GPX3 Overexpression in Cumulus Cells Entails a Poor Prognosis for Uterine Implantation of Morphotype A Embryos
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
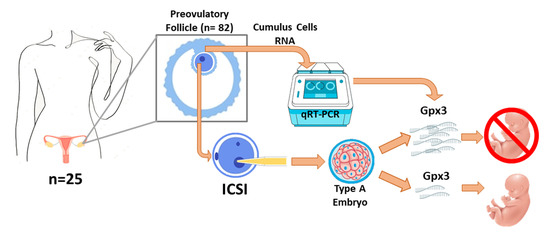
2.1. Subjects
2.2. Experimental Design
2.3. Collection of Cumulus Cells
2.4. Sperm Collection and Preparation
2.5. Embryo Classification
2.6. RNA Isolation, Reverse Transcription, and Real-Time PCR
2.7. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sunderam, S.; Kissin, D.M.; Crawford, S.B.; Folger, S.G.; Boulet, S.L.; Warner, L.; Barfield, W.D. Assisted Reproductive Technology Surveillance-United States, 2015. MMWR Surveill. Summ. 2018, 67, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Krisher, R.L.; Schlenker, T. Culture of Human Preimplantation Embryos in a Clinical ART Setting. Methods Mol. Biol. 2019, 2006, 355–371. [Google Scholar] [PubMed]
- Alpha Scientists in Reproductive Medicine; ESHRE Special Interest Group of Embryology. Istanbul consensus workshop on embryo assessment: Proceedings of an expert meeting. Hum. Reprod. 2011, 26, 1270–1283. [Google Scholar] [CrossRef]
- Ardoy, M.; Caderón, G.; Cuadros, J.; Figueroa, M.J.; Herrer, R.; Moreno, J.M.; Ortiz, A.; Prados, F.; Rodríguez, L.; Pedro, J.S.; et al. Cuadernos de Embriología Clínica. In Criterios ASEBIR de Valoración Morfológica de Oocitos, Embriones Tempranos y Blastocistos Humanos, 3rd ed.; Góbalo. Agencia Creativa Digital: Madrid, Spain, 2015. [Google Scholar]
- Yu, L.; Jia, C.; Lan, Y.; Song, R.; Zhou, L.; Li, Y.; Liang, Y.; Wang, S. Analysis of embryo intactness and developmental potential following slow freezing and vitrification. Syst. Biol. Reprod. Med. 2017, 63, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Alonso, M.; Miravet-Valenciano, J.; López, P.; Simón, C. Endometrial Receptivity by Endometrial Receptivity Analysis (ERA) for Infertility. In Endometrial Gene Expression: An Emerging Paradigm for Reproductive Disorders; Kwak-Kim, J., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 91–102. [Google Scholar]
- Dayal, R.; Singh, S.; Kumar, P.; Singh, K.; Tripathi, P.K.; Gupta, R.; Singhal, S. Morphological Evaluation and Grading of Human Embryo Quality from Day-1 to Day-3 Embryos for Optimum Conceiving Rate. Int. J. Sci. Res. Sci. Technol. 2020, 7, 225–236. [Google Scholar] [CrossRef]
- Alviggi, C.; Humaidan, P.; Howles, C.M.; Tredway, D.; Hillier, S.G. Biological versus chronological ovarian age: Implications for assisted reproductive technology. Reprod. Biol. Endocrinol. 2009, 7, 101. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.L.; Gilchrist, R.B.; Brown, H.M.; Thompson, J.G. Bidirectional communication between cumulus cells and the oocyte: Old hands and new players? Theriogenology 2016, 86, 62–68. [Google Scholar] [CrossRef]
- Salehi, E.; Aflatoonian, R.; Moeini, A.; Yamini, N.; Asadi, E.; Khosravizadeh, Z.; Tarzjani, M.D.; Harat, Z.N.; Abolhassani, F. Apoptotic biomarkers in cumulus cells in relation to embryo quality in polycystic ovary syndrome. Arch. Gynecol. Obstet. 2017, 296, 1219–1227. [Google Scholar] [CrossRef]
- Uyar, A.; Torrealday, S.; Seli, E. Cumulus and granulosa cell markers of oocyte and embryo quality. Fertil. Steril. 2013, 99, 979–997. [Google Scholar] [CrossRef]
- Walter, J.; Huwiler, F.; Fortes, C.; Grossmann, J.; Roschitzki, B.; Hu, J.; Naegeli, H.; Laczko, E.; Bleul, U. Analysis of the equine “cumulome” reveals major metabolic aberrations after maturation In Vitro. BMC Genom. 2019, 20, 588. [Google Scholar] [CrossRef]
- Iager, A.E.; Kocabas, A.M.; Otu, H.H.; Ruppel, P.; Langerveld, A.; Schnarr, P.; Suarez, M.; Jarrett, J.C.; Conaghan, J.; Rosa, G.J.M.; et al. Identification of a novel gene set in human cumulus cells predictive of an oocyte’s pregnancy potential. Fertil. Steril. 2013, 99, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Akbari Sene, A.; Tabatabaie, A.; Nikniaz, H.; Alizadeh, A.; Sheibani, K.; Mortezapour Alisaraie, M.; Tabatabaie, M.; Ashrafi, M.; Amjadi, F. The myo-inositol effect on the oocyte quality and fertilization rate among women with polycystic ovary syndrome undergoing assisted reproductive technology cycles: A randomized clinical trial. Arch. Gynecol. Obstet. 2019, 299, 1701–1707. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Cho, S.; Seo, S.K.; Park, J.H.; Kim, S.H.; Lee, B.S. Alteration in the intrafollicular thiol-redox system in infertile women with endometriosis. Reproduction 2015, 149, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Scarica, C.; Cimadomo, D.; Dovere, L.; Giancani, A.; Stoppa, M.; Capalbo, A.; Ubaldi, F.M.; Rienzi, L.; Canipari, R. An integrated investigation of oocyte developmental competence: Expression of key genes in human cumulus cells, morphokinetics of early divisions, blastulation, and euploidy. J. Assist. Reprod. Genet. 2019, 36, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Y.; Xue, Q.; Kuai, Y.; Wang, S.; Shang, J. Comparison of Connexin43 in Cumulus Cells Between Poor and Non-Poor Responders Undergoing in Vitro Fertilization. J. Reprod. Med. 2017, 62, 50–54. [Google Scholar]
- Cooper, T.G.; Noonan, E.; von Eckardstein, S.; Auger, J.; Baker, H.W.; Behre, H.M.; Haugen, T.B.; Kruger, T.; Wang, C.; Mbizvo, M.T.; et al. World Health Organization reference values for human semen characteristics. Hum. Reprod. Update 2010, 16, 231–245. [Google Scholar] [CrossRef]
- Kursel, L.E.; Malik, H.S. The cellular mechanisms and consequences of centromere drive. Curr. Opin. Cell Biol. 2018, 52, 58–65. [Google Scholar] [CrossRef]
- Hamel, M.; Dufort, I.; Robert, C.; Léveillé, M.-C.; Leader, A.; Sirard, M.-A. Genomic assessment of follicular marker genes as pregnancy predictors for human IVF. Mol. Hum. Reprod. 2009, 16, 87–96. [Google Scholar] [CrossRef]
- Yung, Y.; Ophir, L.; Yerushalmi, G.M.; Baum, M.; Hourvitz, A.; Maman, E. HAS2-AS1 is a novel LH/hCG target gene regulating HAS2 expression and enhancing cumulus cells migration. J. Ovarian Res. 2019, 12, 21. [Google Scholar] [CrossRef]
- Gebhardt, K.M.; Feil, D.K.; Dunning, K.R.; Lane, M.; Russell, D.L. Human cumulus cell gene expression as a biomarker of pregnancy outcome after single embryo transfer. Fertil. Steril. 2011, 96, 47–52. [Google Scholar] [CrossRef]
- Feuerstein, P.; Cadoret, V.; Dalbies-Tran, R.; Guerif, F.; Bidault, R.; Royere, D. Gene expression in human cumulus cells: One approach to oocyte competence. Hum. Reprod. 2007, 22, 3069–3077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santiquet, N.; Robert, C.; Richard, F.J. The Dynamics of Connexin Expression, Degradation and Localisation Are Regulated by Gonadotropins during the Early Stages of In Vitro Maturation of Swine Oocytes. PLoS ONE 2013, 8, e68456. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hao, C.; Shen, X.; Zhang, Y.; Liu, X. RUNX2, GPX3 and PTX3 gene expression profiling in cumulus cells are reflective oocyte/embryo competence and potentially reliable predictors of embryo developmental competence in PCOS patients. Reprod. Biol. Endocrinol. 2013, 11, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Non-pregnant (0% implantation rate) | Participants (n) | Follicles (n) | Age (Years) | BMI (kg/m2) |
| 18 | 55 | 37.4 ± 6.7 | 26.8 ± 5.3 | |
| Embryo Transfer Code | Transferred Embryos (n) | Embryo Morphotype | Implanted Embryos (n) | |
| 01 | 1 | D | 0 | |
| 02 | 3 | A, D, D | 0 | |
| 03 | 3 | A, A, A | 0 | |
| 04 | 2 | A, B | 0 | |
| 05 | 3 | B, B, C | 0 | |
| 06 | 2 | A, A | 0 | |
| 07 | 1 | A | 0 | |
| 08 | 2 | A, B | 0 | |
| 09 | 1 | B | 0 | |
| 10 | 1 | A | 0 | |
| 11 | 3 | A, A, A | 0 | |
| 12 | 3 | A, A, A | 0 | |
| 13 | 2 | A, B | 0 | |
| 14 | 2 | A, A | 0 | |
| 15 | 1 | A | 0 | |
| 16 | 1 | A | 0 | |
| 17 | 1 | C | 0 | |
| 18 | 1 | D | 0 | |
| 19 | 1 | B | 0 | |
| 20 | 2 | A, B | 0 | |
| 21 | 2 | B, B | 0 | |
| 22 | 2 | A, A | 0 | |
| 23 | 3 | A, A, A | 0 | |
| 24 | 2 | A, B | 0 | |
| 25 | 3 | A, B, B | 0 | |
| 26 | 2 | A, A | 0 | |
| 27 | 2 | D, C | 0 | |
| 28 | 2 | A, A | 0 | |
| 29 | 1 | C | 0 | |
| Pregnant (100% implantation rate) | Participants (n) | Follicles (n) | Age (years) | BMI (kg/m2) |
| 7 | 17 | 37.0 ± 7.2 | 25.7 ± 2.8 | |
| Embryo Transfer Code | Transferred Embryos (n) | Embryo Morphotype | Implanted Embryos (n) | |
| 30 | 2 | A, A | 2 | |
| 31 | 2 | A, B | 2 | |
| 32 | 2 | A, B | 2 | |
| 33 | 3 | A, A, A | 3 | |
| 34 | 1 | A | 1 | |
| 35 | 1 | A | 1 | |
| 36 | 2 | A, A | 2 | |
| 37 | 2 | A, A | 2 | |
| 38 | 2 | A, A | 2 |
| Gene | Sequence | ng of cDNA/Well | T° of Annealing | |
|---|---|---|---|---|
| HAS2 | Forward | 5′-ACTTGTGGATGACCTACGAAGCGATTATCACT-3′ | 120 | 65 °C |
| Reverse | 5′-AAACATCTTGGCGGGAAGTAAACTCGAC-3′ | |||
| CDC42 | Forward | 5′-GAAAGGCCTAAAGAATGTATTTGACGAAGC-3′ | 120 | 58 °C |
| Reverse | 5′-TGGGCCTTGTCTCACACGAGTGCAT-3′ | |||
| CX43 | Forward | 5′-CAGCGACCTTCAAGCAGAGCCAGCAGTCGT-3′ | 120 | 65 °C |
| Reverse | 5′-TGTTGAGTACCACCTCCACCGGATCAAA-3′ | |||
| GPX3 | Forward | 5′-TTACACACATGCCTACAGGTATGCGTGATT-3′ | 120 | 58 °C |
| Reverse | 5′-TGGAGAACTGGAGAGAAAGGGTTGTCACT-3′ | |||
| B-ACTIN | Forward | 5′-GGCCGAGGACTTTGATTGCACATTGTT-3′ | 120 | 58–65 °C |
| Reverse | 5′-CCTTAGAGAGAAGTGGGGTGGCTTTTAGGA-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bejarano, I.; Dorado-Silva, M.; Sarmiento-Soto, H.; Álvarez-Sánchez, N.; Lardone, P.J.; Guerrero, J.M.; Sánchez-Martín, P.; Carrillo-Vico, A. GPX3 Overexpression in Cumulus Cells Entails a Poor Prognosis for Uterine Implantation of Morphotype A Embryos. Biology 2022, 11, 1361. https://doi.org/10.3390/biology11091361
Bejarano I, Dorado-Silva M, Sarmiento-Soto H, Álvarez-Sánchez N, Lardone PJ, Guerrero JM, Sánchez-Martín P, Carrillo-Vico A. GPX3 Overexpression in Cumulus Cells Entails a Poor Prognosis for Uterine Implantation of Morphotype A Embryos. Biology. 2022; 11(9):1361. https://doi.org/10.3390/biology11091361
Chicago/Turabian StyleBejarano, Ignacio, Mónica Dorado-Silva, Helia Sarmiento-Soto, Nuria Álvarez-Sánchez, Patricia Judith Lardone, Juan Miguel Guerrero, Pascual Sánchez-Martín, and Antonio Carrillo-Vico. 2022. "GPX3 Overexpression in Cumulus Cells Entails a Poor Prognosis for Uterine Implantation of Morphotype A Embryos" Biology 11, no. 9: 1361. https://doi.org/10.3390/biology11091361