Black Elderberry Press Cake as a Source of Bioactive Ingredients Using Green-Based Extraction Approaches
Abstract
:Simple Summary
Abstract
1. Introduction
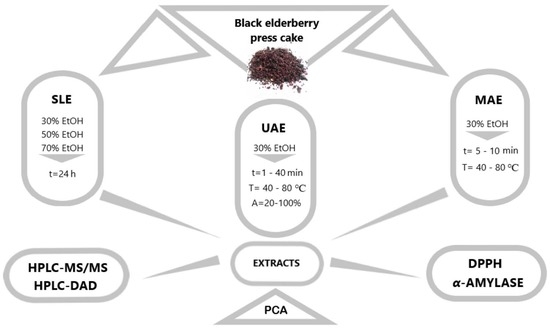
2. Materials and Methods
2.1. Plant Material and Chemicals
2.2. Conventional Solid–Liquid Extraction (SLE)
2.3. Ultrasound-Assisted Extraction (UAE)
2.4. Microwave-Assisted Extraction (MAE)
2.5. Extraction Yield (EY) and Total Phenolic (TPC) and Flavonoid Contents (TFC)
2.6. HPLC-MS/MS Analysis
2.7. HPLC-DAD Analysis
2.8. DPPH Radical Scavenging Activity
2.9. Determination of α-Amylase Inhibitory Activity
2.10. Data Processing
3. Results and Discussion
3.1. Determination of Extraction Efficiency
3.2. HPLC Analysis of Phenolic Compound in Obtained Extracts
3.3. Antioxidant and Enzyme Inhibitory Activities
3.4. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mesías, F.J.; Martín, A.; Hernández, A. Consumers’ growing appetite for natural foods: Perceptions towards the use of natural preservatives in fresh fruit. Food. Res. Int. 2021, 150, 110749. [Google Scholar] [CrossRef] [PubMed]
- De Froidmont-Goertz, I.; Faure, U.; Gajdzinska, M.; Haentjens, W.; Krommer, J.; Lizaso, M.; Lutzeyer, H.J.; Mangan, C.; Markakis, M.; Schoumacher, C. Food 2030 Food Pathways for Action: Research and Innovation Policy as a Driver for Sustainable, Healty and Inclusive Food Systems; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar]
- Awasthi, A.K.; Cheela, V.S.; D’Adamo, I.; Iacovidou, E.; Islam, M.R.; Johnson, M.; Miller, T.R.; Parajuly, K.; Parchomenko, A.; Radhakrishan, L.; et al. Zero waste approach towards a sustainable waste management. Resour. Environ. Sustain. 2021, 3, 100014. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 3 August 2022).
- Uhl, K.R.; Fyhrie, K.J.; Brodt, S.B.; Mitchell, A.E. Blue Elderberry (Sambucus nigra ssp. cerulea): A Robust and Underutilized Fruit for Value-Added Products. ACS Food Sci. Technol. 2022, 2, 347–358. [Google Scholar] [CrossRef]
- Wieland, L.S.; Piechotta, V.; Feinberg, T.; Ludeman, E.; Hutton, B.; Kanji, S.; Garritty, C. Elderberry for prevention and treatment of viral respiratory illnesses: A systematic review. BMC Complement. Med. Ther. 2021, 21, 112. [Google Scholar] [CrossRef]
- Technavio. Available online: https://www.technavio.com/report/elderberry-market-industry-analysis (accessed on 27 July 2022).
- Przybylska-Balcerek, A.; Szablewski, T.; Szwajkowska-Michałek, L.; Świerk, D.; Cegielska-Radziejewska, R.; Krejpcio, Z.; Suchowilska, E.; Tomczyk, Ł.; Stuper-Szablewska, K. Sambucus Nigra Extracts—Natural Antioxidants and Antimicrobial Compounds. Molecules 2021, 26, 2910. [Google Scholar] [CrossRef]
- Speer, H.; D’Cunha, N.M.; Alexopoulos, N.I.; McKune, A.J.; Naumovski, N. Anthocyanins and human health—A focus on oxidative stress, inflammation and disease. Antioxidants 2020, 9, 366. [Google Scholar] [CrossRef]
- Daneshzad, E.; Shab-Bidar, S.; Mohammadpour, Z.; Djafarian, K. Effect of anthocyanin supplementation on cardio-metabolic biomarkers: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2019, 38, 1153–1165. [Google Scholar] [CrossRef]
- Les, F.; Cásedas, G.; Gómez, C.; Moliner, C.; Valero, M.S.; López, V. The role of anthocyanins as antidiabetic agents: From molecular mechanisms to in vivo and human studies. J. Physiol. Biochem. 2021, 77, 109–131. [Google Scholar] [CrossRef]
- Vidović, S.; Vladić, J.; Nastić, N.; Jokić, S. Subcritical and Supercritical Extraction in Food By-product and Food Waste Valorization. In Innovative Food Processing Technologies, 1st ed.; Knoerzer, K., Muthukumarappan, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 705–721. [Google Scholar]
- Vladić, J.; Nastić, N.; Stanojković, T.; Žižak, Ž.; Čakarević, J.; Popović, L.; Vidovic, S. Subcritical water for recovery of polyphenols from comfrey root and biological activities of extracts. Acta Chim. Slov. 2019, 66, 473–783. [Google Scholar] [CrossRef] [Green Version]
- Mandal, S.C.; Mandal, V.; Das, A.K. Classification of Extraction Methods. In Essentials of Botanical Extraction, 1st ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 83–136. [Google Scholar]
- Bélafi-Bakó, K.; Cserjési, P.; Beszédes, S.; Csanádi, Z.; Hodúr, C. Berry pectins: Microwave-assisted extraction and rheological properties. Food Bioprocess Technol. 2012, 5, 1100–1105. [Google Scholar] [CrossRef]
- Kitrytė, V.; Laurinavičienė, A.; Syrpas, M.; Pukalskas, A.; Venskutonis, P.R. Modeling and optimization of supercritical carbon dioxide extraction for isolation of valuable lipophilic constituents from elderberry (Sambucus nigra L.) pomace. J. CO2 Util. 2020, 35, 225–235. [Google Scholar] [CrossRef]
- Kiprovski, B.; Malenčić, Đ.; Ljubojević, M.; Ognjanov, V.; Veberic, R.; Hudina, M.; Mikulic-Petkovsek, M. Quality parameters change during ripening in leaves and fruits of wild growing and cultivated elderberry (Sambucus nigra) genotypes. Sci. Hortic. 2021, 277, 109792. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Olech, M.; Oniszczuk, T.; Wojtunik-Kulesza, K.; Wójtowicz, A. Extraction methods, LC-ESI-MS/MS analysis of phenolic compounds and antiradical properties of functional food enriched with elderberry flowers or fruits. Arab. J. Chem. 2019, 12, 4719–4730. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Finn, C.E. Anthocyanins and other polyphenolics in American elderberry (Sambucus canadensis) and European elderberry (S. nigra) cultivars. J. Sci. Food Agric. 2007, 87, 2665–2675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Harborne, J.B. Methods of Plant Analysis. In Phytochemical Methods; Springer: Dordrecht, The Netherlands, 1984; pp. 1–36. [Google Scholar]
- Espín, J.C.; Soler-Rivas, C.; Wichers, H.J. Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2, 2-diphenyl-1-picrylhydrazyl radical. J. Agric. Food Chem. 2000, 48, 648–656. [Google Scholar] [CrossRef]
- Šavikin, K.; Živković, J.; Alimpić, A.; Zdunić, G.; Janković, T.; Duletić-Laušević, S.; Menković, N. Activity-guided fractionation of pomegranate extract and its antioxidant, antidiabetic and antineurodegenerative properties. Ind. Crops Prod. 2018, 113, 142–149. [Google Scholar] [CrossRef]
- Pastor, K.; Ačanski, M.; Vujić, Đ.; Kondić-Špika, A. Binary simple sugar profiling in corn and small grain flour authentication using GC/EI-qMS approach. Chromatographia 2016, 79, 1553–1559. [Google Scholar] [CrossRef]
- Yildiz, O.; Gurkan, H.; Sahingil, D.; Degirmenci, A.; Er Kemal, M.; Kolayli, S.; Hayaloglu, A.A. Floral authentication of some monofloral honeys based on volatile composition and physicochemical parameters. Eur. Food Res. Technol. 2022, 248, 2145–2155. [Google Scholar] [CrossRef]
- Wroniak, M.; Raczyk, M.; Kruszewski, B.; Symoniuk, E.; Dach, D. Effect of Deep Frying of Potatoes and Tofu on Thermo-Oxidative Changes of Cold Pressed Rapeseed Oil, Cold Pressed High Oleic Rapeseed Oil and Palm Olein. Antioxidants 2021, 10, 1637. [Google Scholar] [CrossRef]
- Karpiński, P.; Kruszewski, B.; Stachelska, M.A.; Szabłowska, E. Development of volatile profile of Kumpiak podlaski dry-cured ham during traditional ripening. Int. J. Food Sci. Technol. 2020, 55, 3630–3638. [Google Scholar] [CrossRef]
- Vidović, S.; Tomšik, A.; Vladić, J.; Jokić, S.; Aladić, K.; Pastor, K.; Jerković, I. Supercritical Carbon Dioxide Extraction of Allium ursinum: Impact of Temperature and Pressure on the Extracts Chemical Profile. Chem. Biodivers. 2021, 18, e2100058. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef] [PubMed]
- Živković, J.; Vladić, J.; Naffati, A.; Nastić, N.; Šavikin, K.; Tomić, M.; Vidović, S. Comparative Chemical Profiling of Underexploited Arctostaphylos uva-ursi L. Herbal Dust Extracts Obtained by Conventional, Ultrasound-Assisted and Subcritical Water Extractions. Waste Biomass Valorization 2022, 13, 4147–4155. [Google Scholar] [CrossRef]
- Maran, J.P.; Priya, B. Ultrasound-assisted extraction of polysaccharide from Nephelium lappaceum L. fruit peel. Int. J. Biol. Macromol. 2014, 70, 530–536. [Google Scholar] [CrossRef]
- Li, Y.; Lai, P.; Chen, J.; Shen, H.; Tang, B.; Wu, L.; Weng, M. Extraction optimization of polyphenols, antioxidant and xanthine oxidase inhibitory activities from Prunus salicina L. Food Sci. Technol. 2016, 36, 520–525. [Google Scholar] [CrossRef] [Green Version]
- Al-Dhabi, N.A.; Ponmurugan, K.; Jeganathan, P.M. Development and validation of ultrasound-assisted solid-liquid extraction of phenolic compounds from waste spent coffee grounds. Ultrason. Sonochem. 2017, 34, 206–213. [Google Scholar] [CrossRef]
- Duymuş, H.G.; Göger, F.; Başer, K.H.C. In vitro antioxidant properties and anthocyanin compositions of elderberry extracts. Food Chem. 2014, 155, 112–119. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green extraction methods for polyphenols from plant matrices and their byproducts: A review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [Green Version]
- Vladić, J.; Janković, T.; Živković, J.; Tomić, M.; Zdunić, G.; Šavikin, K.; Vidović, S. Comparative study of subcritical water and microwave-assisted extraction techniques impact on the phenolic compounds and 5-hydroxymethylfurfural content in pomegranate peel. Plant Foods Hum. Nutr. 2020, 75, 553–560. [Google Scholar] [CrossRef]
- Radványi, D.; Juhász, R.; Kun, S.; Szabó-Nótin, B.; Barta, J. Preliminary study of extraction of biologically active compounds from elderberry (Sambucus nigra L.) pomace. Acta Aliment. 2013, 42, 63–72. [Google Scholar] [CrossRef]
- Seabra, I.J.; Braga, M.E.; Batista, M.T.; de Sousa, H.C. Effect of solvent (CO2/ethanol/H2O) on the fractionated enhanced solvent extraction of anthocyanins from elderberry pomace. J. Supercrit. Fluids 2010, 54, 145–152. [Google Scholar] [CrossRef]
- Seabra, I.J.; Braga, M.E.; Batista, M.T.; de Sousa, H.C. Fractioned high pressure extraction of anthocyanins from elderberry (Sambucus nigra L.) pomace. Food Bioprocess Technol. 2010, 3, 674–683. [Google Scholar] [CrossRef] [Green Version]
- Waldbauer, K.; Seiringer, G.; Sykora, C.; Dirsch, V.M.; Zehl, M.; Kopp, B. Evaluation of apricot, bilberry, and elderberry pomace constituents and their potential to enhance the endothelial nitric oxide synthase (eNOS) activity. Acs Omega 2018, 3, 10545–10553. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Zhang, L.; Rocchetti, G.; Lucini, L.; Pateiro, M.; Munekata, P.E.; Lorenzo, J.M. Elderberry (Sambucus nigra L.) as potential source of antioxidants. Characterization, optimization of extraction parameters and bioactive properties. Food Chem. 2020, 330, 127266. [Google Scholar] [CrossRef]
- Radojković, M.; Vujanović, M.; Majkić, T.; Zengin, G.; Beara, I.; Catauro, M.; Montesano, D. Evaluation of Sambucus nigra L. biopotential as an unused natural resource. Appl. Sci. 2021, 11, 11207. [Google Scholar] [CrossRef]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Oxidative stress and stress-activated signaling pathways: A unifying hypothesis of type 2 diabetes. Endocr. Rev. 2002, 23, 599–622. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, S.; Sil, P.C. Role of plant-derived polyphenols in reducing oxidative stress-mediated diabetic complications. React. Oxyg. Species 2018, 5, 15–34. [Google Scholar] [CrossRef]
- Martín-Gallán, P.; Carrascosa, A.; Gussinyé, M.; Domínguez, C. Biomarkers of diabetes-associated oxidative stress and antioxidant status in young diabetic patients with or without subclinical complications. Free Radic. Biol. Med. 2003, 34, 1563–1574. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Lebelo, S.L. Antioxidant effects and mechanisms of medicinal plants and their bioactive compounds for the prevention and treatment of type 2 diabetes: An updated review. Oxid. Med. Cell. Longev. 2020, 2020, 1356893. [Google Scholar] [CrossRef] [Green Version]
- Ho, G.T.T.; Kase, E.T.; Wangensteen, H.; Barsett, H. Phenolic elderberry extracts, anthocyanins, procyanidins, and metabolites influence glucose and fatty acid uptake in human skeletal muscle cells. J. Agric. Food Chem. 2017, 65, 2677–2685. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.S.; Silva, P.; Silva, A.M.; Nunes, F.M. Effect of harvesting year and elderberry cultivar on the chemical composition and potential bioactivity: A three-year study. Food Chem. 2020, 302, 125366. [Google Scholar] [CrossRef] [PubMed]

| Extraction | Samples | Extraction Conditions | EY * (%) | TPC * (mg GAE/g DE) | TFC * (mg CE/g DE) |
|---|---|---|---|---|---|
| SLE | SLE-1 | 30% EtOH, room temperature, 24 h | 26.58 ± 0.24 d–g | 150.50 ± 5.96 cde | 48.52 ± 1.75 hij |
| SLE-2 | 50% EtOH, room temperature, 24 h | 15.48 ± 0.71 kl | 146.02 ± 8.27 c–f | 49.04 ± 3.23 g–j | |
| SLE-3 | 70% EtOH, room temperature, 24 h | 12.84 ± 0.14 m | 141.77 ± 8.00 d–h | 49.51 ± 3.67 f–j | |
| UAE | UAE-1 | 30% EtOH, 40 °C, A 20%, 11 min | 19.68 ± 0.90 i | 134.41 ± 0.16 f–j | 55.84 ± 0.37 a–d |
| UAE-2 | 30% EtOH, 50 °C, A 20%, 19 min | 28.48 ± 0.89 bcd | 122.38 ± 3.08 j | 57.98 ± 0.76 ab | |
| UAE-3 | 30% EtOH, 60 °C, A 20%, 40 min | 28.02 ± 0.50 cde | 128.07 ± 3.04 ij | 51.46 ± 0.52 c-i | |
| UAE-4 | 30% EtOH, 40 °C, A 60%, 2 min | 26.06 ± 0.79 fgh | 129.30± 1.08 hij | 56.39 ± 1.45 abc | |
| UAE-5 | 30% EtOH, 50 °C, A 60%, 4 min | 26.40 ± 0.42 e–h | 132.95 ± 0.86 g–j | 48.65 ± 0.49 g–j | |
| UAE-6 | 30% EtOH, 60 °C, A 60%, 7 min | 29.04 ± 1.24 bc | 143.23 ± 2.57 c–h | 53.11 ± 1.44 b–g | |
| UAE-7 | 30% EtOH, 70 °C, A 60%, 13 min | 30.16 ± 0.60 ab | 136.48 ± 1.84 e–j | 50.12 ± 0.52 e–j | |
| UAE-8 | 30% EtOH, 80 °C, A 60%, 23 min | 28.18 ± 0.51 cde | 132.85 ± 1.40 g–j | 56.56 ± 0.66 abc | |
| UAE-9 | 30% EtOH, 40 °C, A 100%, 1 min | 24.50 ± 0.93 h | 134.55 ± 0.66 f–j | 58.39 ± 0.44 a | |
| UAE-10 | 30% EtOH, 50 °C, A 100%, 2 min | 25.56 ± 0.63 gh | 130.51 ± 3.17 g–j | 51.44 ± 0.39 c–i | |
| UAE-11 | 30% EtOH, 60 °C, A 100%, 4 min | 27.90 ± 0.18 c–f | 138.75 ± 4.16 e–i | 51.72 ± 0.57 c–i | |
| UAE-12 | 30% EtOH, 70 °C, A 100%, 6 min | 29.44 ± 0.91 abc | 125.58 ± 2.12 ij | 50.99 ± 0.89 d–i | |
| UAE-13 | 30% EtOH, 80 °C, A 100%, 10 min | 31.28 ± 1.09 a | 130.64 ± 2.42 g–j | 53.98 ± 0.61 a–f | |
| MAE | MAE-1 | 30% EtOH, 40 °C, 5 min | 13.33 ± 0.01 m | 156.08 ± 2.50 bc | 52.26 ± 0.90 c–h |
| MAE-2 | 30% EtOH, 60 °C, 5 min | 8.50 ± 0.30 n | 166.02 ± 3.31 ab | 54.30 ± 1.40 a–e | |
| MAE-3 | 30% EtOH, 80 °C, 5 min | 9.27 ± 0.01 n | 122.00 ± 4.80 j | 40.29 ± 0.59 m | |
| MAE-4 | 30% EtOH, 100 °C, 5 min | 17.27 ± 0.50 jk | 154.07 ± 5.24 bcd | 45.33 ± 0.93 j–m | |
| MAE-5 | 30% EtOH, 120 °C, 5 min | 18.80 ± 0.31 ij | 150.90 ± 5.16 cde | 48.06 ± 1.19 h–k | |
| MAE-6 | 30% EtOH, 40 °C, 10 min | 14.41 ± 0.24 lm | 123.77 ± 6.29 ij | 42.32 ± 0.92 lm | |
| MAE-7 | 30% EtOH, 60 °C, 10 min | 16.53 ± 0.34 k | 157.92 ± 2.44 abc | 47.75 ± 0.51 h–k | |
| MAE-8 | 30% EtOH, 80 °C, 10 min | 16.57 ± 0.17 k | 171.86 ± 4.51 a | 50.92 ± 0.73 d–i | |
| MAE-9 | 30% EtOH, 100 °C, 10 min | 20.63 ± 0.20 i | 137.90 ± 2.70 e–i | 43.15 ± 1.01 klm | |
| MAE-10 | 30% EtOH, 120 °C, 10 min | 25.77 ± 0.28 gh | 145.17 ± 6.53 c–g | 46.50 ± 0.65 i–j |
| Peak | Rt (min) | [M − H]− (m/z) | [M + H]+ (m/z) | MS2 (m/z) | MS3 (m/z) | Compounds ** |
|---|---|---|---|---|---|---|
| 1 | 6.55 | 373 | 355(100), 343(80), 193(33) | 193(100) | Ferulic acid hexoside derivative 1 * | |
| 2 | 9.24 | 153 | 123(100), 109(27) | Protocatechuic acid | ||
| 3 | 9.46 | 449 | 287(100) | Cyanidin-3-galactoside | ||
| 4 | 11.03 | 581 | 287(100) | Cyanidin-3-sambubioside | ||
| 5 | 11.14 | 449 | 287(100) | Cyanidin-3-glucoside | ||
| 6 | 13.14 | 371 | 325(100), 163(51) | p-coumaric acid hexoside derivative * | ||
| 7 | 14.86 | 353 | 191(100), 179(5) | Chlorogenic acid (3-caffeoylquinic acid) | ||
| 8 | 15.40 | 597 | 285(100), 241(9) | Kaempferol derivative 1 * | ||
| 9 | 15.42 | 599 | 581(100) | 287(100) | Cyanidin-3-sambubioside derivative | |
| 10 | 16.07 | 465 | 285(100), 241(11) | Kaempferol derivative 2 * | ||
| 12 | 17.12 | 571 | 487(100) | 337(100), 355(48), 193(10) | Ferulic acid hexoside derivative 2 * | |
| 12 | 17.73 | 755 | 300(100), 591(83), 301(44), 271(19) | Quercetin trisaccharide * | ||
| 13 | 19.07 | 609 | 301(100), 300(23), 179(3) | Rutin | ||
| 14 | 19.63 | 463 | 301(100), 300(16), 179(1) | Isoquercitrin | ||
| 15 | 19.77 | 593 | 285(100) | 257(100), 267(44), 241(38), 229(37) | Kaempferol-3-rutinoside | |
| 16 | 20.65 | 549 | 531(100), 353(94) | 353(100) | Dicaffeoylquinic acid derivative * |
| Samples | C3Gal */** (mg/g DE) | C3Glu */** (mg/g DE) | C3Sam */**/*** (mg/g DE) | RUT */** (mg/g DE) | IQ */** (mg/g DE) | KMP-D1 */**/**** (mg/g DE) | KMP-D2 */**/**** (mg/g DE) | CGA */** (mg/g DE) |
|---|---|---|---|---|---|---|---|---|
| SLE-1 | 1.47 ± 0.04 n | 12.41 n ± 0.19 n | 17.64 ± 0.26 l | 4.17 ± 0.12 n | 2.72 ± 0.08 l | 1.34 ± 0.01 m | 0.25 ± 0.01 n | 1.99 ± 0.04 m |
| UAE-1 | 2.54 ± 0.05 g | 25.18 ± 0.38 hi | 33.67 ± 0.51 ef | 7.79 ± 0.23 jk | 3.77 ± 0.08 f | 3.40 ± 0.08 k | 0.67 ± 0.01 klm | 2.85 ± 0.04 h |
| UAE-2 | 2.28 ± 0.05 ij | 22.59 ± 0.34 k | 30.22 ± 0.45 gh | 7.19 ± 0.22 kl | 2.92 ± 0.06 jkl | 3.30 ± 0.05 kl | 0.69 ± 0.02 jkl | 2.10 ± 0.01 j–m |
| UAE-3 | 1.96 ± 0.06 m | 21.68 ± 0.33 kl | 26.19 ± 0.39 jk | 8.04 ± 0.24 ij | 3.01 ± 0.09 ijk | 3.75 ± 0.09 j | 0.69 ± 0.01 jkl | 2.11 ± 0.02 j–m |
| UAE-4 | 2.37 ± 0.07 hi | 22.62 ± 0.34 k | 31.75 ± 0.48 fg | 6.73 ± 0.20 l | 3.13 ± 0.09 hij | 3.03 ± 0.05 l | 0.70 ± 0.01 jk | 2.20 ± 0.04 jkl |
| UAE-5 | 2.41 ± 0.07 ghi | 26.59 ± 0.40 gh | 35.04 ±0.53 de | 8.47 ± 0.25 hi | 3.23 ± 0.06 ghi | 3.93 ± 0.08 ij | 0.77 ± 0.02 ghi | 2.21 ± 0.00 jk |
| UAE-6 | 2.19 ± 0.04 jkl | 22.77 ± 0.34 jk | 28.34 ± 0.43 hi | 8.86 ± 0.27 fgh | 2.89 ± 0.06 kl | 4.17 ± 0.06 f–i | 0.66 ± 0.02 klm | 2.10 ± 0.01 j–m |
| UAE-7 | 2.20 ± 0.07 jk | 19.63 ± 0.29 m | 24.99 ± 0.37 jk | 9.03 ± 0.27 fgh | 2.81 ± 0.08 kl | 4.28 ± 0.05 fg | 0.65 ± 0.01 lm | 2.07 ± 0.02 lm |
| UAE-8 | 2.08 ± 0.06 klm | 21.59 ± 0.32 kl | 25.98 ± 0.39 jk | 9.09 ± 0.27 fg | 3.04 ± 0.09 ijk | 4.28 ± 0.07 fg | 0.73 ± 0.01 ij | 2.16 ± 0.03 jkl |
| UAE-9 | 2.47 ± 0.07 gh | 24.49 ± 0.37 ij | 33.82 ± 0.51 ef | 4.82 ± 0.14 m | 3.42 ± 0.10 g | 3.27 ± 0.01 kl | 0.80 ± 0.01 fg | 2.40 ± 0.05 i |
| UAE-10 | 2.44 ± 0.05 gh | 27.27 ± 0.41 g | 34.93 ± 0.52 de | 8.70 ± 0.26 gh | 3.35 ± 0.09 gh | 4.04 ± 0.06 ghi | 0.81 ± 0.00 fg | 2.38 ± 0.03 i |
| UAE-11 | 2.36 ± 0.07 hi | 26.75 ± 0.40 gh | 32.65 ± 0.49 f | 9.78 ± 0.29 e | 3.18 ± 0.06 hi | 4.65 ± 0.11 e | 0.80 ± 0.01 fg | 2.23 ± 0.00 j |
| UAE-12 | 2.20 ± 0.07 jk | 21.67 ± 0.33 kl | 26.66 ± 0.40 ij | 8.99 ± 0.27 fgh | 3.02 ± 0.03 ijk | 4.27 ± 0.06 fgh | 0.69 ± 0.02 jkl | 2.08 ± 0.06 klm |
| UAE-13 | 2.09 ± 0.06 klm | 19.86 ± 0.30 lm | 24.55 ± 0.37 jk | 9.07 ± 0.27 fgh | 2.88 ± 0.06 kl | 4.33 ± 0.09 f | 0.75 ± 0.01 hi | 2.00 ± 0.07 m |
| MAE-1 | 2.92 ± 0.06 de | 42.38 ± 0.64 bc | 54.15 ± 0.81 b | 9.47 ± 0.28 ef | 5.12 ± 0.08 c | 3.99 ± 0.01 hij | 0.79 ± 0.01 fgh | 4.03 ± 0.07 c |
| MAE-2 | 3.98 ± 0.08 b | 60.99 ± 0.92 a | 73.22 ± 1.10 a | 16.60 ± 0.50 b | 8.04 ± 0.16 a | 7.11 ± 0.16 b | 0.70 ± 0.01 jk | 6.17 ± 0.12 a |
| MAE-3 | 4.15 ± 0.12 a | 62.22 ± 0.93 a | 73.24 ± 1.10 a | 18.16 ± 0.54 a | 7.41 ± 0.22 b | 8.06 ± 0.23 a | 1.02 ± 0.02 c | 5.91 ± 0.01 b |
| MAE-4 | 3.10 ± 0.09 c | 39.92 ± 0.60 de | 47.84 ± 0.72 c | 12.96 ± 0.39 c | 4.58 ± 0.14 de | 6.02 ± 0.11 c | 0.96 ± 0.01 d | 3.47 ± 0.01 e |
| MAE-5 | 2.04 ± 0.04 lm | 19.10 ± 0.29 m | 24.47 ± 0.37 k | 12.88 ± 0.39 c | 4.56 ± 0.14 de | 6.06 ± 0.03 c | 1.10 ± 0.01 b | 3.32 ± 0.01 f |
| MAE-6 | 2.83 ± 0.06 ef | 41.25 ± 0.62 cd | 52.79 ± 0.79 b | 9.47 ± 0.28 ef | 4.72 ± 0.12 d | 4.04 ± 0.04 ghi | 0.63 ± 0.00 m | 3.67 ± 0.01 d |
| MAE-7 | 3.05 ± 0.09 cd | 43.80 ± 0.66 b | 54.73 ± 0.82 b | 10.44 ± 0.31 d | 4.43 ± 0.13 e | 4.66 ± 0.06 e | 0.75 ± 0.02 hi | 3.35 ± 0.01 ef |
| MAE-8 | 2.78 ± 0.08 ef | 38.81 ± 0.58 e | 46.12 ± 0.69 c | 12.36 ± 0.37 c | 4.59 ± 0.14 de | 5.68 ± 0.06 d | 0.88 ± 0.01 e | 3.45 ± 0.00 ef |
| MAE-9 | 2.70 ± 0.08 f | 30.46 ± 0.46 f | 36.75 ± 0.55 d | 12.81 ± 0.38 c | 4.36 ± 0.13 e | 6.10 ± 0.18 c | 1.21 ± 0.04 a | 3.02 ± 0.07 g |
| MAE-10 | 0.58 ± 0.02 o | 3.25 ± 0.05 o | 4.51 ± 0.07 m | 8.56 ± 0.26 ghi | 3.17 ± 0.11 hi | 4.10 ± 0.00 f–i | 0.82 ± 0.02 f | 2.38 ± 0.02 i |
| Samples | DPPH * IC50 (μg/mL) | A-amylase * IC50 (mg/mL) |
|---|---|---|
| SLE-1 | 14.96 ± 0.77 b– | 6.88 ± 0.68 a |
| UAE-1 | 14.67 ± 1.39 b–e | 3.98 ± 0.21 d–g |
| UAE-2 | 16.20 ± 1.33 a–d | 4.64 ± 0.25 b–e |
| UAE-3 | 12.82 ± 0.72 d–g | 4.63 ± 0.45 b–e |
| UAE-4 | 14.06 ± 0.95 b–e | 4.45 ± 0.31 b–f |
| UAE-5 | 14.63 ± 1.31 b–e | 4.91 ± 0.29 bcd |
| UAE-6 | 14.46 ± 0.94 b–e | 5.08 ± 0.45 bc |
| UAE-7 | 16.65 ± 1.32 abc | 4.79 ± 0.25 b–e |
| UAE-8 | 14.85 ± 1.46 b–e | 4.52 ± 0.41 b–f |
| UAE-9 | 13.51 ± 0.88 c–f | 4.18 ± 0.22 b–f |
| UAE-10 | 18.62 ± 1.59 a | 4.47 ± 0.44 b–f |
| UAE-11 | 17.29 ± 1.49 ab | 4.54 ± 0.25 b–f |
| UAE-12 | 16.37 ± 1.55 a–d | 4.31 ± 0.37 b–f |
| UAE-13 | 18.74 ± 1.69 a | 4.32 ± 0.41 b–f |
| MAE-1 | 12.09 ± 1.01 e–h | 3.00 ± 0.20 gh |
| MAE-2 | 7.88 ± 0.69 i | 2.47 ± 0.13 h |
| MAE-3 | 6.89 ± 0.64 i | 2.18 ± 0.17 h |
| MAE-4 | 11.97 ± 1.09 e–h | 3.57 ± 0.24 fg |
| MAE-5 | 13.33 ± 1.28 c–f | 3.75 ± 0.28 efg |
| MAE-6 | 10.45 ± 0.92 f–i | 3.81 ± 0.21 efg |
| MAE-7 | 8.60 ± 0.63 hi | 4.04 ± 0.27 c–g |
| MAE-8 | 9.45 ± 0.86 ghi | 4.08 ± 0.38 c–f |
| MAE-9 | 7.80 ± 0.76 i | 3.92 ± 0.23 d–g |
| MAE-10 | 10.43 ± 1.02 f–i | 5.14 ± 0.40 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutavski, Z.; Nastić, N.; Živković, J.; Šavikin, K.; Veberič, R.; Medič, A.; Pastor, K.; Jokić, S.; Vidović, S. Black Elderberry Press Cake as a Source of Bioactive Ingredients Using Green-Based Extraction Approaches. Biology 2022, 11, 1465. https://doi.org/10.3390/biology11101465
Mutavski Z, Nastić N, Živković J, Šavikin K, Veberič R, Medič A, Pastor K, Jokić S, Vidović S. Black Elderberry Press Cake as a Source of Bioactive Ingredients Using Green-Based Extraction Approaches. Biology. 2022; 11(10):1465. https://doi.org/10.3390/biology11101465
Chicago/Turabian StyleMutavski, Zorana, Nataša Nastić, Jelena Živković, Katarina Šavikin, Robert Veberič, Aljaž Medič, Kristian Pastor, Stela Jokić, and Senka Vidović. 2022. "Black Elderberry Press Cake as a Source of Bioactive Ingredients Using Green-Based Extraction Approaches" Biology 11, no. 10: 1465. https://doi.org/10.3390/biology11101465