Incident Chlamydia trachomatis Infection in a High School Population
Abstract
:Simple Summary
Abstract
1. Introduction
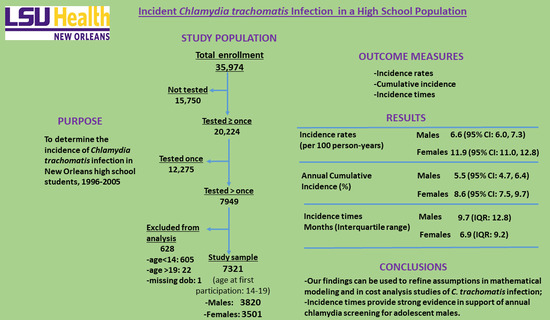
2. Materials and Methods
2.1. Setting and Design
2.2. Study Subjects and Eligibility
2.3. Data Analysis
2.3.1. Incidence Rates Denominators
2.3.2. Cumulative Incidence Denominators
2.3.3. Incidence Numerators
2.3.4. Incidence Time Calculations
2.3.5. Statistical Analyses
3. Results
3.1. Incidence Rates
3.2. Cumulative Incidence
3.3. Incidence Times
4. Discussion
4.1. Strengths
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kleinbaum, D.G.; Kupper, L.L.; Morgenstern, H. Epidemiologic Research: Principles and Quantitative Methods; Van Nostrand Reinhold: New York, NY, USA, 1982; pp. 96–116, 120–122. [Google Scholar]
- Rothman, K.J.; Greenland, S. Measures of Disease Frequency. In Modern Epidemiology, 2nd ed.; Rothman, K.J., Greenland, S., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1998; pp. 30,32,88. [Google Scholar]
- Szklo, M.; Nieto, F.J. Epidemiology: Beyond the Basics; Aspen Publishers, Inc: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance, 2019; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2021. [Google Scholar]
- O’Connell, C.M.; Ferone, M.E. Chlamydia trachomatis Genital Infections. Microbial. Cell. 2016, 3, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Seraceni, S.; De Seta, F.; Colli, C.; Del Savio, R.; Pesel, G.; Zanin, V.; D’Agaro, P.; Contini, C.; Comar, M. High Prevalence of HPV Multiple Genotypes in Women with Persistent Chlamydia trachomatis Infection. Infect. Agent. Cancer 2014, 9, 30. [Google Scholar] [CrossRef]
- Chesson, H.W.; Spicknall, I.H.; Bingham, A.; Brisson, M.; Eppink, S.T.; Farnham, P.G.; Kreisel, K.M.; Kumar, S.; Laprise, J.-F.; Peterman, T.A.; et al. The Estimated Direct Lifetime Medical Costs of Sexually Transmitted Infections Acquired in the United States in 2018. Sex. Trasm. Dis. 2021, 48, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Buimer, M.; Van Doornum, G.J.J.; Ching, S.; Peerbooms, P.G.H.; Plier, P.K.; Ram, D.; Lee, H.H. Detection of Chlamydia trachomatis and Neisseria gonorrhoeae by Ligase Chain Reaction-Based Assays with Clinical Specimens from Various Sites: Implications for Diagnostic Testing and Screening. J. Clin. Microbiol. 1996, 34, 2395–2400. [Google Scholar] [CrossRef] [PubMed]
- Lewis, F.M.T.; Dittus, P.; Salmon, M.E.; Nsuami, M.J. School-Based Sexually Transmitted Disease Screening: Review and Programmatic Guidance. Sex. Transm. Dis. 2016, 43 (Suppl. S1), S18–S27. [Google Scholar] [CrossRef] [PubMed]
- Nsuami, M.J.; Nsa, M.; Brennan, C.; Cammarata, C.L.; Martin, D.H.; Taylor, S.N. Chlamydia Positivity in New Orleans Public High Schools, 1996-2005: Implications for Clinical and Public Health Practices. Acad. Pediatr. 2013, 13, 308–315. [Google Scholar] [CrossRef]
- Low, N.; Forster, M.; Taylor, S.N.; Nsuami, M.J. Repeat Chlamydia Screening Among Adolescents: Cohort Study in a School-Based Programme in New Orleans. Sex. Transm. Infect. 2013, 89, 20–24. [Google Scholar] [CrossRef]
- Burstein, G.R.; Gaydos, C.A.; Diener-West, M.; Howell, M.R.; Zenilman, J.M.; Quinn, T.C. Incident Chlamydia trachomatis Infections Among Inner-city Adolescent Females. JAMA 1998, 280, 521–526. [Google Scholar] [CrossRef]
- Freeman, J.; Hutchinson, G.B. Prevalence, Incidence and Duration. Am. J. Epidemiol. 1980, 112, 707–723. [Google Scholar] [CrossRef]
- Meyers, D.S.; Halvorson, H.; Luckhaupt, S. Screening for Chlamydial Infection: An Evidence Update for the US Preventive Services Task Force. Ann. Intern. Med. 2007, 147, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Abbott Laboratories. Ligase Chain Reaction Amplification Technique. In Chlamydia trachomatis Assay; LCx Probe System Package Insert: Abbott Park, IL, USA, 1996. [Google Scholar]
- Becton Dickinson. BDProbeTecTM ET Chlamydia trachomatis and Neisseria gonorrhoeae Amplification DNA Assays; BDProbeTec ET System Package Insert; Becton Dickinson: Franklin Lakes, NJ, USA, 2000; pp. 1–104. [Google Scholar]
- Ingram, D.D.; Malec, D.J.; Makuc, D.M.; Kruszon-Moran, D.; Gindi, R.M.; Albert, M.; Beresovsky, V.; Hamilton, B.E.; Holmes, J.; Schiller, J.; et al. National Center for Health Statistics Guidelines for Analysis of Trends: Data Evaluation and Methods Research. Vital Health Stat. 2018, 2, 179. [Google Scholar]
- Centers for Disease Control and Prevention. Trends in HIV-Related Behaviors Among High School Students—United States, 1991–2005. MMWR 2006, 55, 851–854. [Google Scholar]
- Gronowski, A.M.; Copper, S.; Baorto, D.; Murray, P.R. Reproducibility Problems with the Abbott Laboratories LCx Assay for Chlamydia trachomatis and Neisseria gonorrhoeae. J. Clin. Microbiol. 2000, 38, 2416–2418. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Recall of LCx® Neisseria gonorrhoeae Assay and Implications for Laboratory Testing for N. gonorrhoeae and Chlamydia trachomatis. MMWR 2002, 51, 709. [Google Scholar]
- Mallinson, H.; Hopwood, J.; Mutton, K. Resolution of the Recent Performance Problem of Abbott LCx Chlamydia trachomatis Assay: Issues of Repeat Testing for Confirmation of Chlamydial Infection. Sex. Transm. Infect. 2002, 78, 225–226. [Google Scholar] [CrossRef]
- Groseclose, S.L.; Zaidi, A.A.; DeLisle, S.J.; Levine, W.C.; St Louis, M.E. Estimated Incidence and Prevalence of Genital Chlamydia trachomatis Infections in the United States, 1996. Sex. Transm. Dis. 1999, 26, 339–344. [Google Scholar] [CrossRef]
- World Health Organization. Estimation of the Incidence and Prevalence of Sexually Transmitted Infections; Report of a WHO Consultation; No. WHO/HIV/2002.14, WHO/CDS/CSR/NCS/2002.6; World Health Organization: Treviso, Italy, 2002. [Google Scholar]
- Weinstock, H.; Berman, S.; Cates, W., Jr. Sexually Transmitted Diseases Among American Youth: Incidence and Prevalence Estimates, 2000. Perspect. Sex. Reprod. Health 2004, 36, 6–10. [Google Scholar] [CrossRef]
- Anderson, R.M.; May, R.M. Infectious Diseases of Humans: Dynamics and Control; Oxford Science Publications: Oxford, UK, 1991. [Google Scholar]
- Boily, M.-C.; Mâsse, B. Mathematical Models of Disease Transmission: A Precious Tool for the Study of Sexually Transmitted Diseases. Can. J. Public Health 1997, 88, 255–265. [Google Scholar] [CrossRef]
- Chesson, H.W.; Collins, D.; Koski, K. Formulas for Estimating the Costs Averted by Sexually Transmitted Infection (STI) Prevention Programs in the United States. Cost. Eff. Resour. Alloc. 2008, 6, 10. [Google Scholar] [CrossRef]
- Committee on Practice and Ambulatory Medicine. Recommendations for preventive pediatric health care. Pediatrics 2000, 105, 645–646. [Google Scholar] [CrossRef]
- Hollblad-Fadiman, K.; Goldman, S.M. American College of Preventive Medicine Practice Policy Statement: Screening for Chlamydia trachomatis. Am. J. Prev. Med. 2003, 24, 287–292. [Google Scholar] [CrossRef]
- American Medical Association. Guidelines for Adolescent Preventive Services (GAPS); American Medical Association Recommendations Mongraph: Chicago, IL, USA, 1997; Available online: http://www.ama-assn.org/ama/upload/mm/39/gapsmono.pdf. (accessed on 19 April 2022).
- U.S. Preventive Services Task Force. Screening for Chlamydial Infection: US Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 2007, 147, 128–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Number Observed | Person-Years at Risk | Students with Incident Chlamydia (n) | Incident Cases per 100 Person-Years (95% CI) | Incidence Time (Months) Median (IQR) | |
|---|---|---|---|---|---|
| Total | 3820 | 6251 | 415 | 6.6 (6.0, 7.3) | 9.7 (12.8) |
| Age at first participation (years) | |||||
| 14 | 299 | 703 | 24 | 3.4 (2.2, 5.0) | 25.9 (12.2) |
| 15 | 1122 | 2307 | 111 | 4.8 (4.0, 5.8) | 17.4 (17.1) |
| 16 | 1179 | 1881 | 152 | 8.1 (6.9, 9.4) | 11.3 (11.4) |
| 17 | 808 | 954 | 90 | 9.4 (7.7, 11.5) | 5.9 (3.5) |
| 18 or 19 | 412 | 405 | 38 | 9.4 (6.7, 12.7) | 6.0 (3.4) |
| Chlamydia test result at first participation | |||||
| Positive | 173 | 173 | 39 | 22.5 (16.6, 29.5) | 6.6 (10.2) |
| Negative | 3647 | 6078 | 376 | 6.2 (5.6, 6.8) | 10.5 (12.9) |
| Had a positive gonorrhea test during follow-up | |||||
| Yes | 104 | 141 | 52 | 36.9 (28.9, 45.4) | 9.6 (12.8) |
| No | 3713 | 6105 | 363 | 6.0 (5.4, 6.6) | 9.7 (12.8) |
| Nucleic acid amplification test | |||||
| PCR/LCx assay (1995–1996 to 1999–2000) | 1805 | 2543 | 134 | 5.3 (4.4, 6.2) | 6.5 (11.2) |
| PCR/LCx and BD assays (prior to and after 2000) | 1007 | 2290 | 162 | 7.1 (6.1, 8.2) | 14.7 (17.2) |
| BD assay (2000–2001 to 2004–2005) | 1008 | 1418 | 119 | 8.4 (7.0, 10.0) | 7.6 (11.1) |
| Number Observed | Person-Years at Risk | Students with Incident Chlamydia (n) | Incident Cases per 100 Person-Years (95% CI) | Incidence Time (Months) Median (IQR) | |
|---|---|---|---|---|---|
| Total | 3501 | 5143 | 610 | 11.9 (11.0, 12.8) | 6.9 (9.2) |
| Age at first participation | |||||
| 14 | 280 | 537 | 55 | 10.2 (7.8, 13.1) | 12.7 (19.4) |
| 15 | 1189 | 2093 | 227 | 10.9 (9.6, 12.3) | 8.7 (11.3) |
| 16 | 1186 | 1623 | 211 | 13.0 (11.4, 14.7) | 6.8 (8.4) |
| 17 | 596 | 648 | 85 | 13.1 (10.6, 16.0) | 6.2 (3.1) |
| 18 or 19 | 250 | 242 | 32 | 13.2 (9.2, 18.2) | 5.5 (2.7) |
| Chlamydia test result at first participation | |||||
| Positive | 354 | 405 | 99 | 24.4 (20.3, 28.9) | 6.1 (6.5) |
| Negative | 3147 | 4738 | 511 | 10.8 (9.9, 11.7) | 7.5 (9.8) |
| Had a positive gonorrhea test during follow-up | |||||
| Yes | 240 | 296 | 121 | 40.9 (35.2, 46.7) | 6.4 (10.8) |
| No | 3253 | 4839 | 489 | 10.1 (9.3, 11.0) | 7.3 (8.9) |
| Nucleic acid amplification test | |||||
| PCR/LCx assay (1995–1996 to 1999–2000) | 1737 | 2216 | 248 | 11.2 (9.9, 12.6) | 6.0 (6.2) |
| PCR/LCx and BD assays (prior to and after 2000) | 833 | 1709 | 192 | 11.2 (9.8, 12.8) | 12.6 (13.6) |
| BD assay (2000–2001 to 2004–2005) | 931 | 1217 | 170 | 14.0 (12.1, 16.0) | 6.5 (6.0) |
| Male Students | Female Students | |||
|---|---|---|---|---|
| Potential Risk Factor | Adjusted Hazard Ratio (95% CI) | p-Value | Adjusted Hazard Ratio (95% CI) | p-Value |
| Age at first participation | 1.31 (1.19–1.44) | <0.001 | 1.04 (0.96–1.13) | 0.317 |
| Positive chlamydia at first participation | 2.76 (1.97–3.88) | <0.001 | 1.59 (1.27–2.00) | <0.001 |
| Positive gonorrhea during follow-up | 5.34 (3.97–7.17) | <0.001 | 3.68 (2.98–4.55) | <0.001 |
| PCR/LCx and BD assays vs. PCR/LCx assay | 1.36 (1.08–1.73) | 0.010 | 1.00 (0.82–1.21) | 0.963 |
| BD assay vs. PCR/LCx assay | 1.37 (1.07–1.76) | 0.013 | 1.18 (0.97–1.44) | 0.094 |
| APC % (95% CI) | p-Value | |
|---|---|---|
| Male Students | 6.6 (−1.2, 15.1) | 0.091 |
| Female Students | 0.1 (−5.3, 5.7) | 0.980 |
| Years Tested Consecutively | Years Followed up from Year 1 | Number Observed (n) | Number with Incident Infection (n) | Cumulative Incidence a (%) | Annual Incidence b % (95% CI) |
|---|---|---|---|---|---|
| Male Students | 2 | 1396 | 153 | 11.0 | 5.5 (4.7, 6.4) |
| 2 | 1 | 824 | 61 | 7.4 | 7.4 (5.7, 9.4) |
| 3 | 2 | 402 | 60 | 14.9 | 7.5 (5.7, 9.5) |
| 4 | 3 | 170 | 32 | 18.8 | 6.3 (4.3, 8.7) |
| Female Students | 2 | 1215 | 208 | 17.1 | 8.6 (7.5, 9.7) |
| 2 | 1 | 747 | 102 | 13.7 | 13.7 (11.3, 16.3) |
| 3 | 2 | 327 | 65 | 19.9 | 9.9 (7.8, 12.5) |
| 4 | 3 | 141 | 41 | 29.1 | 9.7 (7.0, 12.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nsuami, M.J.; Nsa, W.; Cammarata, C.L.; Martin, D.H.; Taylor, S.N. Incident Chlamydia trachomatis Infection in a High School Population. Biology 2022, 11, 1363. https://doi.org/10.3390/biology11091363
Nsuami MJ, Nsa W, Cammarata CL, Martin DH, Taylor SN. Incident Chlamydia trachomatis Infection in a High School Population. Biology. 2022; 11(9):1363. https://doi.org/10.3390/biology11091363
Chicago/Turabian StyleNsuami, M. Jacques, Wato Nsa, Catherine L. Cammarata, David H. Martin, and Stephanie N. Taylor. 2022. "Incident Chlamydia trachomatis Infection in a High School Population" Biology 11, no. 9: 1363. https://doi.org/10.3390/biology11091363