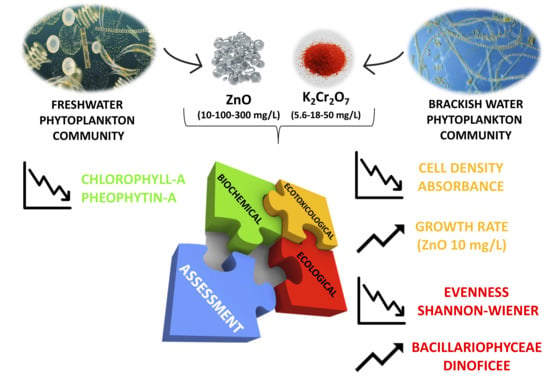
A Multidisciplinary Approach to Evaluate the Effects of Contaminants of Emerging Concern on Natural Freshwater and Brackish Water Phytoplankton Communities
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Supply of Phytoplankton Cultures
2.3. Ecotoxicological Tests
2.4. Preparation of the Algal Inoculums
2.5. Ecotoxicological Assays
2.6. Taxonomical Identification of Phytoplankton, Cell Count, and Ecotoxicological Parameters
2.7. Biochemical Analyses
2.8. Quality Assurance and Quality Control (QA/QC)
2.9. Statistical Analysis
3. Results
3.1. Phytoplankton Community in the Inoculum
3.1.1. Freshwater
3.1.2. Brackish Water
3.2. Freshwater–K2Cr2O7 and ZnO NPs Ecotoxicity Test
3.3. Trend in Ecotoxicological Indexes: Freshwater
3.4. Brackish Water: K2Cr2O7 and ZnO Ecotoxicity Test
3.5. Trend in Ecotoxicological Indexes: Brackish Water
3.6. Trend in Phytoplankton Abundance
3.6.1. Freshwater
3.6.2. Brackish Water
4. Discussion
4.1. Effect of ZnO NPs and K2Cr2O7 on Natural Phytoplankton Communities
4.2. Ecological Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Smital, T. Acute and chronic effects of emerging contaminants. In Emerging Contaminants from Industrial and Municipal Waste; Barceló, D., Petrovic, M., Eds.; Springer: Berlin, Heidelberg, 2008; pp. 105–142. [Google Scholar]
- Petrisor, I.G. Emerging contaminants—the growing problem. Environ. Forensics 2004, 5, 183–184. [Google Scholar] [CrossRef]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. Technol. 2017, 323, 361–380. [Google Scholar] [CrossRef] [Green Version]
- Rathi, B.S.; Kumar, P.S.; Show, P.L. A review on effective removal of emerging contaminants from aquatic systems: Current trends and scope for further research. J. Hazard. Mater. 2021, 409, 124413. [Google Scholar] [CrossRef]
- Gogoi, A.; Mazumder, P.; Tyagi, V.K.; Chaminda, G.T.; An, A.K.; Kumar, M. Occurrence and fate of emerging contaminants in water environment: A review. Ground. Sustain. Dev. 2018, 6, 169–180. [Google Scholar] [CrossRef]
- Taheran, M.; Naghdi, M.; Brar, S.K.; Verma, M.; Surampalli, R.Y. Emerging contaminants: Here today, there tomorrow! Environ. Nanotechnol. Monit. Manag. 2018, 10, 122–126. [Google Scholar] [CrossRef]
- Valbonesi, P.; Profita, M.; Vasumini, I.; Fabbri, E. Contaminants of emerging concern in drinking water: Quality assessment by combining chemical and biological analysis. Sci. Total Environ. 2021, 758, 143624. [Google Scholar] [CrossRef]
- Sturikova, H.; Krystofova, O.; Huska, D.; Adam, V. Zinc, zinc nanoparticles and plants. J. Hazard. Mater. 2018, 349, 101–110. [Google Scholar] [CrossRef]
- Shen, Z.; Chen, Z.; Hou, Z.; Li, T.; Lu, X. Ecotoxicological effect of zinc oxide nanoparticles on soil microorganisms. Front. Env. Sci. Eng. 2015, 9, 912–918. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, W.; Gao, H.; Li, Y.; Tong, X.; Li, K.; Zhu, X.; Wang, Y.; Chen, Y. Behavior and potential impacts of metal-based engineered nanoparticles in aquatic environments. Nanomaterials 2017, 7, 21. [Google Scholar] [CrossRef]
- Nowack, B.; Bucheli, T.D. Occurrence, behavior and effects of nanoparticles in the environment. Environ. Pollut. 2007, 150, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Tourinho, P.S.; Van Gestel, C.A.; Lofts, S.; Svendsen, C.; Soares, A.M.; Loureiro, S. Metal-based nanoparticles in soil: Fate, behavior, and effects on soil invertebrates. Environ. Toxicol. Chem. 2012, 31, 1679–1692. [Google Scholar] [CrossRef] [PubMed]
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef] [Green Version]
- Kimbrough, D.E.; Cohen, Y.; Winer, A.M.; Creelman, L.; Mabuni, C. A critical assessment of chromium in the environment. Crit. Rev. Environ. Sci. Tec. 1999, 29, 1–46. [Google Scholar] [CrossRef]
- He, X.; Li, P. Surface water pollution in the middle Chinese Loess Plateau with special focus on hexavalent chromium (Cr6+): Occurrence, sources and health risks. Expos. Health 2020, 12, 385–401. [Google Scholar] [CrossRef]
- Ertani, A.; Mietto, A.; Borin, M.; Nardi, S. Chromium in agricultural soils and crops: A review. Water Air Soil Pollut. 2017, 228, 190. [Google Scholar] [CrossRef]
- Tseng, C.H.; Lee, I.H.; Chen, Y.C. Evaluation of hexavalent chromium concentration in water and its health risk with a system dynamics model. Sci. Total Environ. 2019, 669, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Stanin, F. The transport and fate of Cr(VI) in the Environment. In Chromium (VI) Handbook; CRC Press: Boca Raton, FL, USA, 2004; pp. 161–204. [Google Scholar]
- Benjamin, L.V.; Kutty, R. Sub-lethal effects of potassium dichromate on hematological and histological parameters in climbing perch, Anabas testudineus (Anabantidae). Int. J. Aquat. Biol. 2019, 7, 140–145. [Google Scholar]
- Gopi, R.A.; Ayyappan, S.; Chandrasehar, G.; Krishna, V.; Goparaju, A. Effect of potassium dichromate on the survival and reproduction of Daphnia magna. Bull. Env. Pharmacol. 2012, 1, 89–94. [Google Scholar]
- Kikuchi, M.; Syudo, A.; Hukumori, M.; Naito, C.; Sawai, J. Changes in aquatic toxicity of potassium dichromate as a function of water quality parameters. Chemosphere 2017, 170, 113–117. [Google Scholar] [CrossRef]
- Sathicq, M.B.; Gómez, N. Effects of hexavalent chromium on phytoplankton and bacterioplankton of the Río de la Plata estuary: An ex-situ assay. Environ. Monit. Assess. 2018, 190, 1–9. [Google Scholar] [CrossRef]
- Kalantzi, I.; Mylona, K.; Toncelli, C.; Bucheli, T.D.; Knauer, K.; Pergantis, S.A.; Pitta, P.; Tsiola, A.; Tsapakis, M. Ecotoxicity of silver nanoparticles on plankton organisms: A review. J. Nanopart. Res. 2019, 21. [Google Scholar]
- European Commission. Aquatic Toxicity. Available online: https://ec.europa.eu/jrc/en/eurl/ecvam/alternative-methods-toxicity-testing/validated-test-methods/aquatic-toxicity (accessed on 10 August 2021).
- Vallina, S.M.; Cermeno, P.; Dutkiewicz, S.; Loreau, M.; Montoya, J.M. Phytoplankton functional diversity increases ecosystem productivity and stability. Ecol. Model. 2017, 361, 184–196. [Google Scholar] [CrossRef]
- Moore, J.K.; Abbott, M.R. Phytoplankton chlorophyll distributions and primary production in the Southern Ocean. J. Geophys. Res. Oceans 2000, 105, 28709–28722. [Google Scholar] [CrossRef]
- Gorbunov, M.Y.; Falkowski, P.G. Using chlorophyll fluorescence kinetics to determine photosynthesis in aquatic ecosystems. Limnol. Oceanogr. 2021, 66, 1–13. [Google Scholar] [CrossRef]
- Leavitt, P.R.; Hodgson, D.A. Sedimentary pigments. In Tracking Environmental Change Using Lake Sediments; Smol, J.P., Birks, H.J.B., Last, W.M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 295–325. [Google Scholar]
- ISO 8692:1989. Water Quality—Freshwater Algal Growth Inhibition Test with Scenedesmus subspicatus and Selenastrum capricornutum. Available online: https://www.iso.org/standard/16096.html (accessed on 10 August 2021).
- ISO 10253:2016. Water Quality—Marine Algal Growth Inhibition Test with Skeletonema sp. and Phaeodactylum tricornutum. Available online: https://www.iso.org/standard/66657.html. (accessed on 10 August 2021).
- Tang, Y.; Xin, H.; Yang, S.; Guo, M.; Malkoske, T.; Yin, D.; Xia, S. Environmental risks of ZnO nanoparticle exposure on Microcystis aeruginosa: Toxic effects and environmental feedback. Aquat. Toxicol. 2018, 204, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef]
- BS EN 15204:2006. Water Quality. Guidance Standard on the Enumeration of Phytoplankton Using Inverted Microscopy (Utermoehl technique). Available online: https://shop.bsigroup.com/ProductDetail?pid=000000000030085818 (accessed on 10 August 2021).
- ISPRA. Metodologie di Studio del Plancton Marino. Available online: https://www.isprambiente.gov.it/files/pubblicazioni/manuali-lineeguida/9171_MLG56_2010.pdf (accessed on 10 August 2021).
- ICRAM. Guida al Riconoscimento del Plancton dei Mari Italiani. Available online: https://www.isprambiente.gov.it/it/pubblicazioni/manuali-e-linee-guida/guida-al-riconoscimento-del-plancton-dei-mari (accessed on 10 August 2021).
- Lavoie, I.; Hamilton, P.B.; Compeau, S.; Grenier, M.; Dillon, P. Guide D’identification des Diatomè es des Riviéres de l’Est du Canada; Presses de l’Université du Québec: Québec City, QC, Canada, 2008. [Google Scholar]
- OECD-Guideline for Testing of Chemicals. No. 201, Freshwater Algae and Cyanobacteria, Growth Inhibition Test. Available online: https://www.oecd-ilibrary.org/docserver/9789264069923-en.pdf?expires=1628806714&id=id&accname=guest&checksum=B2E5000024A220A26026171B48670D1D (accessed on 10 August 2021).
- Aravantinou, A.F.; Tsarpali, V.; Dailianis, S.; Manariotis, I.D. Effect of cultivation media on the toxicity of ZnO nanoparticles to freshwater and marine microalgae. Ecotoxicol. Environ. Saf. 2015, 114, 109–116. [Google Scholar] [CrossRef]
- Longhi, M.L.; Beisner, B.E. Patterns in taxonomic and functional diversity of lake phytoplankton. Freshw. Biol. 2010, 55, 1349–1366. [Google Scholar] [CrossRef]
- Jun, S.; Dongyan, L. The application of diversity indices in marine phytoplankton studies. Acta Oceanol. Sin. 2004, 26, 62–75. [Google Scholar]
- APAT—IRSA/CNR. Manuali e Linee Guida 29/2003. Metodologie Analitiche per il Controllo della Qualità delle Acque. Par. 9020. Available online: https://www.isprambiente.gov.it/it/pubblicazioni/manuali-e-linee-guida/metodi-analitici-per-le-acque (accessed on 10 August 2021).
- Chia, M.A.; Lorenzi, A.S.; Ameh, I.; Dauda, S.; Cordeiro-Araújo, M.K.; Agee, J.T.; Okpanachi, I.Y.; Adesalu, A.T. Susceptibility of phytoplankton to the increasing presence of active pharmaceutical ingredients (APIs) in the aquatic environment: A review. Aquat. Toxicol. 2021, 234, 105809. [Google Scholar] [CrossRef]
- Rodrigues, L.H.R.; Arenzon, A.; Raya-Rodrigues, M.T.; Fontoura, N.F. Algal density assessed by spectrophotometry: A calibration curve for the unicellular algae Pseudokirchneriella subcapitata. J. Environ. Chem. Ecotoxicol. 2011, 3, 225–228. [Google Scholar]
- Miao, A.J.; Zhang, X.Y.; Luo, Z.; Chen, C.S.; Chin, W.C.; Santschi, P.H.; Quigg, A. Zinc oxide–engineered nanoparticles: Dissolution and toxicity to marine phytoplankton. Environ. Toxicol. Chem. 2010, 29, 2814–2822. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneshwari, M.; Iswarya, V.; Chandrasekaran, N.; Mukherjee, A. A review on ecotoxicity of zinc oxide nanoparticles on freshwater algae. In Nanomaterials: Ecotoxicity, Safety, and Public Perception; Rai, M., Biswas, J.K., Eds.; Springer: Cham, Switzerland, 2018; pp. 191–206. [Google Scholar]
- Gunawan, C.; Sirimanoonphan, A.; Teoh, W.Y.; Marquis, C.P.; Amal, R. Submicron and nano formulations of titaniumdioxide and zinc oxide stimulate unique cellular toxicological responses in the green microalga Chlamydomonas reinhardtii. J. Hazard. Mater. 2013, 260, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.D.; Santhanam, P.; Ananth, S.; Devi, A.S.; Nandakumar, R.; Prasath, B.B.; Jeyanthi, S.; Jayalakshmi, T.; Ananthi, P. Effect of different dosages of zinc on the growth and biomass in five marine microalgae. Int. J. Fish Aquat. 2014, 6, 1–8. [Google Scholar]
- Wong, P.T.S.; Chau, Y.K. Zinc toxicity to freshwater algae. Toxic. Assess. 1990, 5, 167–177. [Google Scholar] [CrossRef]
- Kusk, K.O.; Nyholm, N. Toxic effects of chlorinated organic compounds and potassium dichromate on growth rate and photosynthesis of marine phytoplankton. Chemosphere 1992, 25, 875–886. [Google Scholar] [CrossRef]
- Kadiiska, M.B.; Xiang, Q.H.; Mason, R.P. In vivo free radical generation by chromium (VI): An electron spin resonance spin-trapping investigation. Chem. Res. Toxicol. 1994, 7, 800–805. [Google Scholar] [CrossRef]
- Cervantes, C.; Campos-García, J.; Devars, S.; Gutiérrez-Corona, F.; Loza-Tavera, H.; Torres-Guzmán, J.C.; Moreno-Sánchez, R. Interactions of chromium with microorganisms and plants. FEMS Microbiol. Rev. 2001, 25, 335–347. [Google Scholar] [CrossRef]
- Bharagava, R.N.; Mishra, S. Hexavalent chromium reduction potential of Cellulosimicrobium sp. isolated from common effluent treatment plant of tannery industries. Ecotoxicol. Environ. Saf. 2018, 147, 102–109. [Google Scholar]
- Hasle, G.R.; Lange, C.B. Freshwater and brackish water Thalassiosira (Bacillariophyceae): Taxa with tangentially undulated valves. Phycologia 1989, 28, 120–135. [Google Scholar] [CrossRef]
- El Gammal, M.A.M.; Nageeb, M.; Al-Sabeb, S. Phytoplankton abundance in relation to the quality of the coastal water–Arabian Gulf, Saudi Arabia. Egypt. J. Aquat. Res. 2017, 43, 275–282. [Google Scholar] [CrossRef]
- Pistocchi, R.; Dao, L.T.H.; Mikulic, P.; Beardall, J. Metal Pollution in Water: Toxicity, Tolerance and Use of Algae as a Potential Remediation Solution. In Grand Challenges in Algae Biotechnology; Hallmann, A., Rampelotto, P.H., Eds.; Springer: Cham, Switzerland, 2019; pp. 471–500. [Google Scholar]
- Shen, Q.H.; Zhi, T.T.; Cheng, L.H.; Xu, X.H.; Chen, H.L. Hexavalent chromium detoxification by nonliving Chlorella vulgaris cultivated under tuned conditions. Chem. Eng. Technol. 2013, 228, 993–1002. [Google Scholar] [CrossRef]
- Monni, S.; Uhlig, C.; Hansen, E.; Magel, E. Ecophysiological responses of Empetrum nigrum to heavy metal pollution. Environ. Pollut. 2001, 112, 121–129. [Google Scholar] [CrossRef]
- Baho, D.L.; Pomati, F.; Leu, E.; Hessen, D.O.; Moe, S.J.; Norberg, J.; Nizzetto, L. A single pulse of diffuse contaminants alters the size distribution of natural phytoplankton communities. Sci. Total Environ. 2019, 683, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Ptacnik, R.; Solimini, A.G.; Andersen, T.; Tamminen, T.; Brettum, P.; Lepistö, L.; Willén, E.; Rekolainen, S. Diversity predicts stability and resource use efficiency in natural phytoplankton communities. Proc. Natl. Acad. Sci. USA 2008, 105, 5134–5138. [Google Scholar] [CrossRef] [Green Version]
- Echeveste, P.; Dachs, J.; Berrojalbiz, N.; Agustí, S. Decrease in the abundance and viability of oceanic phytoplankton due to trace levels of complex mixtures of organic pollutants. Chemosphere 2010, 81, 161–168. [Google Scholar] [CrossRef]
- Walsh, G.E. Chapter 12 Toxic effects of pollutants on Plankton. In Principles of Ecotoxicology; Butler, G.C., Ed.; Wiley and Sons: New York, NY, USA, 1978; pp. 1–18. [Google Scholar]
- Hammer, K.J.; Kragh, T.; Sand-Jensen, K. Inorganic carbon promotes photosynthesis, growth, and maximum biomass of phytoplankton in eutrophic water bodies. Freshw. Biol. 2019, 64, 1956–1970. [Google Scholar] [CrossRef] [Green Version]
- Hlaili, A.S.; Niquil, N.; Legendre, L. Planktonic food webs revisited: Reanalysis of re-sults from the linear inverse approach. Prog. Oceanogr. 2014, 120, 216–229. [Google Scholar] [CrossRef]
- Lampert, W. A method for determining food selection by zooplankton. Limnol. Oceanogr. 1974, 19, 995–998. [Google Scholar] [CrossRef]
- Moustaka-Gouni, M.; Vardaka, E.; Michaloudi, E.; Kormas, K.A.; Tryfon, E.; Mihalatou, H.; Gkelis, S.; Lanaras, T. Plankton food web structure in a eutrophic polymicticlake with a history in toxic cyanobacterial blooms. Limnol. Oceanogr. 2006, 51, 715–727. [Google Scholar] [CrossRef] [Green Version]
- Saab, M.A.-A.; Hassoun, A.E.R. Effects of organic pollution on environmental conditions and the phytoplankton community in the central Lebanese coastal waters with special attention to toxic algae. Reg. Stud. Mar. Sci. 2017, 10, 38–51. [Google Scholar] [CrossRef]








| Test | NC | Cr 50 mg/L | Cr 18 mg/L | Cr 5.6 mg/L |
|---|---|---|---|---|
| Total cell | 49,288 ± 20.87 | 47,025 ± 25.89 | 44,946 ± 36.98 | 45,723 ± 45.73 |
| Density (cell/mL) | 1972 a ± 16.78 | 1881 a ± 17.45 | 1798 b ± 89.12 | 1829 a ± 19.45 |
| Absorbance | 0.079 a ± 0.004 | 0.069 a ± 0.002 | 0.054 b ± 0.007 | 0.064 a ± 0.009 |
| Number of species | 14 | 13 | 12 | 12 |
| Shannon-Wiener | 1.99 a ± 0.78 | 1.73 a ± 0.67 | 1.91 a ± 0.58 | 1.94 a ± 0.29 |
| Evenness | 0.16 a ± 0.09 | 0.13 a ± 0.08 | 0.16 a ± 0.12 | 0.16 a ± 0.09 |
| Chl-a (μg/L) | 0.002 a ± 0.001 | 0.002 a ± 0.002 | 0.002 a ± 0.001 | 0.001 a ± 0.001 |
| Phe-a (μg/L) | 0.003 a ± 0.002 | 0.001 a ± 0.002 | 0.001 a ± 0.002 | 0.003 a ± 0.001 |
| Growth rate | 0.21 a ± 0.08 | 0.20 a ± 0.09 | 0.18 a ± 0.08 | 0.19 a ± 0.08 |
| Growth inhibition (%) | - | 7.3 ± 2.23 | 14.4 ± 3.34 | 11.7 ± 2.16 |
| Test | NC | Zn 300 mg/L | Zn 100 mg/L | Zn 10 mg/L |
| Total cell | 49288 ± 20.87 | 34665 ± 78.67 | 45707 ± 18.43 | 123634 ± 21.35 |
| Cell density (cell/mL) | 1972 a ± 16.78 | 1387 b ± 28.54 | 1828 a ± 15.67 | 4945 b ± 12.71 |
| Absorbance | 0.079 a ± 0.020 | 0.027 b ± 0.002 | 0.090 a ± 0.001 | 0.305 b ± 0.04 |
| Number of species | 14 | 12 | 12 | 14 |
| Shannon–Wiener | 1.89 a ± 0.78 | 1.77 a ± 0.67 | 1.80 a ± 0.56 | 1.58 a ± 0.23 |
| Evenness | 0.13 a ± 0.09 | 0.15 a ± 0.09 | 0.15 a ± 0.08 | 0.11 a ± 0.06 |
| Chl-a (μg/L) | 0.002 a ± 0.001 | 0.001 a ± 0.001 | 0.002 a ± 0.001 | 0.008 b ± 0.002 |
| Phe-a (μg/L) | 0.003 a ± 0.002 | 0.003 a ± 0.002 | 0.002 a ± 0.001 | 0.001 a ± 0.001 |
| Growth rate | 0.21 a ± 0.08 | 0.10 b ± 0.09 | 0.19 a ± 0.13 | 0.52 b ± 0.18 |
| Growth inhibition (%) | - | 55.03 ± 6.67 | 11.79 ± 3.45 | −143.81 ± 56.24 |
| Test | NC | Cr 50 mg/L | Cr 18 mg/L | Cr 5.6 mg/L |
|---|---|---|---|---|
| Total cell | 35,482 ± 21.17 | 36,893 ± 16.78 | 23,979 | 36,966 |
| Density (cell/mL) | 1419.28 a ± 14.56 | 1475.72 a ± 21.56 | 959.16 b ± 11.86 | 1478.64 a ± 10.76 |
| Absorbance | 0.208 a ± 0.01 | 0.250 b ± 0.001 | 0.134 b ± 0.005 | 0.260 b ± 0.002 |
| Number of species | 11 | 11 | 12 | 13 |
| Shannon–Wiener | 2.02 a ± 0.07 | 1.44 b ± 0.12 | 1.92 a ± 0.15 | 1.89 a ± 0.21 |
| Evenness | 0.18 a ± 0.05 | 0.13 b ± 0.08 | 0.16 a ± 0.02 | 0.14 a ± 0.3 |
| Chl-a (μg/L) | 0.012 a ± 0.002 | <LOQ | <LOQ | 0.012 a ± 0.08 |
| Phe-a (μg/L) | 0.032 ± 0.001 | <LOQ | <LOQ | <LOQ |
| Growth rate | 0.57 a ± 0.11 | 0.56 a ± 0.19 | 0.44 a ± 0.12 | 0.59 a ± 0.17 |
| Growth inhibition (%) | - | −2.27 ± 1.23 | 22.82 ± 4.57 | −2.39 ± 1.23 |
| Test | NC | Zn 300 mg/L | Zn 100 mg/L | Zn 10 mg/L |
| Total cell | 35482 ± 21.17 | - | 13882 ± 12.34 | 45504 ± 12.47 |
| Density (cell/mL) | 1419.28 a ± 14.56 | - | 555.28 b ± 16.54 | 1820.16 b ± 16.75 |
| Absorbance | 0.208 a ± 0.01 | - | 0.082 b ± 0.015 | 0.249 b ± 0.06 |
| Number of species | 11 | - | 9 | 13 |
| Shannon–Wiener | 2.02 a ± 0.07 | - | 1.85 a ± 0.03 | 1.77 a ± 0.08 |
| Evenness | 0.18 a ± 0.05 | - | 0.21 a ± 0.07 | 0.14 a ± 0.05 |
| Chl-a (μg/L) | 0.012 a ± 0.002 | - | <LOQ | 0.084 b ± 0.002 |
| Phe-a (μg/L) | 0.032 a ± 0.001 | - | <LOQ | 0.290 b ± 0.18 |
| Growth rate | 0.57 a ± 0.10 | - | 0.26 b ± 0.15 | 0.66 b ± 0.09 |
| Growth inhibition (%) | - | - | 54.65 ± 6.23 | −14.49 ± 4.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastorino, P.; Broccoli, A.; Bagolin, E.; Anselmi, S.; Cavallo, A.; Prearo, M.; Renzi, M. A Multidisciplinary Approach to Evaluate the Effects of Contaminants of Emerging Concern on Natural Freshwater and Brackish Water Phytoplankton Communities. Biology 2021, 10, 1039. https://doi.org/10.3390/biology10101039
Pastorino P, Broccoli A, Bagolin E, Anselmi S, Cavallo A, Prearo M, Renzi M. A Multidisciplinary Approach to Evaluate the Effects of Contaminants of Emerging Concern on Natural Freshwater and Brackish Water Phytoplankton Communities. Biology. 2021; 10(10):1039. https://doi.org/10.3390/biology10101039
Chicago/Turabian StylePastorino, Paolo, Andrea Broccoli, Elisa Bagolin, Serena Anselmi, Andrea Cavallo, Marino Prearo, and Monia Renzi. 2021. "A Multidisciplinary Approach to Evaluate the Effects of Contaminants of Emerging Concern on Natural Freshwater and Brackish Water Phytoplankton Communities" Biology 10, no. 10: 1039. https://doi.org/10.3390/biology10101039