Mixed Nickel-Cobalt-Molybdenum Metal Oxide Nanosheet Arrays for Hybrid Supercapacitor Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Hybrid Supercapacitor Fabrication
2.3. Materials Characterization
2.4. Measurements of Electrochemical Performance
3. Results and Discussion
3.1. Structure and Morphology Characterizations
3.2. Electrochemical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where do batteries end and supercapacitors begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Song, Y.; Xia, Y. Electrochemical capacitors: Mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Liu, Y.; Liu, Z.; Zhang, L.; Chen, X. Design of architectures and materials in in-plane micro-supercapacitors: Current status and future challenges. Adv. Mater. 2017, 29, 1602802. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitor. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Tetard, L.; Zhai, L.; Thomas, J. Supercapacitor electrode materials: Nanostructures from 0 to 3 dimensions. Energy Environ. Sci. 2014, 8, 702–730. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Yu, M.; Wang, Z.; Han, Y.; Tong, Y.; Lu, X.; Yang, S. Recent progress in the development of anodes for asymmetric supercapacitors. J. Mater. Chem. A 2016, 4, 4634–4658. [Google Scholar] [CrossRef]
- Dong, X.W.; Zhang, Y.Y.; Wang, W.J.; Zhao, R. Rational construction of 3D NiCo2O4@CoMoO4 core/shell nanoarrays as a positive electrode for asymmetric supercapacitor. J. Alloys Compd. 2017, 729, 716–723. [Google Scholar] [CrossRef]
- Zhang, L.; Hui, K.N.; Hui, K.S.; Lee, H. High-performance hybrid supercapacitor with 3D hierarchical porous flower-like layered double hydroxide grown on nickel foam as binder-free electrode. J. Power Sources 2016, 318, 76–85. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, D.; Chen, S.; Guan, Y.; Xiong, J. Construction of hierarchical NiCo2S4@Ni(OH)2 core-shell hybrid nanosheet arrays on Ni foam for high-performance aqueous hybrid supercapacitors. Electrochim. Acta 2016, 193, 116–127. [Google Scholar] [CrossRef]
- Yin, C.; Yang, C.; Jiang, M.; Deng, C.; Yang, L.; Li, J.; Qian, D. A novel and facile one-pot solvothermal synthesis of PEDOT-PSS/Ni-Mn-Co-O hybrid as an advanced supercapacitor electrode material. ACS Appl. Mater. Interfaces 2016, 8, 2741–2752. [Google Scholar] [CrossRef] [PubMed]
- Sk, M.M.; Yue, C.Y.; Ghosh, K.; Jena, R.K. Review on advances in porous nanostructured nickel oxides and their composite electrodes for high-performance supercapacitors. J. Power Sources 2016, 308, 121–140. [Google Scholar] [CrossRef]
- Guan, C.; Liu, X.; Ren, W.; Li, X.; Cheng, C.; Wang, J. Rational design of metal-organic framework derived hollow NiCo2O4 arrays for flexible supercapacitor and electrocatalysis. Adv. Energy Mater. 2017, 7, 1602391. [Google Scholar] [CrossRef]
- Meher, S.K.; Rao, G.R. Effect of microwave on the nanowire morphology, optical, magnetic, and pseudocapacitance behavior of Co3O4. J. Phys. Chem. C 2011, 115, 25543–25556. [Google Scholar] [CrossRef]
- Raj, S.; Srivastava, S.K.; Kar, P.; Roy, P. Three-dimensional NiCo2O4/NiCo2S4 hybrid nanostructures on Ni-foam as high-performance supercapacitor electrode. RSC Adv. 2016, 6, 95760–95767. [Google Scholar] [CrossRef]
- Hu, W.; Chen, R.; Xie, W.; Zou, L.; Qin, N.; Bao, D. CoNi2S4 nanosheet arrays supported on nickel foams with ultrahigh capacitance for aqueous asymmetric supercapacitor applications. ACS Appl. Mater. Interfaces 2014, 6, 19318–19326. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.Y.; Yu, L.; Wang, X.; Song, S.; Lou, X.W. Formation of onion-like NiCo2S4 particles via sequential ion-exchange for hybrid supercapacitors. Adv. Mater. 2017, 29, 1605051. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Casalongue, H.S.; Liang, Y.; Dai, H. Ni(OH)2 nanoplates grown on graphene as advanced electrochemical pseudocapacitor materials. J. Am. Chem. Soc. 2010, 132, 7472–7477. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fu, N.; Zhang, G.; Xu, M.; Lu, W.; Zhou, L.; Huang, H. Design of hierarchical Ni-Co@Ni-Co layered double hydroxide core-shell structured nanotube array for high-performance flexible all-solid-sate battery-type supercapacitors. Adv. Funct. Mater. 2017, 27, 1605307. [Google Scholar] [CrossRef]
- Jagadale, A.D.; Guan, G.; Li, X.; Du, X.; Ma, X.; Hao, X.; Abudula, A. Ultrathin nanoflakes of cobalt-manganese layered double hydroxide with high reversibility for asymmetric supercapacitor. J. Power Sources 2016, 306, 526–534. [Google Scholar] [CrossRef]
- Cheng, J.; Yan, H.; Lu, Y.; Qiu, K.; Hou, X.; Xu, J.; Han, L.; Liu, X.; Kim, J.; Luo, Y. Mesoporous CuCo2O4 nanograss as multi-functional electrodes for supercapacitors and electro-catalysts. J. Mater. Chem. A 2015, 3, 9769–9776. [Google Scholar] [CrossRef]
- Sahoo, S.; Naik, K.K.; Late, D.J.; Rout, C.S. Electrochemical synthesis of a ternary transition metal sulfide nanosheets on nickel foam and energy storage application. J. Alloys Compd. 2017, 695, 154–161. [Google Scholar] [CrossRef]
- Duan, C.; Zhao, J.; Qin, L.; Yang, L.; Zhou, Y. Ternary Ni-Co-Mo oxy-hydroxide nanoflakes grown on carbon cloth for excellent supercapacitor electrodes. Mater. Lett. 2017, 208, 65–68. [Google Scholar] [CrossRef]
- Sahoo, S.; Mondal, R.; Late, D.J.; Rout, C.S. Electrodeposited nickel cobalt manganese based mixed sulfide nanosheets for high performance supercapacitor application. Microporous Mesoporous Mater. 2017, 244, 101–108. [Google Scholar] [CrossRef]
- Wu, C.; Cai, J.; Zhang, Q.; Zhou, X.; Zhu, Y.; Shen, P.; Zhang, K. Hierarchical mesoporous zinc-nickel-cobalt ternary oxide nanowire arrays on nickel foam as high-performance electrodes for supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 26512–26521. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Y.; Shi, F.; Zhang, Y.; Zhang, J.; Gu, C.; Wang, X.; Tu, J. Spinel manganese-nickel-cobalt ternary oxide nanowire array for high-performance electrochemical capacitor applications. ACS Appl. Mater. Interface 2014, 6, 18040–18047. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, J.H.; Chung, S.Y.; Choi, J.W. Enhanced pseudocapacitance in multicomponent transition-metal oxides by local distortion of oxygen octahedral. Angew. Chem. Int. Ed. 2016, 55, 3958–3962. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wei, H.; She, Y.; Tang, X.; Zhou, M.; Zang, Z.; Du, J.; Gao, C.; Guo, Y.; Bao, D. Flower-like nickel-zinc-cobalt mixed metal oxide nanowire arrays for electrochemical capacitor applications. J. Alloys Compd. 2017, 708, 146–153. [Google Scholar] [CrossRef]
- Cai, D.; Liu, B.; Wang, D.; Liu, Y.; Wang, L.; Li, H.; Wang, Y.; Wang, C.; Li, Q.; Wang, T. Facile hydrothermal synthesis of hierarchical ultrathin mesoporous NiMoO4 nanosheets for high performance Supercapacitors. Electrochim. Acta 2014, 115, 358–363. [Google Scholar] [CrossRef]
- Singh, A.K.; Sarkar, D.; Khan, G.G.; Mandal, K. Unique hydrogenated Ni-NiO core-shell 1D nano-heterostructures with superior electrochemical performance as supercapacitor. J. Mater. Chem. A 2013, 1, 12759–12767. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Direct synthesis of highly porous interconnected carbon nanosheets and their application as high-performance supercapacitors. ACS Nano 2014, 8, 5059–5078. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gao, Y.; Wang, C.; Yang, G. A simple electrochemical route to access amorphous mixed-metal hydroxides for supercapacitor electrode materials. Adv. Energy Mater. 2015, 5, 1401767. [Google Scholar] [CrossRef]
- Li, L.; Hui, K.S.; Hui, K.N.; Xia, Q.; Fu, J. Facile synthesis of NiAl layered double hydroxide nanoplates for high-performance asymmetric supercapacitor. J. Alloys Compd. 2017, 721, 803–812. [Google Scholar] [CrossRef] [Green Version]
- Hsu, A.R.; Chien, H.H.; Liao, C.Y.; Lee, C.C.; Tsai, J.H.; Hsu, C.C.; Cheng, I.C.; Chen, J.Z. Scan-mode atmospheric-pressure plasma jet processed reduced graphene oxides for quasi-solid-state gel-electrolyte supercapacitors. Coatings 2018, 8, 52. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Huang, Z.; Ren, L.; Qi, X.; Wei, X.; Zhong, J. Facile hydrothermal synthesis of NiMoO4@CoMoO4 hierarchical nanospheres for supercapacitor applications. Phys. Chem. Chem. Phys. 2015, 17, 20795–20804. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, B.; Meyrick, D.; Lee, Y.S.; Selvan, R.K. Synthesis and improved electrochemical performances of nano b-NiMoO4-CoMoO4·xH2O composites for asymmetric supercapacitors. RSC Adv. 2013, 3, 16542–16548. [Google Scholar] [CrossRef]
- Yin, Z.; Chen, Y.J.; Zhao, Y.; Li, C.; Zhu, C.; Zhang, X. Hierarchical nanosheet-based CoMoO4-NiMoO4 nanotubes for applications in asymmetric supercapacitor and oxygen evolution reaction. J. Mater. Chem. A 2015, 3, 22750–22758. [Google Scholar] [CrossRef]
- Godillot, G.; Taberna, P.L.; Daffos, B.; Simon, P.; Delmas, C.; Guerlou-Demourgues, L. High power density aqueous hybrid supercapacitor combining activated carbon and highly conductive spinel cobalt oxide. J. Power Sources 2016, 331, 277–284. [Google Scholar] [CrossRef]
- Li, L.; Cheah, Y.; Ko, Y.; Teh, P.; Wee, G.; Wong, C.; Peng, S.; Srinivasan, M. The facile synthesis of hierarchical porous flower-like NiCo2O4 with superior lithium storage properties. J. Mater. Chem. A 2013, 1, 10935–10941. [Google Scholar] [CrossRef]
- Peng, S.; Li, L.; Wu, H.B.; Srinivasan, M.; Lou, X.W. Controlled growth of NiMoO4 nanosheet and nanorod arrays on various conductive substrates as advanced electrodes for asymmetric supercapacitors. Adv. Energy Mater. 2015, 5, 1401172. [Google Scholar] [CrossRef]
- Cai, D.; Wang, D.; Liu, B.; Wang, Y.; Liu, Y.; Wang, L.; Li, H.; Huang, H.; Li, Q.; Wang, T. Comparison of the electrochemical performance of NiMoO4 nanorods and hierarchical nanospheres for supercapacitor applications. ACS Appl. Mater. Interfaces 2013, 5, 12905–12910. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Che, Q.; Li, H.; Zhang, X. Metal oxides: Mesoporous NiCo2O4 nanowire arrays grown on carbon textiles as binder-free flexible electrodes for energy storage. Adv. Funct. Mater. 2014, 24, 2630–2637. [Google Scholar] [CrossRef]
- Veerasubramani, G.K.; Krishnamoorthy, K.; Sang, J.K. Improved electrochemical performances of binder-free CoMoO4 nanoplate arrays@Ni foam electrode using redox additive electrolyte. J. Power Sources 2016, 306, 378–386. [Google Scholar] [CrossRef]
- Ding, R.; Qi, L.; Jia, M.; Wang, H. Facile synthesis of mesoporous spinel NiCo2O4 nanostructures as highly efficient electrocatalysts for urea electro-oxidation. Nanoscale 2013, 6, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Yue, C.Y. Development of 3D MoO3/graphene aerogel and sandwich-type polyaniline decorated porous MnO2-graphene hybrid film based high performance all-solid-state asymmetric supercapacitors. Electrochim. Acta 2018, 276, 47–63. [Google Scholar] [CrossRef]
- Riley, L.A.; Lee, S.H.; Gedvilias, L.; Dillon, A.C. Optimization of MoO3 nanoparticles as negative-electrode material in high-energy lithium ion batteries. J. Power Sources 2010, 195, 588–592. [Google Scholar] [CrossRef]
- Guo, K.; Cui, S.; Hou, H.; Chen, W.; Mi, L. Hierarchical ternary Ni-Co-Se nanowires for high-performance supercapacitor device design. Dalton Trans. 2016, 45, 19358–19465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, R.; Song, P.; Zhao, J.; Guo, X.; Zhang, D.; Yuan, B. CoMoO4 and Ni1/3Co2/3MoO4 nanosheets with high performance supercapacitor and nonenzymatic glucose detection properties. RSC Adv. 2015, 5, 84451–84456. [Google Scholar] [CrossRef]
- Chandrasekaran, N.I.; Muthukumar, H.; Sekar, A.D.; Manickam, M. Hollow nickel-aluminium-manganese layered triple hydroxide nanospheres with tunable architecture for supercapacitor application. Mater. Chem. Phys. 2017, 195, 247–258. [Google Scholar] [CrossRef]







| Electrode Materials | Specific Capacitance | Rate Capability | Cycling Performance | Ref. |
|---|---|---|---|---|
| Ni-Zn-Co oxide nanowire arrays | 776 F g−1 at 2 A g−1 | 73.8% at 32 A g−1 | 88.9% of the maximum value after 10,000 cycles | [29] |
| Ni-Co-Mo oxy-hydroxide nanoflakes | 2562 F g−1 at 1 A g−1 | 88.4% at 10 A g−1 | about 91% of its original capacitance after 1000 cycles | [24] |
| Ni-Co-Mo sulfide nanosheets | 2717 F g−1 at 1 A g−1 | 83.6% at 10 A g−1 | about 80% capacitance retention after 1000 cycles | [25] |
| Zn-Ni-Co oxides nanowire arrays | 2482 F g−1 at 1 A g−1 | 91.9% at 5 A g−1 | 94% capacitance retention over 3000 cycles | [26] |
| Mn-Ni-Co oxide nanowire array | 638 F g−1 at 1 A g−1 | 63.3% at 20 A g−1 | 93.6% of the maximum value after 6000 cycles | [27] |
| Ni0.8-Co0.2-Se nanowires | 86 F g−1 at 1 A g−1 | Not reported | exceeding 95% over the 2000 cycles test | [48] |
| Ni1/3Co2/3MoO4 nanosheets | 1103 F g−1 at 1 A g−1 | 84.3% at 10 A g−1 | remaining 85.18% at after 1000 cycles | [49] |
| Hollow Ni-Al-Mn layered hydroxide nanospheres | 1756 F g−1 at 4 A g−1 | Not reported | 89.5% of initial values after 4000 cycles | [50] |
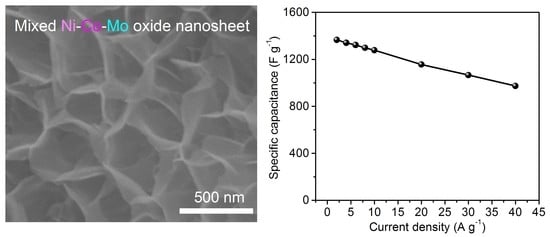
| Ni-Co-Mo oxide nanosheet arrays | 1366 F g−1 at 2 A g−1 | 71.3% at 40 A g−1 | 89.75% of the maximum value after 5000 cycles | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
She, Y.; Tang, B.; Li, D.; Tang, X.; Qiu, J.; Shang, Z.; Hu, W. Mixed Nickel-Cobalt-Molybdenum Metal Oxide Nanosheet Arrays for Hybrid Supercapacitor Applications. Coatings 2018, 8, 340. https://doi.org/10.3390/coatings8100340
She Y, Tang B, Li D, Tang X, Qiu J, Shang Z, Hu W. Mixed Nickel-Cobalt-Molybdenum Metal Oxide Nanosheet Arrays for Hybrid Supercapacitor Applications. Coatings. 2018; 8(10):340. https://doi.org/10.3390/coatings8100340
Chicago/Turabian StyleShe, Yin, Bin Tang, Dongling Li, Xiaosheng Tang, Jing Qiu, Zhengguo Shang, and Wei Hu. 2018. "Mixed Nickel-Cobalt-Molybdenum Metal Oxide Nanosheet Arrays for Hybrid Supercapacitor Applications" Coatings 8, no. 10: 340. https://doi.org/10.3390/coatings8100340