Developments of Core/Shell Chitosan-Based Nanofibers by Electrospinning Techniques: A Review
Abstract
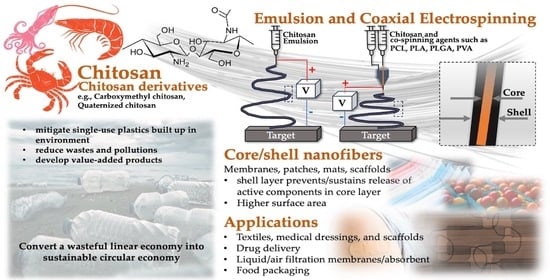
:1. Introduction
2. Overview of Electrospinning of Chitosan
3. Emulsion Electrospinning
4. Coaxial Electrospinning of Chitosan
5. Applications
5.1. Textiles, Medical Dressings, and Scaffolds
5.2. Filtration Membranes/Absorbent
5.3. Food Packaging
6. Conclusions and Challenges
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xue, C.; Wilson, L.D. An Overview of the Design of Chitosan-Based Fiber Composite Materials. J. Compos. Sci. 2021, 5, 160. [Google Scholar] [CrossRef]
- UN Environment Programme. Our Planet is Choking on Plastic. Available online: https://www.unep.org/interactives/beat-plastic-pollution/ (accessed on 29 November 2023).
- OECD. Plastic Pollution is Growing Relentlessly as Waste Management and Recycling Fall Short, Says OECD. Available online: https://www.oecd.org/environment/plastic-pollution-is-growing-relentlessly-as-waste-management-and-recycling-fall-short.htm (accessed on 29 November 2023).
- Lin, Z.; Chen, H.; Li, S.; Li, X.; Wang, J.; Xu, S. Electrospun Food Polysaccharides Loaded with Bioactive Compounds: Fabrication, Release, and Applications. Polymers 2023, 15, 2318. [Google Scholar] [CrossRef]
- Hameed, A.Z.; Raj, S.A.; Kandasamy, J.; Baghdadi, M.A.; Shahzad, M.A. Chitosan: A Sustainable Material for Multifarious Applications. Polymers 2022, 14, 2335. [Google Scholar] [CrossRef]
- Rashki, S.; Asgarpour, K.; Tarrahimofrad, H.; Hashemipour, M.; Ebrahimi, M.S.; Fathizadeh, H.; Khorshidi, A.; Khan, H.; Marzhoseyni, Z.; Salavati-Niasari, M.; et al. Chitosan-based nanoparticles against bacterial infections. Carbohydr. Polym. 2021, 251, 117108. [Google Scholar] [CrossRef] [PubMed]
- Taokaew, S.; Kriangkrai, W. Chitinase-Assisted Bioconversion of Chitinous Waste for Development of Value-Added Chito-Oligosaccharides Products. Biology 2023, 12, 87. [Google Scholar] [CrossRef]
- Rahimi, M.; Mir, S.M.; Baghban, R.; Charmi, G.; Plummer, C.M.; Shafiei-Irannejad, V.; Soleymani, J.; Pietrasik, J. Chitosan-based biomaterials for the treatment of bone disorders. Int. J. Biol. Macromol. 2022, 215, 346–367. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ji, Y.; Zhu, Y.; Wu, X.; Mei, L.; Zhang, H.; Deng, J.; Wang, S. Antibacterial effect of chitosan and its derivative on Enterococcus faecalis associated with endodontic infection. Exp. Ther. Med. 2020, 19, 3805–3813. [Google Scholar] [CrossRef] [PubMed]
- Malatjie, K.I.; Ndlovu, L.N.; Mishra, A.K.; Mishra, S.B. A Review on smart nanotextiles for filtration. Nanotechnol. Environ. Eng. 2023, 8, 449–459. [Google Scholar] [CrossRef]
- Tien, N.D.; Lyngstadaas, S.P.; Mano, J.F.; Blaker, J.J.; Haugen, H.J. Recent Developments in Chitosan-Based Micro/Nanofibers for Sustainable Food Packaging, Smart Textiles, Cosmeceuticals, and Biomedical Applications. Molecules 2021, 26, 2683. [Google Scholar] [CrossRef]
- Li, J.; Tian, X.; Hua, T.; Fu, J.; Koo, M.; Chan, W.; Poon, T. Chitosan Natural Polymer Material for Improving Antibacterial Properties of Textiles. ACS Appl. Bio Mater. 2021, 4, 4014–4038. [Google Scholar] [CrossRef]
- Kalantari, K.; Afifi, A.M.; Jahangirian, H.; Webster, T.J. Biomedical applications of chitosan electrospun nanofibers as a green polymer—Review. Carbohydr. Polym. 2019, 207, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Mirbagheri, M.S.; Akhavan-Mahdavi, S.; Hasan, A.; Kharazmi, M.S.; Jafari, S.M. Chitosan-based electrospun nanofibers for diabetic foot ulcer management; recent advances. Carbohydr. Polym. 2023, 313, 120512. [Google Scholar] [CrossRef]
- Biswal, A.; Purohit, S.S.; Swain, S.K. Chitosan based composite scaffolds in skin wound repair: A review. J. Drug Deliv. Sci. Technol. 2023, 84, 104549. [Google Scholar] [CrossRef]
- Sánchez-Machado, D.I.; Maldonado-Cabrera, A.; López-Cervantes, J.; Maldonado-Cabrera, B.; Chávez-Almanza, A.F. Therapeutic effects of electrospun chitosan nanofibers on animal skin wounds: A systematic review and meta-analysis. Int. J. Pharm. X 2023, 5, 100175. [Google Scholar] [CrossRef] [PubMed]
- Valachová, K.; El Meligy, M.A.; Šoltés, L. Hyaluronic acid and chitosan-based electrospun wound dressings: Problems and solutions. Int. J. Biol. Macromol. 2022, 206, 74–91. [Google Scholar] [CrossRef]
- Chen, S.; Tian, H.; Mao, J.; Ma, F.; Zhang, M.; Chen, F.; Yang, P. Preparation and application of chitosan-based medical electrospun nanofibers. Int. J. Biol. Macromol. 2023, 226, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, A.; Tripathi, D.M.; Sundarrajan, S.; Venugopal, J.R.; Ramakrishna, S.; Kaur, S. Evolution of Electrospinning in Liver Tissue Engineering. Biomimetics 2022, 7, 149. [Google Scholar] [CrossRef]
- Cui, C.; Sun, S.; Wu, S.; Chen, S.; Ma, J.; Zhou, F. Electrospun chitosan nanofibers for wound healing application. Eng. Regen. 2021, 2, 82–90. [Google Scholar] [CrossRef]
- Khan, S.A.; Khan, S.B.; Kamal, T.; Asiri, A.M.; Akhtar, K. Recent Development of Chitosan Nanocomposites for Environmental Applications. Recent Pat. Nanotechnol. 2016, 10, 181–188. [Google Scholar] [CrossRef]
- Anwar, Y. Antibacterial and lead ions adsorption characteristics of chitosan-manganese dioxide bionanocomposite. Int. J. Biol. Macromol. 2018, 111, 1140–1145. [Google Scholar] [CrossRef]
- Shao, Z.; Chen, H.; Wang, Q.; Kang, G.; Wang, X.; Li, W.; Liu, Y.; Zheng, G. High-performance multifunctional electrospun fibrous air filter for personal protection: A review. Sep. Purif. Technol. 2022, 302, 122175. [Google Scholar] [CrossRef] [PubMed]
- El-Aswar, E.I.; Ramadan, H.; Elkik, H.; Taha, A.G. A comprehensive review on preparation, functionalization and recent applications of nanofiber membranes in wastewater treatment. J. Environ. Manag. 2022, 301, 113908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ru, Z.; Sun, Y.; Zhang, M.; Wang, J.; Ge, M.; Liu, H.; Wu, S.; Cao, C.; Ren, X.; et al. Recent advances in applications for air pollutants purification and perspectives of electrospun nanofibers. J. Clean. Prod. 2022, 378, 134567. [Google Scholar] [CrossRef]
- Abdulhamid, M.A.; Muzamil, K. Recent progress on electrospun nanofibrous polymer membranes for water and air purification: A review. Chemosphere 2023, 310, 136886. [Google Scholar] [CrossRef]
- Scaffaro, R.; Citarrella, M.C. Nanofibrous Polymeric Membranes for Air Filtration Application: A Review of Progress after the COVID-19 Pandemic. Macromol. Mater. Eng. 2023, 308, 2300072. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Emerging Chitosan-Based Films for Food Packaging Applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef]
- Kaya, M.; Khadem, S.; Cakmak, Y.S.; Mujtaba, M.; Ilk, S.; Akyuz, L.; Salaberria, A.M.; Labidi, J.; Abdulqadir, A.H.; Deligöz, E. Antioxidative and antimicrobial edible chitosan films blended with stem, leaf and seed extracts of Pistacia terebinthus for active food packaging. RSC Adv. 2018, 8, 3941–3950. [Google Scholar] [CrossRef]
- Zhao, L.; Duan, G.; Zhang, G.; Yang, H.; He, S.; Jiang, S. Electrospun Functional Materials toward Food Packaging Applications: A Review. Nanomaterials 2020, 10, 150. [Google Scholar] [CrossRef]
- Munteanu, B.S.; Vasile, C. Encapsulation of Natural Bioactive Compounds by Electrospinning—Applications in Food Storage and Safety. Polymers 2021, 13, 3771. [Google Scholar] [CrossRef]
- Elamri, A.; Zdiri, K.; Bouzir, D.; Hamdaoui, M. Use of chitosan as antimicrobial, antiviral and antipollution agent in textile finishing. Fibres Text. 2022, 29, 51–70. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Pramanik, S.; Abdelgawad, M.A.; Abualsoud, B.M.; Kadi, A.; Ansari, M.J.; Deepak, A. Recent Advances of Chitosan Formulations in Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 10975. [Google Scholar] [CrossRef]
- Picos-Corrales, L.A.; Morales-Burgos, A.M.; Ruelas-Leyva, J.P.; Crini, G.; García-Armenta, E.; Jimenez-Lam, S.A.; Ayón-Reyna, L.E.; Rocha-Alonzo, F.; Calderón-Zamora, L.; Osuna-Martínez, U.; et al. Chitosan as an Outstanding Polysaccharide Improving Health-Commodities of Humans and Environmental Protection. Polymers 2023, 15, 526. [Google Scholar] [CrossRef]
- Malik, S.; Sundarrajan, S.; Hussain, T.; Nazir, A.; Ayyoob, M.; Berto, F.; Ramakrishna, S. Sustainable nanofibers in tissue engineering and biomedical applications. Mater. Des. Process. Commun. 2021, 3, e202. [Google Scholar] [CrossRef]
- Lauricella, M.; Succi, S.; Zussman, E.; Pisignano, D.; Yarin, A.L. Models of polymer solutions in electrified jets and solution blowing. Rev. Mod. Phys. 2020, 92, 035004. [Google Scholar] [CrossRef]
- Abrishamkar, A.; Nilghaz, A.; Saadatmand, M.; Naeimirad, M.; de Mello, A.J. Microfluidic-assisted fiber production: Potentials, limitations, and prospects. Biomicrofluidics 2022, 16, 061504. [Google Scholar] [CrossRef]
- Song, J.; Kim, M.; Lee, H. Recent Advances on Nanofiber Fabrications: Unconventional State-of-the-Art Spinning Techniques. Polymers 2020, 12, 1386. [Google Scholar] [CrossRef]
- Rocha, J.; Araújo, J.C.; Fangueiro, R.; Ferreira, D.P. Wetspun Polymeric Fibrous Systems as Potential Scaffolds for Tendon and Ligament Repair, Healing and Regeneration. Pharmaceutics 2022, 14, 2526. [Google Scholar] [CrossRef]
- Barhoum, A.; Pal, K.; Rahier, H.; Uludag, H.; Kim, I.S.; Bechelany, M. Nanofibers as new-generation materials: From spinning and nano-spinning fabrication techniques to emerging applications. Appl. Mater. Today 2019, 17, 1–35. [Google Scholar] [CrossRef]
- Ye, P.; Guo, Q.; Zhang, Z.; Xu, Q. High-Speed Centrifugal Spinning Polymer Slip Mechanism and PEO/PVA Composite Fiber Preparation. Nanomaterials 2023, 13, 1277. [Google Scholar] [CrossRef] [PubMed]
- Gholipour-Kanani, A.; Daneshi, P. A Review on Centrifugal and Electro-Centrifugal Spinning as New Methods of Nanofibers Fabrication. J. Text. Polym. 2022, 10, 41–55. [Google Scholar]
- Wang, X.-X.; Yu, G.-F.; Zhang, J.; Yu, M.; Ramakrishna, S.; Long, Y.-Z. Conductive polymer ultrafine fibers via electrospinning: Preparation, physical properties and applications. Prog. Mater. Sci. 2021, 115, 100704. [Google Scholar] [CrossRef]
- Negm, N.A.; Hefni, H.H.H.; Abd-Elaal, A.A.A.; Badr, E.A.; Abou Kana, M.T.H. Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol. 2020, 152, 681–702. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Wang, J.-P.; Sun, X.-B.; Wang, X.-X.; Jiang, J.-Y.; Zhang, Z.-G.; Li, P.; Qu, C.-H.; Long, Y.-Z.; Yu, G.-F. Ultra uniform metal−organic framework-5 loading along electrospun chitosan/polyethylene oxide membrane fibers for efficient PM2.5 removal. J. Clean. Prod. 2021, 291, 125270. [Google Scholar] [CrossRef]
- Lu, S.; Tao, J.; Liu, X.; Wen, Z. Baicalin-liposomes loaded polyvinyl alcohol-chitosan electrospinning nanofibrous films: Characterization, antibacterial properties and preservation effects on mushrooms. Food Chem. 2022, 371, 131372. [Google Scholar] [CrossRef] [PubMed]
- Afshar, S.; Rashedi, S.; Nazockdast, H.; Ghazalian, M. Preparation and characterization of electrospun poly(lactic acid)-chitosan core-shell nanofibers with a new solvent system. Int. J. Biol. Macromol. 2019, 138, 1130–1137. [Google Scholar] [CrossRef]
- Su, S.; Bedir, T.; Kalkandelen, C.; Ozan Başar, A.; Turkoğlu Şaşmazel, H.; Bulent Ustundag, C.; Sengor, M.; Gunduz, O. Coaxial and emulsion electrospinning of extracted hyaluronic acid and keratin based nanofibers for wound healing applications. Eur. Polym. J. 2021, 142, 110158. [Google Scholar] [CrossRef]
- Augustine, R.; Rehman, S.R.U.; Ahmed, R.; Zahid, A.A.; Sharifi, M.; Falahati, M.; Hasan, A. Electrospun chitosan membranes containing bioactive and therapeutic agents for enhanced wound healing. Int. J. Biol. Macromol. 2020, 156, 153–170. [Google Scholar] [CrossRef]
- Abasalta, M.; Asefnejad, A.; Khorasani, M.T.; Saadatabadi, A.R.; Irani, M. Adsorption and sustained release of doxorubicin from N-carboxymethyl chitosan/polyvinyl alcohol/poly(ε-caprolactone) composite and core-shell nanofibers. J. Drug Deliv. Sci. Technol. 2022, 67, 102937. [Google Scholar] [CrossRef]
- Farboudi, A.; Mahboobnia, K.; Chogan, F.; Karimi, M.; Askari, A.; Banihashem, S.; Davaran, S.; Irani, M. UiO-66 metal organic framework nanoparticles loaded carboxymethyl chitosan/poly ethylene oxide/polyurethane core-shell nanofibers for controlled release of doxorubicin and folic acid. Int. J. Biol. Macromol. 2020, 150, 178–188. [Google Scholar] [CrossRef]
- Cui, C.; Sun, S.; Li, X.; Chen, S.; Wu, S.; Zhou, F.; Ma, J. Optimizing the chitosan-PCL based membranes with random/aligned fiber structure for controlled ciprofloxacin delivery and wound healing. Int. J. Biol. Macromol. 2022, 205, 500–510. [Google Scholar] [CrossRef]
- Liu, C.; Deng, D.; Gao, J.; Jin, S.; Zuo, Y.; Li, Y.; Li, J. Construction and Properties of Simvastatin and Calcium Phosphate Dual-Loaded Coaxial Fibrous Membranes with Osteogenic and Angiogenic Functions. J. Bionic Eng. 2022, 19, 1645–1657. [Google Scholar] [CrossRef]
- Duan, M.; Sun, J.; Yu, S.; Zhi, Z.; Pang, J.; Wu, C. Insights into electrospun pullulan-carboxymethyl chitosan/PEO core-shell nanofibers loaded with nanogels for food antibacterial packaging. Int. J. Biol. Macromol. 2023, 233, 123433. [Google Scholar] [CrossRef]
- Al-Abduljabbar, A.; Farooq, I. Electrospun Polymer Nanofibers: Processing, Properties, and Applications. Polymers 2023, 15, 65. [Google Scholar] [CrossRef]
- Nadaf, A.; Gupta, A.; Hasan, N.; Fauziya; Ahmad, S.; Kesharwani, P.; Ahmad, F.J. Recent update on electrospinning and electrospun nanofibers: Current trends and their applications. RSC Adv. 2022, 12, 23808–23828. [Google Scholar] [CrossRef] [PubMed]
- Giaconia, M.A.; dos Passos Ramos, S.; Araújo, T.A.; de Almeida Cruz, M.; Renno, A.C.; Braga, A.R.C. Scaffold Production and Bone Tissue Healing Using Electrospinning: Trends and Gap of Knowledge. Regen. Eng. Transl. Med. 2022, 8, 506–522. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, X.; Shen, Y.; Dong, K.; Shen, L.; Alzalab, A.A.A. Research progress, models and simulation of electrospinning technology: A review. J. Mater. Sci. 2022, 57, 58–104. [Google Scholar] [CrossRef]
- Han, W.; Wang, L.; Li, Q.; Ma, B.; He, C.; Guo, X.; Nie, J.; Ma, G. A Review: Current Status and Emerging Developments on Natural Polymer-Based Electrospun Fibers. Macromol. Rapid Commun. 2022, 43, 2200456. [Google Scholar] [CrossRef]
- Angel, N.; Li, S.; Yan, F.; Kong, L. Recent advances in electrospinning of nanofibers from bio-based carbohydrate polymers and their applications. Trends Food Sci. Technol. 2022, 120, 308–324. [Google Scholar] [CrossRef]
- Ranganathan, S.; Balagangadharan, K.; Selvamurugan, N. Chitosan and gelatin-based electrospun fibers for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 354–364. [Google Scholar] [CrossRef]
- Wu, T.; Ding, M.; Shi, C.; Qiao, Y.; Wang, P.; Qiao, R.; Wang, X.; Zhong, J. Resorbable polymer electrospun nanofibers: History, shapes and application for tissue engineering. Chin. Chem. Lett. 2020, 31, 617–625. [Google Scholar] [CrossRef]
- Pal, P.; Srivas, P.K.; Dadhich, P.; Das, B.; Maulik, D.; Dhara, S. Nano-/Microfibrous Cotton-Wool-Like 3D Scaffold with Core–Shell Architecture by Emulsion Electrospinning for Skin Tissue Regeneration. ACS Biomater. Sci. Eng. 2017, 3, 3563–3575. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Shi, X.; Zhang, X.; Li, L. Electrospinning of polycaprolacton/chitosan core-shell nanofibers by a stable emulsion system. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123956. [Google Scholar] [CrossRef]
- Chen, P.; Liu, L.; Pan, J.; Mei, J.; Li, C.; Zheng, Y. Biomimetic composite scaffold of hydroxyapatite/gelatin-chitosan core-shell nanofibers for bone tissue engineering. Mater. Sci. Eng. C 2019, 97, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Abadi, B.; Goshtasbi, N.; Bolourian, S.; Tahsili, J.; Adeli-Sardou, M.; Forootanfar, H. Electrospun hybrid nanofibers: Fabrication, characterization, and biomedical applications. Front. Bioeng. Biotechnol. 2022, 10, 986975. [Google Scholar] [CrossRef] [PubMed]
- Murillo, L.; Rivero, P.J.; Sandúa, X.; Pérez, G.; Palacio, J.F.; Rodríguez, R.J. Antifungal Activity of Chitosan/Poly(Ethylene Oxide) Blend Electrospun Polymeric Fiber Mat Doped with Metallic Silver Nanoparticles. Polymers 2023, 15, 3700. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, Q.; Hou, G.; Wang, C.; Yan, H. Sustained release of EGF/bFGF growth factors achieved by mussel-inspired core–shell nanofibers with hemostatic and anti-inflammatory effects for promoting wound healing. Eur. Polym. J. 2023, 190, 112003. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Liu, H. Conductive Bicomponent Fibers Containing Polyaniline Produced via Side-by-Side Electrospinning. Polymers 2019, 11, 954. [Google Scholar] [CrossRef]
- Ignatova, M.; Manolova, N.; Rashkov, I. Electrospun Antibacterial Chitosan-Based Fibers. Macromol. Biosci. 2013, 13, 860–872. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Z.; Shi, J.; Wang, C.; Ding, L.; Ding, X.; Teng, G.; Wu, J.; Zhang, J. Preparation and antibacterial properties of chitosan/polyvinyl alcohol nanofibrous mats using different organic acids as solvents. Process Biochem. 2022, 122, 13–28. [Google Scholar] [CrossRef]
- Arkoun, M.; Daigle, F.; Heuzey, M.-C.; Ajji, A. Antibacterial electrospun chitosan-based nanofibers: A bacterial membrane perforator. Food Sci. Nutr. 2017, 5, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Arkoun, M.; Daigle, F.; Heuzey, M.-C.; Ajji, A. Mechanism of Action of Electrospun Chitosan-Based Nanofibers against Meat Spoilage and Pathogenic Bacteria. Molecules 2017, 22, 585. [Google Scholar] [CrossRef] [PubMed]
- Arkoun, M.; Daigle, F.; Holley, R.A.; Heuzey, M.C.; Ajji, A. Chitosan-based nanofibers as bioactive meat packaging materials. Packag. Technol. Sci. 2018, 31, 185–195. [Google Scholar] [CrossRef]
- Dodero, A.; Brunengo, E.; Alloisio, M.; Sionkowska, A.; Vicini, S.; Castellano, M. Chitosan-based electrospun membranes: Effects of solution viscosity, coagulant and crosslinker. Carbohydr. Polym. 2020, 235, 115976. [Google Scholar] [CrossRef]
- Dodero, A.; Scarfi, S.; Mirata, S.; Sionkowska, A.; Vicini, S.; Alloisio, M.; Castellano, M. Effect of Crosslinking Type on the Physical-Chemical Properties and Biocompatibility of Chitosan-Based Electrospun Membranes. Polymers 2021, 13, 831. [Google Scholar] [CrossRef]
- Christ, H.-A.; Menzel, H. Electrospinning and Photocrosslinking of Highly Modified Fungal Chitosan. Macromol. Mater. Eng. 2023, 308, 2200430. [Google Scholar] [CrossRef]
- Cui, H.; Bai, M.; Li, C.; Liu, R.; Lin, L. Fabrication of chitosan nanofibers containing tea tree oil liposomes against Salmonella spp. in chicken. LWT 2018, 96, 671–678. [Google Scholar] [CrossRef]
- Bayat, S.; Amiri, N.; Pishavar, E.; Kalalinia, F.; Movaffagh, J.; Hashemi, M. Bromelain-loaded chitosan nanofibers prepared by electrospinning method for burn wound healing in animal models. Life Sci. 2019, 229, 57–66. [Google Scholar] [CrossRef]
- Sun, C.; Yin, H.; He, J.; Zou, L.; Xu, Y. Fabrication and characterization of nanofibrous gelatin/chitosan-poly (ethylene oxide) membranes by electrospinning with acetic acid as solvent. J. Polym. Res. 2021, 28, 482. [Google Scholar] [CrossRef]
- Rahnama, S.; Movaffagh, J.; Shahroodi, A.; Jirofti, N.; Fazly Bazzaz, B.S.; Beyraghdari, M.; Hashemi, M.; Kalalinia, F. Development and characterization of the electrospun melittin-loaded chitosan nanofibers for treatment of acne vulgaris in animal model. J. Ind. Text. 2022, 52, 15280837221112410. [Google Scholar] [CrossRef]
- Sun, T.-C.; Yan, B.-Y.; Ning, X.-C.; Hui, C.; Xu, L.; Ding, Y.-N.; Yang, X.-L.; Ramakrishna, S.; Long, Y.-Z.; Zhang, J. Cool and hot chitosan/platelet-derived growth factor nanofibers for outdoors burns. Int. J. Biol. Macromol. 2022, 218, 409–419. [Google Scholar] [CrossRef]
- Derakhshi, M.; Naseri, M.; Vafaeipour, Z.; Malaekeh-Nikouei, B.; Jafarian, A.H.; Ansari, L. Enhanced wound-healing efficacy of electrospun mesoporous hydroxyapatite nanoparticle-loaded chitosan nanofiber developed using pluronic F127. Int. J. Biol. Macromol. 2023, 240, 124427. [Google Scholar] [CrossRef]
- Jie, X.; Shiu, B.-C.; Zhang, Y.; Wu, H.; Ye, Y.; Fang, R. Chitosan-Urushiol nanofiber membrane with enhanced acid resistance and broad-spectrum antibacterial activity. Carbohydr. Polym. 2023, 312, 120792. [Google Scholar] [CrossRef]
- Guha Ray, P.; Pal, P.; Srivas, P.K.; Basak, P.; Roy, S.; Dhara, S. Surface Modification of Eggshell Membrane with Electrospun Chitosan/Polycaprolactone Nanofibers for Enhanced Dermal Wound Healing. ACS Appl. Bio Mater. 2018, 1, 985–998. [Google Scholar] [CrossRef] [PubMed]
- Doan, H.N.; Vo, P.P.; Baggio, A.; Negoro, M.; Kinashi, K.; Fuse, Y.; Sakai, W.; Tsutsumi, N. Environmentally Friendly Chitosan-Modified Polycaprolactone Nanofiber/Nanonet Membrane for Controllable Oil/Water Separation. ACS Appl. Polym. Mater. 2021, 3, 3891–3901. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, C.; Wang, P.; Zhang, Y.; Zhang, H. Electrospun chitosan/polycaprolactone nanofibers containing chlorogenic acid-loaded halloysite nanotube for active food packaging. Carbohydr. Polym. 2020, 247, 116711. [Google Scholar] [CrossRef] [PubMed]
- Kaliaperumal, C.; Thulasisingh, A. In-vitro and in-vivo assessment of Polycaprolactone-Chitosan-Pectin imbibed nanofiber potentials as a wound healing biomaterial. J. Polym. Res. 2023, 30, 160. [Google Scholar] [CrossRef]
- Mohraz, M.H.; Golbabaei, F.; Yu, I.J.; Mansournia, M.A.; Zadeh, A.S.; Dehghan, S.F. Preparation and optimization of multifunctional electrospun polyurethane/chitosan nanofibers for air pollution control applications. Int. J. Environ. Sci. Technol. 2019, 16, 681–694. [Google Scholar] [CrossRef]
- Ahmadi, P.; Nazeri, N.; Derakhshan, M.A.; Ghanbari, H. Preparation and characterization of polyurethane/chitosan/CNT nanofibrous scaffold for cardiac tissue engineering. Int. J. Biol. Macromol. 2021, 180, 590–598. [Google Scholar] [CrossRef]
- Dehghani, S.; Rezaei, K.; Hamishehkar, H.; Oromiehie, A. The effect of electrospun polylactic acid/chitosan nanofibers on the low density polyethylene/ploy lactic acid film as bilayer antibacterial active packaging films. J. Food Process. Preserv. 2022, 46, e15889. [Google Scholar] [CrossRef]
- Zhu, M.; Xiong, R.; Huang, C. Bio-based and photocrosslinked electrospun antibacterial nanofibrous membranes for air filtration. Carbohydr. Polym. 2019, 205, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, S.; Zhang, R.; Lan, W.; Qin, W. Development of Poly(lactic acid)/Chitosan Fibers Loaded with Essential Oil for Antimicrobial Applications. Nanomaterials 2017, 7, 194. [Google Scholar] [CrossRef] [PubMed]
- Demina, T.S.; Bolbasov, E.N.; Peshkova, M.A.; Efremov, Y.M.; Bikmulina, P.Y.; Birdibekova, A.V.; Popyrina, T.N.; Kosheleva, N.V.; Tverdokhlebov, S.I.; Timashev, P.S.; et al. Electrospinning vs. Electro-Assisted Solution Blow Spinning for Fabrication of Fibrous Scaffolds for Tissue Engineering. Polymers 2022, 14, 5254. [Google Scholar] [CrossRef] [PubMed]
- Galstyan, A.; Strokov, K. Influence of photosensitizer concentration and polymer composition on photoinduced antimicrobial activity of PVA- and PVA-chitosan-based electrospun nanomaterials cross-linked with tailor-made silicon(IV) phthalocyanine. Photochem. Photobiol. Sci. 2022, 21, 1387–1398. [Google Scholar] [CrossRef]
- Sargazi, G.; Afzali, D.; Mostafavi, A.; Shadman, A.; Rezaee, B.; Zarrintaj, P.; Saeb, M.R.; Ramakrishna, S.; Mozafari, M. Chitosan/polyvinyl alcohol nanofibrous membranes: Towards green super-adsorbents for toxic gases. Heliyon 2019, 5, e01527. [Google Scholar] [CrossRef]
- Qiu, H.; Zhu, S.; Pang, L.; Ma, J.; Liu, Y.; Du, L.; Wu, Y.; Jin, Y. ICG-loaded photodynamic chitosan/polyvinyl alcohol composite nanofibers: Anti-resistant bacterial effect and improved healing of infected wounds. Int. J. Pharm. 2020, 588, 119797. [Google Scholar] [CrossRef]
- Mahatmanti, F.W.; Kusumastuti, E.; Wijayati, N. Electrospinning of nanofibers chitosan/PVA-sodium silicate. J. Phys. Conf. Ser. 2021, 1918, 032012. [Google Scholar] [CrossRef]
- Abou-Okeil, A.; Refaei, R.; Khalil, E.M.; Moustafa, S.E.; Ibrahim, H.M. Fabrication of novel green magnetic electrospun nanofibers based on Fe3O4 nanoparticles/PVA/chitosan/collagen. Egypt. J. Chem. 2022, 65, 285–298. [Google Scholar] [CrossRef]
- Wu, S.; Li, K.; Shi, W.; Cai, J. Preparation and performance evaluation of chitosan/polyvinylpyrrolidone/polyvinyl alcohol electrospun nanofiber membrane for heavy metal ions and organic pollutants removal. Int. J. Biol. Macromol. 2022, 210, 76–84. [Google Scholar] [CrossRef]
- Bandatang, N.; Pongsomboon, S.-a.; Jumpapaeng, P.; Suwanakood, P.; Saengsuwan, S. Antimicrobial electrospun nanofiber mats of NaOH-hydrolyzed chitosan (HCS)/PVP/PVA incorporated with in-situ synthesized AgNPs: Fabrication, characterization, and antibacterial activity. Int. J. Biol. Macromol. 2021, 190, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Hadipour-Goudarzi, E.; Hemmatinejad, N.; Shokrgozar, M.A. Fabrication and DOE Optimization of Electrospun Chitosan/Gelatin/PVA Nanofibers for Skin Tissue Engineering. Macromol. Mater. Eng. 2023, 308, 2200562. [Google Scholar] [CrossRef]
- Wang, C.; Fan, J.; Xu, R.; Zhang, L.; Zhong, S.; Wang, W.; Yu, D. Quaternary ammonium chitosan/polyvinyl alcohol composites prepared by electrospinning with high antibacterial properties and filtration efficiency. J. Mater. Sci. 2019, 54, 12522–12532. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Ooi, C.W.; Song, C.P.; Wang, C.-Y.; Liu, B.-L.; Lin, G.-Y.; Chiu, C.-Y.; Chang, Y.-K. Antibacterial efficacy of quaternized chitosan/poly (vinyl alcohol) nanofiber membrane crosslinked with blocked diisocyanate. Carbohydr. Polym. 2021, 262, 117910. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-Y.; Wang, C.-Y.; Chen, K.-H.; Lai, Y.-R.; Chiu, C.-Y.; Lee, H.-C.; Chang, Y.-K. Electrospinning of Quaternized Chitosan-Poly(vinyl alcohol) Composite Nanofiber Membrane: Processing Optimization and Antibacterial Efficacy. Membranes 2022, 12, 332. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Tang, X.; Wu, X.; Li, Y.; Xu, C.; Chen, S.; Wu, P. Electrospun chitosan-based nanofibers loading tea tree oil for fresh salmon fillet shelf-life extension. J. Food Sci. 2023, 88, 3075–3089. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Guo, J.; Li, J.; Shi, X.; Wang, L.; Chen, W.; Mo, X. Repair of skin defects with electrospun collagen/chitosan and fibroin/chitosan compound nanofiber scaffolds compared with gauze dressing. J. Biomater. Tissue Eng. 2017, 7, 386–392. [Google Scholar] [CrossRef]
- Shokraei, S.; Mirzaei, E.; Shokraei, N.; Derakhshan, M.A.; Ghanbari, H.; Faridi-Majidi, R. Fabrication and characterization of chitosan/kefiran electrospun nanofibers for tissue engineering applications. J. Appl. Polym. Sci. 2021, 138, 50547. [Google Scholar] [CrossRef]
- Celebioglu, A.; Saporito, A.F.; Uyar, T. Green Electrospinning of Chitosan/Pectin Nanofibrous Films by the Incorporation of Cyclodextrin/Curcumin Inclusion Complexes: pH-Responsive Release and Hydrogel Features. ACS Sustain. Chem. Eng. 2022, 10, 4758–4769. [Google Scholar] [CrossRef]
- Shavisi, N.; Shahbazi, Y. Chitosan-gum Arabic nanofiber mats encapsulated with pH-sensitive Rosa damascena anthocyanins for freshness monitoring of chicken fillets. Food Packag. Shelf Life 2022, 32, 100827. [Google Scholar] [CrossRef]
- Kang, E.; Moon, E.; Song, W.; Kim, L.H.; Hyung, J.S.; Jo, J.-H.; Park, J.-H.; Kim, M.-S.; Na, J.-G.; Choi, Y.S. Chitosan/oleamide nanofluid as a significant medium for enhancing gas utilization efficiency in C1-gas microbial biotransformation. Chem. Eng. J. 2022, 433, 133846. [Google Scholar] [CrossRef]
- Tao, F.; Cheng, Y.; Tao, H.; Jin, L.; Wan, Z.; Dai, F.; Xiang, W.; Deng, H. Carboxymethyl chitosan/sodium alginate-based micron-fibers fabricated by emulsion electrospinning for periosteal tissue engineering. Mater. Des. 2020, 194, 108849. [Google Scholar] [CrossRef]
- Mouro, C.; Simões, M.; Gouveia, I.C. Emulsion Electrospun Fiber Mats of PCL/PVA/Chitosan and Eugenol for Wound Dressing Applications. Adv. Polym. Technol. 2019, 2019, 9859506. [Google Scholar] [CrossRef]
- Lamarra, J.; Rivero, S.; Pinotti, A.; Lopez, D. Nanofiber mats functionalized with Mentha piperita essential oil stabilized in a chitosan-based emulsion designed via an electrospinning technique. Int. J. Biol. Macromol. 2023, 248, 125980. [Google Scholar] [CrossRef] [PubMed]
- Sosiati, H.; Nur Fatihah, W.; Yusmaniar, Y.; Nur Rahman, M.B. Characterization of the Properties of Electrospun Blended Hybrid Poly(Vinyl Alcohol)_Aloe Vera/Chitosan Nano-Emulsion Nanofibrous Membranes. Key Eng. Mater. 2019, 792, 74–79. [Google Scholar] [CrossRef]
- Li, C.; Luo, X.; Li, L.; Cai, Y.; Kang, X.; Li, P. Carboxymethyl chitosan-based electrospun nanofibers with high citral-loading for potential anti-infection wound dressings. Int. J. Biol. Macromol. 2022, 209, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Xu, W.; Xue, Y.; Chen, L.; Ye, H.; Zhong, E.; Ye, Z.; Gao, J.; Yan, Y. The use of chitosan/PLA nano-fibers by emulsion eletrospinning for periodontal tissue engineering. Artif. Cells Nanomed. Biotechnol. 2018, 46, 419–430. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.; Yan, L.; Mei, X.; Hou, Z. Combining emulsion electrospinning with surface functionalization to fabricate multistructural PLA/CS@ZIF-8 nanofiber membranes toward pH-responsive dual drug delivery. Int. J. Biol. Macromol. 2023, 253, 126506. [Google Scholar] [CrossRef] [PubMed]
- Soon, C.Y.; Rahman, N.A.; Tee, Y.B.; Talib, R.A.; Tan, C.H.; Abdan, K.; Chan, E.W.C. Electrospun biocomposite: Nanocellulose and chitosan entrapped within a poly(hydroxyalkanoate) matrix for Congo red removal. J. Mater. Res. Technol. 2019, 8, 5091–5102. [Google Scholar] [CrossRef]
- Keirouz, A.; Radacsi, N.; Ren, Q.; Dommann, A.; Beldi, G.; Maniura-Weber, K.; Rossi, R.M.; Fortunato, G. Nylon-6/chitosan core/shell antimicrobial nanofibers for the prevention of mesh-associated surgical site infection. J. Nanobiotechnol. 2020, 18, 51. [Google Scholar] [CrossRef]
- Ghasemvand, F.; Kabiri, M.; Hassan-Zadeh, V.; Simchi, A. Chitosan, polyethylene oxide/polycaprolactone electrospun core/shell nanofibrous mat containing rosuvastatin as a novel drug delivery system for enhancing human mesenchymal stem cell osteogenesis. Front. Mol. Biosci. 2023, 10, 1220357. [Google Scholar] [CrossRef]
- Ouerghemmi, S.; Degoutin, S.; Maton, M.; Tabary, N.; Cazaux, F.; Neut, C.; Blanchemain, N.; Martel, B. Core-Sheath Electrospun Nanofibers Based on Chitosan and Cyclodextrin Polymer for the Prolonged Release of Triclosan. Polymers 2022, 14, 1955. [Google Scholar] [CrossRef]
- Ma, L.; Shi, X.; Zhang, X.; Dong, S.; Li, L. Electrospun Cellulose Acetate–Polycaprolactone/Chitosan Core–Shell Nanofibers for the Removal of Cr(VI). Phys. Status Solidi (a) 2019, 216, 1900379. [Google Scholar] [CrossRef]
- Abasalta, M.; Asefnejad, A.; Khorasani, M.T.; Saadatabadi, A.R. Fabrication of carboxymethyl chitosan/poly(ε-caprolactone)/doxorubicin/nickel ferrite core-shell fibers for controlled release of doxorubicin against breast cancer. Carbohydr. Polym. 2021, 257, 117631. [Google Scholar] [CrossRef]
- Hadjianfar, M.; Semnani, D.; Varshosaz, J.; Mohammadi, S.; Rezazadeh Tehrani, S.P. 5FU-loaded PCL/Chitosan/Fe3O4 Core-Shell Nanofibers Structure: An Approach to Multi-Mode Anticancer System. Adv. Pharm. Bull. 2022, 12, 568–582. [Google Scholar] [CrossRef]
- Ghazalian, M.; Afshar, S.; Rostami, A.; Rashedi, S.; Bahrami, S.H. Fabrication and characterization of chitosan-polycaprolactone core-shell nanofibers containing tetracycline hydrochloride. Colloids Surf. A Physicochem. Eng. Asp. 2022, 636, 128163. [Google Scholar] [CrossRef]
- Yousefi, P.; Dini, G.; Movahedi, B.; Vaezifar, S.; Mehdikhani, M. Polycaprolactone/chitosan core/shell nanofibrous mat fabricated by electrospinning process as carrier for rosuvastatin drug. Polym. Bull. 2022, 79, 1627–1645. [Google Scholar] [CrossRef]
- Poornima, B.; Korrapati, P.S. Fabrication of chitosan-polycaprolactone composite nanofibrous scaffold for simultaneous delivery of ferulic acid and resveratrol. Carbohydr. Polym. 2017, 157, 1741–1749. [Google Scholar] [CrossRef]
- Rastegar, A.; Mahmoodi, M.; Mirjalili, M.; Nasirizadeh, N. Platelet-rich fibrin-loaded PCL/chitosan core-shell fibers scaffold for enhanced osteogenic differentiation of mesenchymal stem cells. Carbohydr. Polym. 2021, 269, 118351. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Jiang, S.; Xu, D.; Cheng, G.; Shi, B. Resveratrol-loaded co-axial electrospun poly(ε-caprolactone)/chitosan/polyvinyl alcohol membranes for promotion of cells osteogenesis and bone regeneration. Int. J. Biol. Macromol. 2023, 249, 126085. [Google Scholar] [CrossRef] [PubMed]
- Farboudi, A.; Nouri, A.; Shirinzad, S.; Sojoudi, P.; Davaran, S.; Akrami, M.; Irani, M. Synthesis of magnetic gold coated poly (ε-caprolactonediol) based polyurethane/poly(N-isopropylacrylamide)-grafted-chitosan core-shell nanofibers for controlled release of paclitaxel and 5-FU. Int. J. Biol. Macromol. 2020, 150, 1130–1140. [Google Scholar] [CrossRef]
- Salsabila, D.S.; Widiyanti, P.; Hernando, E.; Hulu, I.M.; Putri Wibowo, T.D. Characterization of Coaxially Electrospun Poly (L-Lactic) Acid/Chitosan with Heparin Modification as Patch Angioplasty Candidate. J. Membr. Sci. Res. 2023, 9, 552200. [Google Scholar]
- dos Santos, D.M.; Chagas, P.A.M.; Leite, I.S.; Inada, N.M.; de Annunzio, S.R.; Fontana, C.R.; Campana-Filho, S.P.; Correa, D.S. Core-sheath nanostructured chitosan-based nonwovens as a potential drug delivery system for periodontitis treatment. Int. J. Biol. Macromol. 2020, 142, 521–534. [Google Scholar] [CrossRef]
- Sattari, S.; Tehrani, A.D.; Adeli, M.; Soleimani, K.; Rashidipour, M. Fabrication of new generation of co-delivery systems based on graphene-g-cyclodextrin/chitosan nanofiber. Int. J. Biol. Macromol. 2020, 156, 1126–1134. [Google Scholar] [CrossRef]
- Kuo, T.-Y.; Jhang, C.-F.; Lin, C.-M.; Hsien, T.-Y.; Hsieh, H.-J. Fabrication and application of coaxial polyvinyl alcohol/chitosan nanofiber membranes. Open Phys. 2017, 15, 1004–1014. [Google Scholar] [CrossRef]
- Chen, J.; Duan, H.; Pan, H.; Yang, X.; Pan, W. Two types of core/shell fibers based on carboxymethyl chitosan and Sodium carboxymethyl cellulose with self-assembled liposome for buccal delivery of carvedilol across TR146 cell culture and porcine buccal mucosa. Int. J. Biol. Macromol. 2019, 128, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Shi, W.; Li, K.; Cai, J.; Xu, C.; Gao, L.; Lu, J.; Ding, F. Chitosan-based hollow nanofiber membranes with polyvinylpyrrolidone and polyvinyl alcohol for efficient removal and filtration of organic dyes and heavy metals. Int. J. Biol. Macromol. 2023, 239, 124264. [Google Scholar] [CrossRef]
- Cai, L.; Shi, H.; Cao, A.; Jia, J. Characterization of gelatin/chitosan ploymer films integrated with docosahexaenoic acids fabricated by different methods. Sci. Rep. 2019, 9, 8375. [Google Scholar] [CrossRef] [PubMed]
- Abasalta, M.; Zibaseresht, R.; Yousefi Zoshk, M.; Foroutan Koudehi, M.; Irani, M.; Hami, Z. Simultaneous loading of clarithromycin and zinc oxide into the chitosan/gelatin/polyurethane core–shell nanofibers for wound dressing. J. Dispers. Sci. Technol. 2022, 44, 2664–2674. [Google Scholar] [CrossRef]
- Kaewklin, P.; Siripatrawan, U.; Suwanagul, A.; Lee, Y.S. Active packaging from chitosan-titanium dioxide nanocomposite film for prolonging storage life of tomato fruit. Int. J. Biol. Macromol. 2018, 112, 523–529. [Google Scholar] [CrossRef]
- Râpă, M.; Gaidau, C.; Mititelu-Tartau, L.; Berechet, M.-D.; Berbecaru, A.C.; Rosca, I.; Chiriac, A.P.; Matei, E.; Predescu, A.-M.; Predescu, C. Bioactive Collagen Hydrolysate-Chitosan/Essential Oil Electrospun Nanofibers Designed for Medical Wound Dressings. Pharmaceutics 2021, 13, 1939. [Google Scholar] [CrossRef] [PubMed]
- Avci, H.; Ghorbanpoor, H.; Nurbas, M. Preparation of origanum minutiflorum oil-loaded core–shell structured chitosan nanofibers with tunable properties. Polym. Bull. 2018, 75, 4129–4144. [Google Scholar] [CrossRef]
- Movahedi, M.; Karbasi, S. A core-shell Electrospun Scaffold of polyhydroxybutyrate-starch/halloysite Nanotubes Containing Extracellular Matrix and Chitosan for Articular Cartilage Tissue Engineering Application. J. Polym. Environ. 2023, 31, 3052–3069. [Google Scholar] [CrossRef]
- Abdelgawad, A.M.; Hudson, S.M.; Rojas, O.J. Antimicrobial wound dressing nanofiber mats from multicomponent (chitosan/silver-NPs/polyvinyl alcohol) systems. Carbohydr. Polym. 2014, 100, 166–178. [Google Scholar] [CrossRef]
- Dobrovolskaya, I.P.; Yudin, V.E.; Popryadukhin, P.V.; Ivan’kova, E.M.; Shabunin, A.S.; Kasatkin, I.A.; Morgantie, P. Effect of chitin nanofibrils on electrospinning of chitosan-based composite nanofibers. Carbohydr. Polym. 2018, 194, 260–266. [Google Scholar] [CrossRef]
- Fatullayeva, S.; Tagiyev, D.; Zeynalov, N.; Mammadova, S.; Aliyeva, E. Recent advances of chitosan-based polymers in biomedical applications and environmental protection. J. Polym. Res. 2022, 29, 259. [Google Scholar] [CrossRef]
- Marin, L.; Andreica, B.-I.; Anisiei, A.; Cibotaru, S.; Bardosova, M.; Materon, E.M.; Oliveira, O.N. Quaternized chitosan (nano)fibers: A journey from preparation to high performance applications. Int. J. Biol. Macromol. 2023, 242, 125136. [Google Scholar] [CrossRef] [PubMed]
- Milewska, A.; Chi, Y.; Szczepanski, A.; Barreto-Duran, E.; Dabrowska, A.; Botwina, P.; Obloza, M.; Liu, K.; Liu, D.; Guo, X.; et al. HTCC as a Polymeric Inhibitor of SARS-CoV-2 and MERS-CoV. J. Virol. 2021, 95, e01622-20. [Google Scholar] [CrossRef] [PubMed]
- Elamri, A.; Zdiri, K.; Hamdaoui, M.; Harzallah, O. Chitosan: A biopolymer for textile processes and products. Text. Res. J. 2022, 93, 1456–1484. [Google Scholar] [CrossRef]
- Mi, X.; Vijayaragavan, K.S.; Heldt, C.L. Virus adsorption of water-stable quaternized chitosan nanofibers. Carbohydr. Res. 2014, 387, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yao, H.; Luo, J.; Li, Z.; Wei, J. Functionalization of Electrospun Nanofiber for Bone Tissue Engineering. Polymers 2022, 14, 2940. [Google Scholar] [CrossRef] [PubMed]
- Tamilarasi, G.P.; Sabarees, G.; Manikandan, K.; Gouthaman, S.; Alagarsamy, V.; Solomon, V.R. Advances in electrospun chitosan nanofiber biomaterials for biomedical applications. Mater. Adv. 2023, 4, 3114–3139. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, C.; Gao, F.; Pan, G. Needleless electrospinning for scaled-up production of ultrafine chitosan hybrid nanofibers used for air filtration. RSC Adv. 2016, 6, 105988–105995. [Google Scholar] [CrossRef]
- Poshina, D.N.; Khadyko, I.A.; Sukhova, A.A.; Serov, I.V.; Zabivalova, N.M.; Skorik, Y.A. Needleless Electrospinning of a Chitosan Lactate Aqueous Solution: Influence of Solution Composition and Spinning Parameters. Technologies 2020, 8, 2. [Google Scholar] [CrossRef]
- Bochek, A.M.; Zabivalova, N.M.; Brazhnikova, E.N.; Anferova, M.S.; Lavrent’ev, V.K. Chitosan—Polyamide Composite Nanofibers Produced by Needleless Electrospinning. Fibre Chem. 2019, 50, 391–395. [Google Scholar] [CrossRef]
- Grimmelsmann, N.; Homburg, S.V.; Ehrmann, A. Needleless Electrospinning of Pure and Blended Chitosan. IOP Conf. Ser. Mater. Sci. Eng. 2017, 225, 012098. [Google Scholar] [CrossRef]
- Mouro, C.; Gomes, A.P.; Gouveia, I.C. Emulsion Electrospinning of PLLA/PVA/Chitosan with Hypericum perforatum L. as an Antibacterial Nanofibrous Wound Dressing. Gels 2023, 9, 353. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.-W.; Lin, M.-C.; Huang, C.-H.; Lai, M.-F.; Shiu, B.-C.; Lin, J.-H. Preparation of Needleless Electrospinning Polyvinyl Alcohol/Water-Soluble Chitosan Nanofibrous Membranes: Antibacterial Property and Filter Efficiency. Polymers 2022, 14, 1054. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Prabhakaran, M.P.; Tian, L.; Ding, X.; Ramakrishna, S. Drug-loaded emulsion electrospun nanofibers: Characterization, drug release and in vitro biocompatibility. RSC Adv. 2015, 5, 100256–100267. [Google Scholar] [CrossRef]
- Ghosh, S.; Yadav, A.; Gurave, P.M.; Srivastava, R.K. Unique Fiber Morphologies from Emulsion Electrospinning—A Case Study of Poly(ε-caprolactone) and Its Applications. Colloids Interfaces 2023, 7, 19. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Hou, C.; Li, T.; Xin, B. The release kinetic of drug encapsulated poly(L-lactide-co-ε-caprolactone) core-shell nanofibers fabricated by emulsion electrospinning. J. Macromol. Sci. Part A 2022, 59, 489–503. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, F.; Zhang, H. Emulsion electrospinning: Fundamentals, food applications and prospects. Trends Food Sci. Technol. 2018, 80, 175–186. [Google Scholar] [CrossRef]
- Hebishy, E.; Collette, L.; Iheozor-Ejiofor, P.; Onarinde, B.A. Stability and antimicrobial activity of lemongrass essential oil in nanoemulsions produced by high-intensity ultrasounds and stabilized by soy lecithin, hydrolyzed whey proteins, gum Arabic, or their ternary admixture. J. Food Process. Preserv. 2022, 46, e16840. [Google Scholar] [CrossRef]
- Yoon, J.; Yang, H.-S.; Lee, B.-S.; Yu, W.-R. Recent Progress in Coaxial Electrospinning: New Parameters, Various Structures, and Wide Applications. Adv. Mater. 2018, 30, 1704765. [Google Scholar] [CrossRef]
- Jiang, S.; Chen, Y.; Duan, G.; Mei, C.; Greiner, A.; Agarwal, S. Electrospun nanofiber reinforced composites: A review. Polym. Chem. 2018, 9, 2685–2720. [Google Scholar] [CrossRef]
- Naeimirad, M.; Zadhoush, A.; Kotek, R.; Esmaeely Neisiany, R.; Nouri Khorasani, S.; Ramakrishna, S. Recent advances in core/shell bicomponent fibers and nanofibers: A review. J. Appl. Polym. Sci. 2018, 135, 46265. [Google Scholar] [CrossRef]
- Ozkan, O.; Sasmazel, H.T. Antibacterial Performance of PCL-Chitosan Core-Shell Scaffolds. J. Nanosci. Nanotechnol. 2018, 18, 2415–2421. [Google Scholar] [CrossRef]
- Morais, D.S.; Guedes, R.M.; Lopes, M.A. Antimicrobial Approaches for Textiles: From Research to Market. Materials 2016, 9, 498. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.A.; El-Zairy, E.M.; Eid, B.M.; Emam, E.; Barkat, S.R. A new approach for imparting durable multifunctional properties to linen-containing fabrics. Carbohydr. Polym. 2017, 157, 1085–1093. [Google Scholar] [CrossRef]
- Colobatiu, L.; Gavan, A.; Mocan, A.; Bogdan, C.; Mirel, S.; Tomuta, I. Development of bioactive compounds-loaded chitosan films by using a QbD approach—A novel and potential wound dressing material. React. Funct. Polym. 2019, 138, 46–54. [Google Scholar] [CrossRef]
- Shukla, R.; Kashaw, S.K.; Jain, A.P.; Lodhi, S. Fabrication of Apigenin loaded gellan gum–chitosan hydrogels (GGCH-HGs) for effective diabetic wound healing. Int. J. Biol. Macromol. 2016, 91, 1110–1119. [Google Scholar] [CrossRef]
- Güneş, S.; Tıhmınlıoğlu, F. Hypericum perforatum incorporated chitosan films as potential bioactive wound dressing material. Int. J. Biol. Macromol. 2017, 102, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Andreica, B.-I.; Anisiei, A.; Rosca, I.; Sandu, A.-I.; Pasca, A.S.; Tartau, L.M.; Marin, L. Quaternized chitosan/chitosan nanofibrous mats: An approach toward bioactive materials for tissue engineering and regenerative medicine. Carbohydr. Polym. 2023, 302, 120431. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Chen, Y.; Wang, X.; Xu, X.; Shen, Y.; Khan, A.u.R.; Aldalbahi, A.; Fetz, A.E.; Bowlin, G.L.; El-Newehy, M.; et al. Electrospinning nanofiber scaffolds for soft and hard tissue regeneration. J. Mater. Sci. Technol. 2020, 59, 243–261. [Google Scholar] [CrossRef]
- Kazemi Asl, S.; Rahimzadegan, M.; Ostadrahimi, R. The recent advancement in the chitosan hybrid-based scaffolds for cardiac regeneration after myocardial infarction. Carbohydr. Polym. 2023, 300, 120266. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wei, W.; Tremblay, P.-L.; Zhang, T. Electrostimulation of fibroblast proliferation by an electrospun poly (lactide-co-glycolide)/polydopamine/chitosan membrane in a humid environment. Colloids Surf. B Biointerfaces 2022, 220, 112902. [Google Scholar] [CrossRef]
- Tavakoli, M.; Labbaf, S.; Mirhaj, M.; Salehi, S.; Seifalian, A.M.; Firuzeh, M. Natural polymers in wound healing: From academic studies to commercial products. J. Appl. Polym. Sci. 2023, 140, e53910. [Google Scholar] [CrossRef]
- Qasim, S.B.; Zafar, M.S.; Najeeb, S.; Khurshid, Z.; Shah, A.H.; Husain, S.; Rehman, I.U. Electrospinning of Chitosan-Based Solutions for Tissue Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2018, 19, 407. [Google Scholar] [CrossRef]
- Barzegar, S.; Zare, M.R.; Shojaei, F.; Zareshahrabadi, Z.; Koohi-Hosseinabadi, O.; Saharkhiz, M.J.; Iraji, A.; Zomorodian, K.; Khorram, M. Core-shell chitosan/PVA-based nanofibrous scaffolds loaded with Satureja mutica or Oliveria decumbens essential oils as enhanced antimicrobial wound dressing. Int. J. Pharm. 2021, 597, 120288. [Google Scholar] [CrossRef]
- Jahan, I.; Zhang, L. Natural Polymer-Based Electrospun Nanofibrous Membranes for Wastewater Treatment: A Review. J. Polym. Environ. 2022, 30, 1709–1729. [Google Scholar] [CrossRef]
- Rabiee, N.; Sharma, R.; Foorginezhad, S.; Jouyandeh, M.; Asadnia, M.; Rabiee, M.; Akhavan, O.; Lima, E.C.; Formela, K.; Ashrafizadeh, M.; et al. Green and Sustainable Membranes: A review. Environ. Res. 2023, 231, 116133. [Google Scholar] [CrossRef]
- Phan, D.-N.; Khan, M.Q.; Nguyen, N.-T.; Phan, T.-T.; Ullah, A.; Khatri, M.; Kien, N.N.; Kim, I.-S. A review on the fabrication of several carbohydrate polymers into nanofibrous structures using electrospinning for removal of metal ions and dyes. Carbohydr. Polym. 2021, 252, 117175. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Kumari, S.; Selamat, J.; Shameli, K.; Sazili, A.Q. Reducing Meat Perishability through Pullulan Active Packaging. J. Food Qual. 2020, 2020, 8880977. [Google Scholar] [CrossRef]
- Molognoni, L.; Daguer, H.; Motta, G.E.; Merlo, T.C.; Lindner, J.D.D. Interactions of preservatives in meat processing: Formation of carcinogenic compounds, analytical methods, and inhibitory agents. Food Res. Int. 2019, 125, 108608. [Google Scholar] [CrossRef] [PubMed]
- Trajkovska Petkoska, A.; Daniloski, D.; D’Cunha, N.M.; Naumovski, N.; Broach, A.T. Edible packaging: Sustainable solutions and novel trends in food packaging. Food Res. Int. 2021, 140, 109981. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H.W.; Pulvirenti, A. Recent advances on chitosan-based films for sustainable food packaging applications. Food Packag. Shelf Life 2020, 26, 100551. [Google Scholar] [CrossRef]
- Tang, S.; Yang, J.; Lin, L.; Peng, K.; Chen, Y.; Jin, S.; Yao, W. Construction of physically crosslinked chitosan/sodium alginate/calcium ion double-network hydrogel and its application to heavy metal ions removal. Chem. Eng. J. 2020, 393, 124728. [Google Scholar] [CrossRef]
- Yadav, N.; Monisha, M.; Niranjan, R.; Dubey, A.; Patil, S.; Priyadarshini, R.; Lochab, B. Antibacterial performance of fully biobased chitosan-grafted-polybenzoxazine films: Elaboration and properties of released material. Carbohydr. Polym. 2021, 254, 117296. [Google Scholar] [CrossRef]
- Yu, Z.; Li, B.; Chu, J.; Zhang, P. Silica in situ enhanced PVA/chitosan biodegradable films for food packages. Carbohydr. Polym. 2018, 184, 214–220. [Google Scholar] [CrossRef]
- Tripathi, S.; Mehrotra, G.K.; Dutta, P.K. Chitosan–silver oxide nanocomposite film: Preparation and antimicrobial activity. Bull. Mater. Sci. 2011, 34, 29–35. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, X.; Li, Y.-C.; Xiao, H.; Wang, X. Novel chitosan films with laponite immobilized Ag nanoparticles for active food packaging. Carbohydr. Polym. 2018, 199, 210–218. [Google Scholar] [CrossRef]
- Kumar, S.; Shukla, A.; Baul, P.P.; Mitra, A.; Halder, D. Biodegradable hybrid nanocomposites of chitosan/gelatin and silver nanoparticles for active food packaging applications. Food Packag. Shelf Life 2018, 16, 178–184. [Google Scholar] [CrossRef]
- Lou, C.; Liu, X.; Yang, C.; Ye, F.; Zhou, Q. One-step synthesis of silver nanoparticles exposed on the chitosan-covered polyamide 6 electrospinning nanofibers. J. Appl. Polym. Sci. 2023, 140, e53501. [Google Scholar] [CrossRef]
- Sun, X.; Yin, L.; Zhu, H.; Zhu, J.; Hu, J.; Luo, X.; Huang, H.; Fu, Y. Enhanced Antimicrobial Cellulose/Chitosan/ZnO Biodegradable Composite Membrane. Membranes 2022, 12, 239. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Yue, L.; Wang, Z. High antibacterial activity of chitosan—Molybdenum disulfide nanocomposite. Carbohydr. Polym. 2019, 215, 226–234. [Google Scholar] [CrossRef]
- Doğan, C.; Doğan, N.; Gungor, M.; Eticha, A.K.; Akgul, Y. Novel active food packaging based on centrifugally spun nanofibers containing lavender essential oil: Rapid fabrication, characterization, and application to preserve of minced lamb meat. Food Packag. Shelf Life 2022, 34, 100942. [Google Scholar] [CrossRef]











| Techniques | Co-Spinning Agents | Applications | Ref. |
|---|---|---|---|
| Uniaxial electrospinning | |||
| (Blend electrospinning) | PEO | Air filtration | [45] |
| Food packaging | [73,74,75] | ||
| Biomedical/pharmaceutical products | [76,77,78] | ||
| PEO/tea tree oil liposomes | Food packaging | [79] | |
| PEO/bromelain liposomes | Wound dressing | [80] | |
| PEO/gelatin | Wound dressing | [81] | |
| PEO/melittin | Acne treatment | [82] | |
| PEO/platelet-derived growth factor | Wound dressing | [83] | |
| PEO/Pluronic® F-127/ Hydroxyapatite | Wound dressing | [84] | |
| PEO/urushiol | Antibacterial membrane | [85] | |
| PCL | Wound dressing, separation membrane | [86,87] | |
| PCL/chlorogenic acid-loaded halloysite nanotube | Food packaging | [88] | |
| PCL/pectin | Wound dressing | [89] | |
| PU | Air filtration | [90] | |
| PU/carbon nanotubes | Cardiac tissue engineering | [91] | |
| PLA | Food packaging | [92] | |
| PLA/SiO2 | Air filtration | [93] | |
| PLA/cinnamon essential oil | Food packaging | [94] | |
| PLA/PCL/gelatin | Tissue engineering | [95] | |
| PVA | Antibacterial membrane | [96] | |
| Air filtration | [97] | ||
| PVA/indocyanine green | Wound dressing | [98] | |
| PVA/sodium silicate | Separation membrane | [99] | |
| PVA/collagen/Fe3O4 | Antibacterial membrane | [100] | |
| PVA/baicalin liposomes | Food packaging | [46] | |
| PVA/PVP | Separation membrane, medical purpose | [101,102] | |
| PVA/gelatin | Wound dressing | [103] | |
| PVA a | Air filtration | [104] | |
| Food packaging, wound dressing | [105,106] | ||
| PVA a/tea tree oil | Food packaging | [107] | |
| Collagen and fibroin | Wound dressing | [108] | |
| Kefiran | Tissue engineering | [109] | |
| Pectin/cyclodextrin/curcumin | Biomedical products | [110] | |
| Gum Arabic/anthocyanins | Food packaging | [111] | |
| Oleamide | Culture medium of C1 gas utilizing microorganism | [112] | |
| Emulsion electrospinning | |||
| PCL | Wound dressing | [64,65] | |
| PCL/alginate b | Bone tissue engineering | [113] | |
| PCL/PVA | Wound dressing | [114] | |
| PVA | Cosmetics and pharmaceuticals | [115] | |
| PVA/aloe vera | Wound dressing | [116] | |
| PVA b | Wound dressing | [117] | |
| PLA | Periodontal tissue engineering | [118] | |
| PLA | Drug delivery | [119] | |
| Poly(hydroxyalkanoate)/cellulose | Separation membrane | [120] | |
| Coaxial electrospinning | |||
| PEO | Bone tissue engineering | [53,66] | |
| Surgical antimicrobial membrane | [121] | ||
| Drug delivery/controlled drug release | [122,123] | ||
| PEO b | Controlled drug release Food packaging | [51] [54] | |
| PCL | Separation membrane | [124] | |
| Controlled drug release | [52,125,126,127,128,129] | ||
| Tissue engineering Controlled drug release | [130,131] | ||
| PCL/PVA b | Controlled drug release | [50] | |
| PCL-Diol-b-PU c | Controlled drug release | [132] | |
| PLA | Controlled drug release | [47] | |
| PLLA | Patch graft in carotid endarterectomy | [133] | |
| PVA | Drug delivery/controlled drug release | [134,135,136] | |
| PVA b,d | Drug delivery | [137] | |
| PVA/PVP Gelatin | Separation membrane Food packaging | [138] [139] | |
| PU/gelatin | Wound dressing | [140] | |
| Cellulose | Anode materials for lithium-ion batteries | [141] | |
| Collagen/essential oils | Wound dressing | [142] | |
| Origanum minutiflorum oil | Biomedical products | [143] | |
| Extracellular matrix components | Articular cartilage tissue engineering | [144] |
| Emulsifier/ Stabilizer | Water Phase | Oil Phase | Condition | Fiber Diameter (nm) | Ref. |
|---|---|---|---|---|---|
| Span 80 | Carboxymethyl chitosan and alginate (1:1) in distilled water | PCL | 8 mm (syringe), 15 cm b, 16 kV, 0.6 mL/h c, 25 °C, 55% RH, | ~2380 | [113] |
| Span 80 | Chitosan in 2% acetic acid (electrospinning on the surface of PLA fibers) | PLA emulsion (dissolved in chloroform) | 0.838 mm a, 15 cm b, 17 kV, 0.012 mL/min c | About 200 | [118] |
| Span 80 | Chitosan (dissolved in 2% acetic acid)/PVP in DMF/DCM (3:7) | PLA | 0.838 mm a, 15 cm b, 23 kV, 0.8 mL/h c, 20 ± 2 °C, 25 ± 5% RH, | N/A | [119] |
| Tween 80 | 10% w/v PVA mixed with 4% w/w chitosan in 14% acetic acid | PCL solution (8% w/v in chloroform/DMF, 30:20) with addition of eugenol (5% w/w based on weight of PCL) | 13 cm b, 75.0 kV, 60 Hz, 25 °C, 35% RH | 200–387 ± 179 | [114] |
| Tween 80 | Nanocellulose and 0.5% chitosan in pH 4 acetic acid | 10% poly(hydroxylalkanoate) (dissolved in chloroform) | 10 cm b, 20 kV, 4 mL/h c, 25.5 ± 0.5 °C, 55 ± 5% RH | N/A | [120] |
| Soybean lecithin | 0.75% w/v chitosan in 0.75% v/v acetic acid | 0.75% w/v PVA with addition of 7.5% v/v Mentha piperita essential oil, crosslinked by w/v sodium citrate | 10 cm b, 18 kV, 0.4 mL/minc | 300–400 | [115] |
| Sodium tripolyphosphate | 5% aloe vera in PVA (10% w/v)/ 1% chitosan emulsion in 1% acetic acid) | 10% w/v PVA | 0.6 mm a, 16.5 cm b, 15 kV, 0.083 mL/min c | 180–366 | [116] |
| β-CD-citral inclusion complexparticles | Carboxymethyl chitosan/PVA | Citral | 12 cm b, 12 kV, 0.07 mL/min c, 25 ± 5 °C, 50 ± 5% RH | 268 ± 62 | [117] |
| – | 4% w/w chitosan in 90% acetic acid | 10% w/w PCL in 3:1 chloroform/methanol | 0.260 mm a, 10 cm b, 22 kV, 3 μL/min c, room temperature, | 413–3770 | [64] |
| – | PCL/chitosan in formic acid/DCM | Formic acid/DCM by varying volume ratio of 7/3, 5/5 and 3/7 (v/v) | 3.810 mm a, 18 cm b, 13 kV, 0.3 mL/h c, 35 °C | 143 ± 49 | [65] |
| Polymers in Core | Polymers in Shell | Active Components in Core | Condition | Fiber Diameter (nm) | Ref. |
|---|---|---|---|---|---|
| 12% w/v PCL in trifluoroethanol | 2% w/v chitosan in 1:1 v/v of 3% w/v acetic acid/ trifluoroethanol | Ciprofloxacin | 0.92/1.64 mm orifice a, 15 kV, 15 cm b, 150–1000 rpm c | 180–340 nm | [52] |
| 13% w/v PCL in trifluoroethanol | 4% w/v chitosan/PEO (8:2 w/w) in trifluoroacetic acid | Simvastatin (calcium phosphate in shell) | 0.5/1.2 mm nozzle a, room temperature, 30–40% RH, 23 kV, 15 cm | 254.2 ± 34.5 nm core 103.6 ± 10.7 nm shell thickness | [53] |
| 7:3 w/w chitosan/PEO in 1:1 v/v acetic acid | 10% w/v PCL in 9:1 v/v chloroform/ DMF | Rosuvastatin | 16–20 kV, 0.5 mL/h, 10–12 cm b | 187–741 nm core 70 nm shell thickness | [122] |
| 8% w/v cyclodextrin, 5% w/w triclosan, and 2% w/w PEO in 50% v/v acetic acid/water | 3.5% w/w of chitosan and PEO (9:1) in 90% v/v acetic acid/ water | Triclosan loaded into cyclodextrin | 0.514/1.372 mm orifice a, 20 °C, 35% RH, 15–20 kV, 0.4–0.9 mL/h (shell), 0.3 mL/h (core), 20 cm b, 100 rpm c | 138–397 nm core 14–87 nm shell thickness | [123] |
| 8:2 of 5% w/w carboxymethyl chitosan/ 5% w/w PEO in water | 10% w/w PU in DMF | Doxorubicin and folic acid incorporated into the UiO-66 | 0.635/1.27 mm orifice a, 25 °C, 50% RH, 25 kV, 0.3–0.8 mL/h (shell), 0.5 mL/h (core), 15 cm b, 200 rpm c | 220–490 nm fiber diameter | [51] |
| 8:2 of 4–6% w/w carboxymethyl chitosan/ 10% w/w PVA in water | 8–12 w/w PCL in 80:20 v/v DCM:DMF | Doxorubicin loaded into nickel ferrite nanoparticles | 0.635/1.27 mm orifice a, 25 kV, 0.5 mL/h (core), 10–16.7 cm b | 305–495 nm fiber diameter | [125] |
| 12% w/w PCL in 70:30 v/v formic acid/ acetic acid | 2:1–1:2 v/v of 12% w/w PCL and 12% w/w chitosan in 70:30 v/v formic acid/acetic acid | 5FU and Fe3O4 nanoparticles | 0.413/1.194 mm orifice a, 15 kV, 0.08–0.1 mL/h (shell), 1.25–2 feeding ratio of shell/core, 14 cm b, 200 rpm c | 272–468 nm fiber diameter | [126] |
| 2% w/w chitosan in 80% v/v acetic acid | 15% w/w PCL in 90% v/v acetic acid | Tetracycline hydrochloride | 0.4/1.7 mm orifice a, 25 °C, 65% RH, 15 kV, 1 mL/h, 15 cm b, 500 rpm c | 285 ± 75 nm fiber diameter | [127] |
| 6% w/v PCL in DCM | 6% w/v chitosan in 80% v/v acetic acid | Rosuvastatin | 0.35/1.1 mm orifice a, 25 °C, 46% RH, 20 kV, 0.5 mL/h, 10 cm b. | 180–270 nm fiber diameter (120 nm core, 60 nm shell thickness) | [128] |
| 2% w/v chitosan in 90% v/v acetic acid | 15% w/v PCL in 1:1 v/v DCM/ ethanol | Resveratrol (ferulic acid in shell) | 0.5/0.83 mm orifice a, 24 °C, 68% RH, 25 kV, 0.4 mL/h (shell), 0.2 mL/h (core), 10–12 cm b | 240 ± 50 nm | [129] |
| 2:8 v/v of 5% w/w carboxymethyl chitosan and 10% w/w PVA | 10% w/w PCL in 80:20 v/v DCM/DMF | Doxorubicin | 0.21/1.6 mm orifice a, 24 °C, 68% RH, 25 kV, 0.5 mL/h (shell), 0.5 mL/h (core), 12 cm b, 1000 rpm c | 410 nm | [50] |
| 4% w/v polyvinyl pyrrolidone K90 and 12.5% w/v phospholipids in absolute ethanol | 3.5% w/v carboxymethyl chitosan in 1:2 ethanol/water and 10% w/v in 1:1 ethanol/water | Carvedilol | 0.413/1.067 mm orifice a, 20 °C, 35% RH,12.5 kV, 0.4 mL/h (shell), 0.13 mL/h (core), 10 cm b | 118–188 nm | [137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taokaew, S.; Chuenkaek, T. Developments of Core/Shell Chitosan-Based Nanofibers by Electrospinning Techniques: A Review. Fibers 2024, 12, 26. https://doi.org/10.3390/fib12030026
Taokaew S, Chuenkaek T. Developments of Core/Shell Chitosan-Based Nanofibers by Electrospinning Techniques: A Review. Fibers. 2024; 12(3):26. https://doi.org/10.3390/fib12030026
Chicago/Turabian StyleTaokaew, Siriporn, and Tapanee Chuenkaek. 2024. "Developments of Core/Shell Chitosan-Based Nanofibers by Electrospinning Techniques: A Review" Fibers 12, no. 3: 26. https://doi.org/10.3390/fib12030026