Shear Bond Strength of Nanohybrid Composite to Biodentine with Three Different Adhesives
Abstract
:1. Introduction
2. Materials and Methods
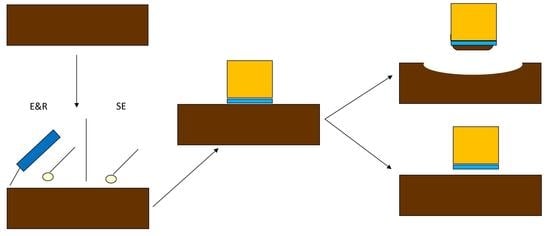
2.1. Specimen Preparation
- Group 1 (n = 60): the adhesion process was done after setting time (12 min). To do so, the specimens were randomly divided into four subgroups of 15 specimens [18,24]:
- o
- Group 1a (n = 15): Optibond FL (Kerr Corp), three-step etch-and-rinse adhesive.
- o
- Group 1b (n = 15): Solobond M (VOCO), two-step etch-and-rinse adhesive.
- o
- Group 1c (n = 15): Scotchbond (3M), universal adhesive, used as a two-step etch-and-rinse adhesive.
- o
- Group 1d (n = 15): Scotchbond (3M), universal adhesive, used as a self-etch adhesive.
- Group 2 (n = 60): the adhesion process was done after 24 h in the incubator. To do so, the specimens were randomly divided into four subgroups of 15 specimens. The distribution followed was the same as for Group 1.
2.2. Shear Bond Strength Measurement
2.3. Observation of Fractured Surfaces
2.4. Statistics
3. Results
3.1. Shear Bond Strength
3.2. Fracture Failure
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sezinando, A. Looking for ideal adhesive—A review. Rev. Port. Estomatol. Med. Dent. Cir. Maxilofac. 2014, 55, 194–206. [Google Scholar] [CrossRef]
- Breschi, L.; Mazzoni, A.; Ruggeri, A.; Cadenaro, M.; Di Lenarda, R.; De Stefano Dorigo, E. Dental adhesion review: Aging and stability of the bonded interface. Dent. Mater. 2008, 24, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Saiz, U.; Bock, T. Testing Adhesion of Direct Restoratives to Dental Hard Tissue—A Review. J. Adhes. Dent. 2010, 12, 343–371. [Google Scholar]
- De Munck, J.; Van Landuyt, K.; Peumans, M.; Potevin, A.; Labrechts, P.; Braem, M.; Van Meerbeek, B. A Critical Review of the Durability of Adhesion to Tooth Tissue: Methods and Results. J. Dent. Res. 2005, 84, 118–132. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; De Munck, J.; Yoshida, Y.; Inoue, S.; Vargas, M.; Vijay, P.; Van Landuyt, K.; Lambrechts, P.; Vanherle, G. Buonocore memorial lecture. Adhesion to enamel and dentin: Current status and future challenges. Oper. Dent. 2003, 28, 215–235. [Google Scholar]
- Bjomdal, L.; Relt, C.; Bruun, G.; Markvart, M.; Kjaeldgaard, M.; Näsman, P.; Thordrup, M.; Dige, I.; Nyvad, B.; Fransson, H. Treatment of deep caries lesions in adults: Randomized clinical trials comparing stepwise vs direct complete excavation, and direct pulp capping vs partial pulpotomy. Eur. J. Oral Sci. 2010, 118, 290–297. [Google Scholar] [CrossRef]
- De Souza Costa, C.A.; Hebling, J.; Hanks, C.T. Current status of pulp capping with dentin adhesive systems: A review. Dent. Mater. 2000, 16, 188–197. [Google Scholar] [CrossRef]
- Akhlaghi, N.; Khademi, A. Outcomes of vital pulp therapy in permanent teeth with different medicaments based on review of the literature. J. Dent. Res. 2015, 12, 406–417. [Google Scholar]
- Hilton, T.J.; Ferracane, J.L.; Mancl, L. Comparison of CaOH with MTA for direct pulp capping. J. Dent. Res. 2013, 92, S16–S22. [Google Scholar] [CrossRef]
- Paula, A.B.; Laranjo, M.; Marto, C.M.; Paulo, S.; Abrantes, A.M.; Casalta-Lopes, J.; Marques-Ferreira, M.; Botelho, M.F.; Carrilho, E. Direct pulp capping: What is the most effective therapy? Systematic review and meta-analysis. J. Evid. Based Dent. Pract. 2018, 18, 298–314. [Google Scholar] [CrossRef]
- Altunsoy, M.; Tanriver, M.; Ok, E.; Kucukylmaz, E. Shear Bond Strength of a Self- adhering Flowable Composite and a Flowable Base Composite to Mineral Trioxide Aggregate, Calcium-enriched Mixture Cement, and Biodentine. J. Endod. 2015, 41, 1691–1695. [Google Scholar] [CrossRef] [PubMed]
- Schwendicke, F.; Brouwer, F.; Schwendicke, A.; Paris, S. Different materials for direct pulp capping: Systematic review and meta-analysis and trial sequential analysis. Clin. Oral Investig. 2016, 20, 1121–1332. [Google Scholar] [CrossRef] [PubMed]
- Kundzina, R.; Stangvaltaite, L.; Eriksen, H.M.; Kerosuo, E. Capping carious exposures in adults: A randomized controlled trial investigating mineral trioxide aggregate versus calcium hydroxide. Int. Endod. J. 2017, 50, 924–932. [Google Scholar] [CrossRef]
- Tran, X.V.; Salehi, H.; Truong, M.T.; Sandra, M.; Sadoine, J.; Jacquot, B.; Cuisinier, F.; Chaussain, C.; Boukpessi, T. Reparative mineralized tissue characterization after direct pulp capping with calcium-silicate-based cements. Materials. 2019, 12, 2102. [Google Scholar] [CrossRef] [PubMed]
- Tran, X.V.; Gorin, C.; Willing, C.; Baroukh, B.; Pellat, B.; Decup, F.; Opsahl Vital, S.; Chaussain, C.; Boukpessi, T. Effect of a calcium-silicate-based restorative cement on pulp repair. J. Dent. Res. 2012, 91, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, A.; Lipski, M.; Parafiniuk, M.; Sporniak-Tutak, K.; Lichota, D.; Kosierkiewicz, A.; Kaczmarek, W.; Buczkowska-Radlinska, J. Response of Human Dental Pulp Capped with Biodentine and mineral trioxide aggregate. J. Endod. 2013, 39, 743–747. [Google Scholar] [CrossRef]
- Camilleri, J. Investigation of biodentine as dentine replacement material. J. Dent. 2013, 41, 600–610. [Google Scholar] [CrossRef]
- Cantekin, K.; Avci, S. Evaluation of shear bond strength of two resin-based composites and glass ionomer cement to pure tricalcium silicate-based cement (Biodentine®). J. Appl. Oral Sci. 2014, 22, 302–306. [Google Scholar] [CrossRef]
- Odabaş, M.; Bani, M.; Tirali, R.E. Shear Bond Strengths of Different Adhesive Systems to Biodentine. Sci. World J. 2013, 10, 626103. [Google Scholar] [CrossRef]
- Malkondu, Ö.; Kazandag, M.K.; Kazazoglu, E. A Review on Biodentine, a Contemporary Dentine Replacement and Repair Material. BioMed Res. Int. 2014, 160951. [Google Scholar] [CrossRef]
- Schmidt, A.; Schäfer, E.; Dammaschke, T. Shear bond Strength of lining materials to calcium-silicate cements at different time intervals. J. Adhes. Dent. 2017, 19, 129–135. [Google Scholar] [PubMed]
- Koubi, G.; Colon, P.; Franquin, J.C.; Hartmann, A.; Richard, G.; Faure, M.O.; Lambert, G. Clinical evaluation of the performance and safety of a new dentine substitute, Biodentine, in the restoration of posterior teeth—A prospective study. Clin. Oral Invest. 2013, 17, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Laurent, P.; Camps, J.; About, I. Biodentine(TM) induces TGF-beta1 release from human pulp cells and early dental pulp mineralization. Int. Endod. J. 2012, 45, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Tulumbalaci, F.; Erkmen, M.; Arikan, V.; Safa, M. Shear Bond strength of different restorative materials to mineral trioxide aggregate and Biodentine. J. Conserv. Dent. 2017, 20, 292–296. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, H.; Dhillon, J.S.; Batra, M.; Saini, M. MTA versus Biodentine: Review of literature with a comparative analysis. J. Clin. Diagn. Res. 2017, 11, ZG01–ZG05. [Google Scholar] [CrossRef]
- Uesrichai, N.; Nirunsittirat, A.; Chuveera, P.; Srisuwan, T.; Sastraruji, T.; Chompu-Inwai, P. Partial pulpotomy with two bioactive cements in permanent teeth of 6- to 18-year-old patients with signs and symptoms indicative of irreversible pulpitis: A noninferiority randomized controlled trial. Int. Endod. J. 2019, 52, 749–759. [Google Scholar] [CrossRef]
- Shafiei, F.; Doozandeh, M.; Gharibpour, F.; Adj, A. Effect of reducing acid-etching duration time on compressive strength and bonding of a universal adhesive to calcium silicate cements. Int. Endod. J. 2019, 52, 530–539. [Google Scholar] [CrossRef]
- Song, M.; Yu, B.; Kim, S.; Hayashi, M.; Smith, C.; Sohn, S.; Kim, E.; Stevenson, R.G.; Kim, R.H. Clinical and molecular perspectives of reparative dentin formation: Lessons learned from pulp-capping materials and the emerging roles of calcium. Dent. Clin. N. Am. 2017, 61, 93–110. [Google Scholar] [CrossRef]
- Karabucak, B.; Li, D.; Lim, J.; Iqbal, M. Vital pulp therapy with mineral trioxide aggregate. Dent. Traumatol. 2005, 21, 240–243. [Google Scholar] [CrossRef]
- Parirokh, M.; Torabinejad, M. Mineral trioxide aggregate: A comprehensive literature review-part1 1: Chemical, physical and antibacterial properties. J. Endod. 2010, 36, 16–27. [Google Scholar] [CrossRef]
- Han, L.; Okiji, T. Uptake of calcium and silicon released from calcium silicate-based endodontic materials into root canal dentine. Int. Endod. J. 2011, 44, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Çolak, H.; Tokay, U.; Uzgur, R.; Uzgur, Z.; Ercan, E.; Hamidi, M.M. The effect of different adhesives and setting times on bond strength between Biodentine and composite. J. Appl. Biomater. Funct. Mat. 2016, 14, e217–e222. [Google Scholar]
- Meraji, N.; Camilleri, J. Bonding over dentin replacement materials. J. Endod. 2017, 43, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Hashem, D.F.; Foxton, R.; Manoharan, A.; Watson, T.F.; Banerjee, A. The physical characteristics of resin composite-calcium silicate interface as part of layered/laminate adhesive restoration. Dent. Mater. 2014, 30, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Deepa, V.L.; Dhamaraju, B.; Bollu, I.P.; Balaji, T.S. Shear bond strength evaluation of resin composite bonded to three different liners: TheraCal LC, Biodentine, and resin-modified glass ionomer cement using universal adhesive: An in vitro study. J. Conserv. Dent. 2016, 19, 166–170. [Google Scholar] [CrossRef]
- Attik, G.N.; Villat, C.; Hallay, F.; Pradelle-Plasse, N.; Bonnet, H.; Moreau, K.; Colon, P.; Grosgogeat, B. In vitro biocompatibility of a dentine substitute cement on human MG63 osteoblasts cells: Biodentine™ versus MTA®. Int. Endod. J. 2014, 47, 1133–1141. [Google Scholar] [CrossRef]
- Aksel, H.; Küçükkaya Eren, S.; Askerbeyli Órs, S.; Karaismailoğlu, E. Surface and vertical dimensional changes of mineral trioxide aggregate and biodentine in different environmental conditions. J. Appl. Oral Sci. 2019, 27, e20180093. [Google Scholar] [CrossRef]
- Cengiz, E.; Ulusoy, N. Microshear bond strength of tri-calcium silicate-based cement to different restorative materials. J. Adhes. Dent. 2016, 18, 231–237. [Google Scholar]


| Material | Manufacturer | Lot Number | Clinical Procedure |
|---|---|---|---|
| Calcium silicate cement | Biodentine® (Septodont, Saint Maur des Fosses, France) | B22106 | Liquid and powder mixing at 4000–4200 rpm for 30 s |
| Nanohybrid composite | Grandio® (VOCO GmbH, Cuxhaven, Germany) | 1643371 | Light cure for 20 s |
| 3-step etch-and-rinse adhesive | Optibond® FL (Kerr Corp, Orange, CA, USA) | 6605713 |
|
| 2-step etch-and-rinse adhesive | Solobond® M (Voco GmbH, Cuxhaven, Germany) | 1637105 |
|
| Universal adhesive | Scotchbond Universal® (3M ESPE, St. Paul, MN, USA) | 80702A |
|
| Adhesive | 12 min MPa (SD) | 24 h MPa (SD) |
|---|---|---|
| Scotchbond Universal 1-step | 13.65 (4.62) a | 19.16 (6.62) a |
| Scotchbond Universal 2-step | 15.63 (5.79) a | 17.73 (7.54) a |
| Solobond M | 16.98 (3.56) a,b | 18.24 (6.60) a |
| Optibond FL | 20.34 (6.63) b | 19.87 (5.24) a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carretero, V.; Giner-Tarrida, L.; Peñate, L.; Arregui, M. Shear Bond Strength of Nanohybrid Composite to Biodentine with Three Different Adhesives. Coatings 2019, 9, 783. https://doi.org/10.3390/coatings9120783
Carretero V, Giner-Tarrida L, Peñate L, Arregui M. Shear Bond Strength of Nanohybrid Composite to Biodentine with Three Different Adhesives. Coatings. 2019; 9(12):783. https://doi.org/10.3390/coatings9120783
Chicago/Turabian StyleCarretero, Víctor, Luís Giner-Tarrida, Lissethe Peñate, and María Arregui. 2019. "Shear Bond Strength of Nanohybrid Composite to Biodentine with Three Different Adhesives" Coatings 9, no. 12: 783. https://doi.org/10.3390/coatings9120783