedicine. Nanomaterials 2019, 9, 1791. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Chen, Y.; Yu, L.; Lin, K.; Wang, X. Magnetic Hyperthermia–Synergistic H2O2 Self-Sufficient Catalytic Suppression of Osteosarcoma with Enhanced Bone-Regeneration Bioactivity by 3D-Printing Composite Scaffolds. Adv. Funct. Mater. 2020, 30, 1907071. [Google Scholar] [CrossRef]
- Meng, J.; Zhang, Y.; Qi, X.; Kong, H.; Wang, C.; Xu, Z.; Xie, S.; Gu, N.; Xu, H. Paramagnetic nanofibrous composite films enhance the osteogenic responses of pre-osteoblast cells. Nanoscale 2010, 2, 2565–2569. [Google Scholar] [CrossRef]
- Meng, J.; Xiao, B.; Zhang, Y.; Liu, J.; Xue, H.; Lei, J.; Kong, H.; Huang, Y.; Jin, Z.; Gu, N.; et al. Super-paramagnetic responsive nanofibrous scaffolds under static magnetic field enhance osteogenesis for bone repair in vivo. Sci. Rep. 2013, 3, 2655. [Google Scholar] [CrossRef]
- Singh, R.K.; Patel, K.D.; Lee, J.H.; Lee, E.-J.; Kim, J.-H.; Kim, T.-H.; Kim, H.-W. Potential of Magnetic Nanofiber Scaffolds with Mechanical and Biological Properties Applicable for Bone Regeneration. PLoS ONE 2014, 9, e91584. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, T.; Chen, J.; Su, J.; Zhi, X.; Pan, P.; Zou, L.; Zhang, Q. Magnetic bioinspired micro/nanostructured composite scaffold for bone regeneration. Colloids Surf. B Biointerfaces 2019, 174, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Chen, H.; Zhang, F.; Wang, L.; Chen, B.; Reynolds, M.A.; Ma, J.; Schneider, A.; Gu, N.; Xu, H.H.K. Injectable calcium phosphate scaffold with iron oxide nanoparticles to enhance osteogenesis via dental pulp stem cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Iconaru, S.L.; Ciobanu, S.C.; Predoi, S.-A.; Buton, N.; Megier, C.; Beuran, M. Development of Iron-Doped Hydroxyapatite Coatings. Coatings 2021, 11, 186. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Guo, S.; Zhao, Y.; Qi, J.; Zhang, R.; Ren, J.; Cheng, H.; Zong, M.; Wu, X.; et al. A review of carbon nanomaterials/bacterial cellulose composites for nanomedicine applications. Carbohydr. Polym. 2024, 323, 121445. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Zhao, T.; Zhou, Y.; Li, S.; Li, J.; Leblanc, R.M. Bone Tissue Engineering via Carbon-Based Nanomaterials. Adv. Healthc. Mater. 2020, 9, 1901495. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, T.; Liang, R.; Wei, M. Application of Zero-Dimensional Nanomaterials in Biosensing. Front. Chem. 2020, 8, 320. [Google Scholar] [CrossRef] [PubMed]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1965, 318, 162–163. [Google Scholar] [CrossRef]
- Vandrovcova, M.; Vacik, J.; Svorcik, V.; Slepicka, P.; Kasalkova, N.; Vorlicek, V.; Lavrentiev, V.; Vosecek, V.; Grausova, L.; Lisa, V.; et al. Fullerene C60 and hybrid C60/Ti films as substrates for adhesion and growth of bone cells. Phys. Stat. Sol. A 2008, 205, 2252–2261. [Google Scholar] [CrossRef]
- Grausova, L.; Vacik, J.; Bilkova, P.; Vorlicek, V.; Svorcik, V.; Soukup, D.; Bacakova, M.; Lisa, V.; Bacakova, L. Regionally selective adhesion and growth of human osteoblast-like MG 63 cells on micropatterned fullerene C60 layers. J. Optoelectron. Adv. Mater. 2008, 10, 2071–2076. [Google Scholar]
- Grausova, L.; Vacik, J.; Vorlicek, V.; Svorcik, V.; Slepicka, P.; Bilkova, P.; Vandrovcova, M.; Lisa, V.; Bacakova, L. Fullerene C60 films of continuous and micropatterned morphology as substrates for adhesion and growth of bone cells. Diam. Relat. Mater. 2009, 18, 578–586. [Google Scholar] [CrossRef]
- Kostyuk, S.V.; Proskurnina, E.V.; Savinova, E.A.; Ershova, E.S.; Kraevaya, O.A.; Kameneva, L.V.; Umryukhin, P.E.; Dolgikh, O.A.; Kutsev, S.I.; Troshin, P.A. Effects of Functionalized Fullerenes on ROS Homeostasis Determine Their Cytoprotective or Cytotoxic Properties. Nanomaterials 2020, 10, 1405. [Google Scholar] [CrossRef] [PubMed]
- Trpkovic, A.; Todorovic-Markovic, B.; Trajkovic, V. Toxicity of pristine versus functionalized fullerenes: Mechanisms of cell damage and the role of oxidative stress. Arch. Toxicol. 2012, 86, 1809–1827. [Google Scholar] [CrossRef] [PubMed]
- Yau, H.; Bayazıt, M.K.; Steinke, J.; Shaffer, M. Diamond Rings or Dumbbells: Controlling the Structure of Poly(ethylene glycol)–Fullerene C60 Adducts by Varying Linking Chain Length. Macromolecules 2014, 47, 4870–4875. [Google Scholar] [CrossRef]
- Piotrowski, P.; Klimek, K.; Ginalska, G.; Kaim, A. Beneficial Influence of Water-Soluble PEG-Functionalized C60 Fullerene on Human Osteoblast Growth In Vitro. Materials 2021, 14, 1566. [Google Scholar] [CrossRef]
- Osswald, S.; Yushin, G.; Vadym, M.; Kucheyev, S.O.; Gogotsi, Y. Control of sp2/sp3 Carbon Ratio and Surface Chemistry of Nanodiamond Powders by Selective Oxidation in Air. J Am. Chem. Soc. 2006, 128, 11635–11642. [Google Scholar] [CrossRef]
- Grausova, L.; Bacakova, L.; Kromka, A.; Potocky, S.; Vanecek, M.; Nesladek, M.; Lisa, V. Nanodiamond as promising material for bone tissue engineering. J. Nanosci. Nanotechnol. 2009, 9, 3524–3534. [Google Scholar] [CrossRef]
- Zhang, Q.; Mochalin, V.N.; Neitzel, I.; Hazeli, K.; Niu, J.; Kontsos, A.; Zhou, J.G.; Lelkes, P.I.; Gogotsi, Y. Mechanical properties and biomineralization of multifunctional nanodiamond-PLLA composites for bone tissue engineering. Biomaterials 2012, 33, 5067–5075. [Google Scholar] [CrossRef] [PubMed]
- Lanao, F.R.P.; Jonker, A.M.; Wolke, J.G.; Jansen, J.A.; van Hest, J.C.; Leeuwenburgh, S.C. Physicochemical properties and applications of poly(lactic-co-glycolic acid) for use in bone regeneration. Tissue Eng. Part B Rev. 2013, 19, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, X. The utilization of carbon-based nanomaterials in bone tissue regeneration and engineering: Respective featured applications and future prospects. Med. Nov. Technol. Devices 2022, 16, 100168. [Google Scholar] [CrossRef]
- Khajuria, D.K.; Kumar, V.B.; Gigi, D.; Gedanken, A.; Karasik, D. Accelerated Bone Regeneration by Nitrogen-Doped Carbon Dots Functionalized with Hydroxyapatite Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 19373–19385. [Google Scholar] [CrossRef]
- Shafiei, S.; Omidi, M.; Nasehi, F.; Golzar, H.; Mohammadrezaei, D.; Rezai Rad, M.; Khojasteh, A. Eggshell-derived calcium phosphate/carbon dot nanofibrous scaffolds for bone tissue engineering: Fabrication and characterization. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, S.; Kumar, M.; Mandal, B.B.; Karak, N. A renewable resource based carbon dot decorated hydroxyapatite nanohybrid and its fabrication with waterborne hyperbranched polyurethane for bone tissue engineering. RSC Adv. 2016, 6, 26066–26076. [Google Scholar] [CrossRef]
- Li, Q.; Ohulchanskyy, T.; Liu, R.; Koynov, K.; Wu, D.; Best, A.; Kumar, R.; Bonoiu, A.; Prasad, P. Photoluminescent Carbon Dots as Biocompatible Nanoprobes for Targeting Cancer Cells in Vitro. J. Phys. Chem. C 2010, 114, 12062–12068. [Google Scholar] [CrossRef]
- Yang, S.T.; Cao, L.; Luo, P.G.; Lu, F.; Wang, X.; Wang, H. Carbon dots for optical imaging in vivo. J. Am. Chem. Soc. 2009, 131, 11308–11309. [Google Scholar] [CrossRef] [PubMed]
- Ates, M.; Eker, A.A.; Eker, B. Carbon nanotube-based nanocomposites and their applications. J. Adhes. Sci. Technol. 2017, 31, 1977–1997. [Google Scholar] [CrossRef]
- Calabrese, G.; De Luca, G.; Nocito, G.; Rizzo, M.G.; Lombardo, S.P.; Chisari, G.; Forte, S.; Sciuto, E.L.; Conoci, S. Carbon Dots: An Innovative Tool for Drug Delivery in Brain Tumors. Int. J. Mol. Sci. 2021, 22, 11783. [Google Scholar] [CrossRef]
- Mehra, N.K.; Mishra, V.; Jain, N.K. A review of ligand tethered surface engineered carbon nanotubes. Biomaterials 2014, 35, 1267–1283. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, H.; Niu, X.; Yu, B.; Fan, Y.; Feng, Q.; Cui, F.Z.; Watari, F. The use of carbon nanotubes to induce osteogenic differentiation of human adipose-derived MSCs in vitro and ectopic bone formation in vivo. Biomaterials 2012, 33, 4818–4827. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Kim, T.; Kim, Y.S.; Choi, H.S.; Lim, H.J.; Yang, S.J.; Park, C.R. Surface modifications for the effective dispersion of carbon nanotubes in solvents and polymers. Carbon 2012, 50, 3–33. [Google Scholar] [CrossRef]
- Lawton, K.; Le, H.; Tredwin, C.; Handy, R.D. Carbon Nanotube Reinforced Hydroxyapatite Nanocomposites As Bone Implants: Nanostructure, Mechanical Strength And Biocompatibility. Int. J. Nanomed. 2019, 14, 7947–7962. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Q.; Li, B.; Kang, J.; Yu, Z.; Li, Y.; Song, X.; Liang, C.; Wang, H. Fabrication and properties of carbon nanotube-reinforced hydroxyapatite composites by a double in situ synthesis process. Carbon 2016, 101, 159–167. [Google Scholar] [CrossRef]
- Hirata, E.; Uo, M.; Takita, H.; Akasaka, T.; Watari, F.; Yokoyama, A. Multiwalled carbon nanotube-coating of 3D collagen scaffolds for bone tissue engineering. Carbon 2011, 49, 3284–3291. [Google Scholar] [CrossRef]
- Shao, S.; Zhou, S.; Li, L.; Li, J.; Luo, C.; Wang, J.; Li, X.; Weng, J. Osteoblast function on electrically conductive electrospun PLA/MWCNTs nanofibers. Biomaterials 2011, 32, 2821–2833. [Google Scholar] [CrossRef]
- Martínez de Arenaza, I.; Obarzanek-Fojt, M.; Sarasua, J.R.; Meaurio, E.; Meyer, F.; Raquez, J.M.; Dubois, P.; Bruinink, A. Pyrene-end-functionalized poly(L-lactide) as an efficient carbon nanotube dispersing agent in poly(L-lactide): Mechanical performance and biocompatibility study. Biomed. Mater. 2015, 10, 045003. [Google Scholar] [CrossRef]
- Lin, C.; Wang, Y.; Lai, Y.; Yang, W.; Jiao, F.; Zhang, H.; Ye, S.; Zhang, Q. Incorporation of carboxylation multiwalled carbon nanotubes into biodegradable poly(lactic-co-glycolic acid) for bone tissue engineering. Colloids Surf. B Biointerfaces 2011, 83, 367–375. [Google Scholar] [CrossRef]
- Cheng, Q.; Rutledge, K.; Jabbarzadeh, E. Carbon nanotube-poly(lactide-co-glycolide) composite scaffolds for bone tissue engineering applications. Ann. Biomed. Eng. 2013, 41, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Mikael, P.E.; Amini, A.R.; Basu, J.; Arellano-Jimenez, J.M.; Laurencin, C.T.; Sanders, M.M.; Carter, B.C.; Nukavarapu, S.P. Functionalized carbon nanotube reinforced scaffolds for bone regenerative engineering: Fabrication, in vitro and in vivo evaluation. Biomed. Mater. 2014, 9, 035001. [Google Scholar] [CrossRef]
- Pan, L.; Pei, X.; He, R.; Wan, Q.; Wang, J. Multiwall carbon nanotubes/polycaprolactone composites for bone tissue engineering application. Colloids Surf. B Biointerfaces. 2012, 93, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Dorj, B.; Won, J.E.; Kim, J.H.; Choi, S.J.; Shin, U.S.; Kim, H.W. Robocasting nanocomposite scaffolds of poly(caprolactone)/hydroxyapatite incorporating modified carbon nanotubes for hard tissue reconstruction. J. Biomed. Mater. Res. Part A 2013, 101, 1670–1681. [Google Scholar] [CrossRef]
- Shin, S.R.; Li, Y.C.; Jang, H.L.; Khoshakhlagh, P.; Akbari, M.; Nasajpour, A.; Zhang, Y.S.; Tamayol, A.; Khademhosseini, A. Graphene-based materials for tissue engineering. Adv. Drug Deliv. Rev. 2016, 105, 255–274. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, X.; Chen, Y. Graphene accelerates osteoblast attachment and biomineralization. Carbon Lett. 2017, 22, 42–47. [Google Scholar] [CrossRef]
- Nayak, T.R.; Andersen, H.; Makam, V.S.; Khaw, C.; Bae, S.; Xu, X.; Ee, P.L.; Ahn, J.H.; Hong, B.H.; Pastorin, G.; et al. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. ACS Nano 2011, 5, 4670–4678. [Google Scholar] [CrossRef]
- Jakus, A.E.; Shah, R.N. Multi and mixed 3D-printing of graphene-hydroxyapatite hybrid materials for complex tissue engineering. J. Biomed. Mater. Res. A 2017, 105, 274–283. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, X.; Ong’achwa Machuki, J. Three-Dimensionally N-Doped Graphene-Hydroxyapatite/Agarose as an Osteoinductive Scaffold for Enhancing Bone Regeneration. ACS Appl. Bio Mater. 2019, 2, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Paz, E.; Ballesteros, Y.; Abenojar, J.; Del Real, J.C.; Dunne, N.J. Graphene Oxide and Graphene Reinforced PMMA Bone Cements: Evaluation of Thermal Properties and Biocompatibility. Materials 2019, 12, 3146. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, N.; Simchi, A. On the biological performance of graphene oxide-modified chitosan/polyvinyl pyrrolidone nanocomposite membranes: In vitro and in vivo effects of graphene oxide. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 121–131. [Google Scholar] [CrossRef]
- Purohit, S.D.; Singh, H.; Bhaskar, R.; Yadav, I.; Bhushan, S.; Gupta, M.K.; Mishra, N.C. Fabrication of Graphene Oxide and Nanohydroxyapatite Reinforced Gelatin–Alginate Nanocomposite Scaffold for Bone Tissue Regeneration. Front. Mater. 2020, 7, 250. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, J.; Wu, B.; Liu, Z.; Li, M.; Wang, X.; Tang, P.; Wang, Z. Graphene and its Derivatives for Bone Tissue Engineering: In Vitro and In Vivo Evaluation of Graphene-Based Scaffolds, Membranes and Coatings. Front. Bioeng. Biotechnol. 2021, 9, 734688. [Google Scholar] [CrossRef]
- Norahan, M.H.; Amroon, M.; Ghahremanzadeh, R.; Rabiee, N.; Baheiraei, N. Reduced graphene oxide: Osteogenic potential for bone tissue engineering. IET Nanobiotechnol. 2019, 13, 720–725. [Google Scholar] [CrossRef]
- Jiayu, L.; Yu-Shi, H.; Cheng, C.; Wang, Y.; Qiu, L.; Li, D.; Zou, D. Self-Supporting Graphene Hydrogel Film as an Experimental Platform to Evaluate the Potential of Graphene for Bone Regeneration. Adv. Funct. Mater. 2013, 23, 3494–3502. [Google Scholar] [CrossRef]
- Lu, J.; Cheng, C.; He, Y.S.; Lyu, C.; Wang, Y.; Yu, J.; Qiu, L.; Zou, D.; Li, D. Multilayered Graphene Hydrogel Membranes for Guided Bone Regeneration. Adv. Mater. 2016, 28, 4025–4031. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Shin, Y.C.; Lee, S.-M.; Jin, O.S.; Kang, S.H.; Hong, S.W.; Jeong, C.-M.; Huh, J.B.; Han, D.-W. Enhanced Osteogenesis by Reduced Graphene Oxide/Hydroxyapatite Nanocomposites. Sci. Rep. 2015, 5, 18833. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Shin, Y.C.; Jin, O.S.; Kang, S.H.; Hwang, Y.S.; Park, J.C.; Hong, S.W.; Han, D.W. Reduced graphene oxide-coated hydroxyapatite composites stimulate spontaneous osteogenic differentiation of human mesenchymal stem cells. Nanoscale 2015, 7, 11642–11651. [Google Scholar] [CrossRef]
- Liu, W.; Dong, X.; Qin, H.; Sui, L.; Wang, J. Three-dimensional porous reduced graphene oxide/hydroxyapatite membrane for guided bone regeneration. Colloids Surf. B Biointerfaces 2021, 208, 112102. [Google Scholar] [CrossRef] [PubMed]

| Class of Biomaterial | e.g., | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Ceramics | Bioglass, alumina, zirconia, CaP (HA, b-TCP, biphasic calcium phosphate) | Biocompatibility, osteoconductivity. | Slow degradation, shaping issues, fragility. | [48,49,50,51,52,53,54,55,56,57,58] |
| Polymers | Naturals (Col, ALG, chitin, CS) | Biocompatibility, bioactivity. | Immunogenicity due to pathogenic contaminants, quick biodegradability, weak mechanical qualities. | [59,60,61,62,63] |
| Synthetics (polystyrene, PLA, PGA, PCL, PLGA) | Possibility to customize the synthesis procedure and reagents. | Poor mechanical resistance and biocompatibility, toxicity due to the release of ions and other residual particles. | [64,65,66,67,68,69] | |
| Metals | Iron; chromium; stainless steel; titanium- and cobalt-alloys. | High elasticity, resistance, and ductility. | Toxic ion release. | [70,71,72,73,74] |
| Composites | PLA/PGA; PLA/HA; PGA/PCA; HA/PGA; HA/CS; HA/Col | Improvement of biological, chemical, and structural properties compared to individual components. | Laborious manufacturing procedure. | [75,76,77,78] |
| Hydrogels | Naturals (e.g., hyaluronic acid) or synthetic (e.g., PEO) | Rubbery ECM-like nature; flexibility to adjust structural parameters during the production process, minimally invasive implant. | Poor biocompatibility and rapid biodegradability in vivo. | [79,80,81,82,83,84,85,86] |
| Class of NPs | NP Composition | Composite Scaffold | Model In Vitro/In Vivo | Effect of NPs Integration on Scaffold | References |
|---|---|---|---|---|---|
| ORGANIC | |||||
| Natural Polymers | Collagen | Col-Ap | Rodent | Promotes osteoblast proliferation, differentiation, and vascularization; | [99] |
| Col-hydrogel | SBF, mouse | Enhances bone mineralization | [100] | ||
| Collagen-coated HB | MG-63 | Improves osteoblast adhesion and proliferation in vitro | [101] | ||
| Gelatin | Gt-coated PCL/GO | hGMSCs | Enhances degradability rate, bioactivity, and cell adhesion and proliferation | [103] | |
| HA-Gel-CS | Osteoblast cells | Improves cell proliferation | [104] | ||
| 3D Gel-Au NPs | MG-63, rat | Promotes bone regeneration | [106] | ||
| Fibrin | Fibrinogen-BCP | hMSCs, rabbit | Promotes cell proliferation and adhesion, as well as bone healing | [108] | |
| Fibrinogen-CS | Rat | Enhances bone regeneration | [109] | ||
| Magnetic fibrin NPs | Saos-2 | Enhances cell viability and ALP activity | [112] | ||
| Alginate | HA/ALG | human osteoblastic cells, rat | Enhances cell adhesion, proliferation, and osteogenic differentiation | [116,117,118] | |
| Synthetic Polymers | PLA | - | hMSCs | Promotes growth and osteogenic differentiation | [120,121,122] |
| PLA-GO-nHA | Saos-2 | Enhances biocompatibility, tensile strength, and cell proliferation | [123] | ||
| PLGA | UPPE-PLGA-rhBMP-2 | bMSCs | Improves osteogenic differentiation and ALP activity | [129] | |
| Liposome | - | 20S-OHC/PA/HA | bMSCs, mouse | Improves osteogenesis and bone healing | [135] |
| - | 20S-OHC-SA-MeGC hydrogel | MSCs, Rat | Stimulates osteogenesis and bone healing | [137] | |
| Dendrimers | - | G3@nCe/GelMA hydrogel | rMSCs, rat | Improves cell adhesion, proliferation, osteogenic differentiation, and tissue integration | [140] |
| - | PAMAM/hBMP-2 | MSCs | Promotes osteogenic differentiation | [141] | |
| INORGANIC | |||||
| Metals | Ag | CS/CMC/CCNWs-AgNPs | MG63 cells, gram (−) and gram (+) bacteria | Promotes mechanical strength, antimicrobial activity, and cell adhesion and proliferation | [159] |
| - | Mouse | Promotes osteoblast proliferation and differentiation | [160] | ||
| BMP/CS/Ag/HA-Ti |
Osteoblasts, bMSCs, Rabbit, S. epidermidis and E. coli. | Improves osteoinductivity, bone formation, and antibacterial properties | [163] | ||
| Au | - | MSCs, hADMSCs | Promotes osteogenic differentiation | [168,169] | |
| - |
Primary osteoblasts | Stimulates differentiation and mineralization | [170] | ||
| GNPs-ALD | Bone marrow- derived macrophage, mouse | Inhibits osteoclast differentiation and bone resorption | [171] | ||
| Gel-GNP |
ADSCs, rabbit | Improves bone regeneration | [172] | ||
| PEGylated GNPs |
MC3T3-E1, hBMSCs, rBMSCs, rabbit | Improves osteogenic differentiation and bone regeneration | [173] | ||
| Pd | PVA/Alg/Pd | hDPSCs | Improves mechanical support and increases cell viability and matrix mineralization | [185] | |
| Pd/PPy/rGO | E. coli, B. subtilis, P. aeruginosa, and K. pneumoniae | Prevents colonization, adhesion, and biofilm formation | [186] | ||
| Mg-HA-ColI-Pd | hADSCs | Inhibits cell growth and decreases cell differentiation | [90] | ||
| Cu, CuO | CS/nHAp/nCu–Zn | rat osteoprogenitor cells | Enhances antibacterial and osteoproliferative properties | [191] | |
| Ti6Al4V(ELI)-3at.%Cu |
hOB, S. aureus and E. coli | Unaffected by osteoblast behaviour, reduces bacterial adhesion and biofilm formation | [192] | ||
| Cu-MBG | hBMSCs | Promotes osteogenic differentiation, inhibits bacteria viability, and enhances angiogenesis | [193] | ||
| n-HA-CuO | In vitro | Improves mechanical properties, cell viability, and electrical conductivity | [196] | ||
| ZnO | HA/PPy/ZnO/Ti | E. coli and S. aureus, bMSCs. | Promotes antibacterial and osteoinductive activity | [203] | |
| Ti-ZnO |
S. aureus
and P. aeruginosa, Osteoblast and osteoclast cells, rabbit | Inhibits bacterial adhesion, regulates the proliferation and differentiation of osteoblasts and osteoclasts, and promotes new bone formation | [204] | ||
| PCL/nHA/ZnO |
MG-63 cells, E. coli and S. aureus | Improves cell viability, antimicrobial effect, and mechanical strength | [205] | ||
| ZnO@Ti and ZnxMn(1−x)O@Ti | S. aureus and P. aeruginosa | Exhibits antibacterial activity | [206] | ||
| TiO2 | TiO2/HA/Ti | Osteoblast cells | Promotes cell proliferation and osteogenic differentiation | [211] | |
| Ti_TiO2 | S. aureus, hADSCs | Promotes anti-bacterial activity, cell proliferation and differentiation. | [153] | ||
| Ti_TiO2 | MG63 cells | Promotes adhesion and osteogenic differentiation | [212] | ||
| Ceramics | HA | PLA-HA PCL-HA | hADMSCs, osteoblasts, rat | Enhances cell adhesion and mineral deposition | [214,215,216,217] |
| Gellan Gum/HA | 7F2 osteoblast cells | Enhances cell proliferation and ALP activity | [84] | ||
| b-TCP | 3D collagen-b-TCP | Osteoblast cells rat | Enhances cell proliferation and bone formation | [219] | |
| PCL-b-TCP | rat bone mesenchymal stem cells, rat | Enhances cell adhesion, viability, and mineralization, and promotes bone formation | [220,221] | ||
| BCP | Chitosan/gelatin hydrogels | BMSCs, rabbit | Improves physical–chemical properties, exhibits biocompatibility, and promotes new bone formation | [223] | |
| Col-DEX-BCP | hMSCs, mouse | Exhibits good porosity, strength, biocompatibility, and osteoinductivity; promotes bone tissue repair | [224] | ||
| MSNs | - | Osteoblasts | Exhibits non-toxicity, high biocompatibility, and adjustable porosity | [226] | |
| - | Osteoblasts, mouse | Suppresses osteoclast resorption, enhances bone formation and mineralization | [227,228] | ||
| Ti-HA-MSNs | MG-63 cells | Promotes biocompatibility and osteoblast differentiation | [229] | ||
| Magnetics | Fe3O4 and γ-Fe2O3 | PLA-HA-γ-Fe2O3 | Osteoblast cells | Improves proliferation, differentiation, and ECM secretion | [234] |
| PLA-HA-γ-Fe2O3 | Rabbit | Accelerates bone regeneration in the defect | [235] | ||
| PCL-MNPs | Osteoblast cells, rat | Improves chemical–physical, mechanical, and osteogenic properties; promotes bone regeneration | [236] | ||
| CS/Col/Fe3O4/nHAP | MC3T3-E1 rat skull osteoblasts/ Rat | Improves cell adhesion and proliferation, as well as osteogenic differentiation in vitro and mineralization and bone regeneration in vivo | [237] | ||
| IONP-CPC | hDPSCs | Improves cell attachment, osteogenic differentiation, and bone mineralization | [238] | ||
| CARBON-BASED | |||||
| Fullerene | C60NPEG5000 | MG-63 | Promotes osteoblast adhesion, proliferation, and differentiation | [244,245,246] | |
| Nanocrystalline diamond (NCD) | MG-63 | Serves as a support for the adhesion, growth, and differentiation of osteogenic cells | [252] | ||
| Nanodiamonds | ND-ODA/PLLA | SBF | Enhanced mechanical properties and increased mineralization capability | [253] | |
| Carbon dots | NCDs-HA | MC3T3-E1, Zebrafish | Promotes osteogenic differentiation in vitro and bone regeneration in vivo | [256] | |
| PCL/PVA-TCP3-CDs | hBFPSCs | Improves mechanical strength, cell adhesion, and proliferation | [257] | ||
| CD@HAp | MG-63 | Improves mechanical strength, cell adhesion, and proliferation | [258] | ||
| Carbon nanotubes | MWCNTs | MSCs | Allows osteogenic differentiation in vitro, and ectopic bone formation in vivo | [259] | |
| f-MWCNTs HTAB | HOB | Improves mechanical strength, biocompatibility, and ALP activity | [266] | ||
| CNT/HA | L-929 cells, rabbits | Accelerates cell proliferation and improves mechanical properties | [267] | ||
| MWCNT-coated Col sponge | Saos-2 | Improves cell adhesion | [268] | ||
| PLA/MWCNTs | Osteoblast cells | Directs osteoblast outgrowth | [269] | ||
| PLLA/py-end-PLLA/MWCNTs | HBMC | Supports cell adhesion and proliferation and promotes osteogenic differentiation | [270] | ||
| PLGA/c-MWCNT | MSCs | Promotes cell growth and osteoblast differentiation | [271] | ||
| CNT/PLGA | MC3T3-E1 | Enhanced surface roughness, increased cell attachment and proliferation | [272] | ||
| MWNTs/PCL | BMSCs | Enhances cell proliferation and differentiation | [274] | ||
| PCL-HA-imCNT | SBF, MC3T3-E1, rat | Improves the compressive strength and elastic modulus; induces substantial mineralization and cell proliferation. Induces vascularization and bone regeneration in vivo | [275] | ||
| Graphene | Graphene film (GF) | MG-63 | Promotes cell adhesion, activity, and the formation of bone-like apatite | [276] | |
| - | hMSCs | Provides biocompatibility and accelerates bone differentiation | [278] | ||
| HB-3DG-HA | MSCs | Supports cell viability and proliferation, upregulates osteogenic differentiation | [279] | ||
| AG/NG–HA | MSCs | Improves mechanical properties and promotes cell proliferation, viability, and osteogenic differentiation | [280] | ||
| G- or GO-PMMA | MC3-T3 | Influences thermal properties and biocompatibility | [281] | ||
| CS/PVP/GO | rbmMSCs, rats | Promotes cell attachment and viability in vitro; in vivo promotes more efficient wound closure | [282] | ||
| nHAp-GO/GA) | MG-63 | Enhances compressive strength, reduces the biodegradation rate, and improves mineral deposition | [283] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauso, L.V.; La Fauci, V.; Longo, C.; Calabrese, G. Bone Tissue Engineering and Nanotechnology: A Promising Combination for Bone Regeneration. Biology 2024, 13, 237. https://doi.org/10.3390/biology13040237
Bauso LV, La Fauci V, Longo C, Calabrese G. Bone Tissue Engineering and Nanotechnology: A Promising Combination for Bone Regeneration. Biology. 2024; 13(4):237. https://doi.org/10.3390/biology13040237
Chicago/Turabian StyleBauso, Luana Vittoria, Valeria La Fauci, Clelia Longo, and Giovanna Calabrese. 2024. "Bone Tissue Engineering and Nanotechnology: A Promising Combination for Bone Regeneration" Biology 13, no. 4: 237. https://doi.org/10.3390/biology13040237