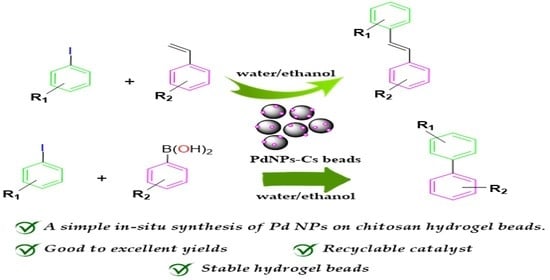
In Situ Decorated Palladium Nanoparticles on Chitosan Beads as a Catalyst for Coupling Reactions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Chitosan Hydrogel Beads (CS)
2.3. Palladium-Supported Chitosan Beads (Pd NPs-CS)
2.4. Characterization
2.5. General Experimental Procedure for the Catalyzed Suzuki–Miyaura Cross-Coupling Reaction
2.6. General Experimental Procedure for the Catalyzed Heck Coupling Reaction
3. Results and Discussion
3.1. Synthesis and Characterizations of Pd NPs-CS Catalyst
3.2. Suzuki–Miyaura Coupling Reaction
3.3. Catalytic Heck Reaction
3.4. Reusability of Pd NPs-CS Catalyst
3.5. Comparison of the Catalytic Activity of Pd NPs-CS Beads
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gilbertson, L.M.; Zimmerman, J.B.; Plata, D.L.; Hutchison, J.E.; Anastas, P.T. Designing Nanomaterials to Maximize Performance and Minimize Undesirable Implications Guided by the Principles of Green Chemistry. Chem. Soc. Rev. 2015, 44, 5758–5777. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Sanderson, H.; Roy, K.; Benfenati, E.; Leszczynski, J. Green Chemistry in the Synthesis of Pharmaceuticals. Chem. Rev. 2022, 122, 3637–3710. [Google Scholar] [CrossRef] [PubMed]
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Bio-Based Active Food Packaging Materials: Sustainable Alternative to Conventional Petrochemical-Based Packaging Materials. Food Res. Int. 2020, 137, 109625. [Google Scholar] [CrossRef] [PubMed]
- Chtchigrovsky, M.; Primo, A.; Gonzalez, P.; Molvinger, K.; Robitzer, M.; Quignard, F.; Taran, F. Functionalized Chitosan as a Green, Recyclable, Biopolymer-Supported Catalyst for the [3+2] Huisgen Cycloaddition. Angew. Chem. Int. Ed. Engl. 2009, 48, 5916–5920. [Google Scholar] [CrossRef]
- Levy-Ontman, O.; Biton, S.; Shlomov, B.; Wolfson, A. Renewable Polysaccharides as Supports for Palladium Phosphine Catalysts. Polymers 2018, 10, 659. [Google Scholar] [CrossRef] [Green Version]
- Sindhu, R.K.; Goyal, A.; Das, J.; Neha; Choden, S.; Kumar, P. Immunomodulatory Potential of Polysaccharides Derived from Plants and Microbes: A Narrative Review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100044. [Google Scholar] [CrossRef]
- D’Ayala, G.G.; Malinconico, M.; Laurienzo, P. Marine Derived Polysaccharides for Biomedical Applications: Chemical Modification Approaches. Molecules 2008, 13, 2069–2106. [Google Scholar] [CrossRef] [Green Version]
- Chandy, T.; Sharma, C.P. Chitosan-as a Biomaterial. Biomater. Artif. Cells Artif. Organs 1990, 18, 1–24. [Google Scholar] [CrossRef]
- Hudson, S.M.; Smith, C. Polysaccharides: Chitin and Chitosan: Chemistry and Technology of Their Use as Structural Materials. In Biopolymers from Renewable Resources; Kaplan, D.L., Ed.; Macromolecular Systems—Materials Approach; Springer: Berlin/Heidelberg, Germany, 1998; pp. 96–118. ISBN 978-3-662-03680-8. [Google Scholar]
- Mathur, N.K.; Narang, C.K. Chitin and Chitosan, Versatile Polysaccharides from Marine Animals. J. Chem. Educ. 1990, 67, 938. [Google Scholar] [CrossRef]
- Quignard, F.; Di Renzo, F.; Guibal, E. From Natural Polysaccharides to Materials for Catalysis, Adsorption, and Remediation. In Carbohydrates in Sustainable Development I; Rauter, A.P., Vogel, P., Queneau, Y., Eds.; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2010; pp. 165–197. ISBN 978-3-642-14837-8. [Google Scholar]
- Luo, Y.; Wang, Q. Recent Development of Chitosan-Based Polyelectrolyte Complexes with Natural Polysaccharides for Drug Delivery. Int. J. Biol. Macromol. 2014, 64, 353–367. [Google Scholar] [CrossRef]
- Felt, O.; Buri, P.; Gurny, R. Chitosan: A Unique Polysaccharide for Drug Delivery. Drug Dev. Ind. Pharm. 1998, 24, 979–993. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Riaz, M.; Yasin, T. Structural and Viscoelastic Properties of Chitosan-Based Hydrogel and Its Drug Delivery Application. Int. J. Biol. Macromol. 2013, 59, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of Chitosan in Food, Pharmaceuticals, Medicine, Cosmetics, Agriculture, Textiles, Pulp and Paper, Biotechnology, and Environmental Chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692. [Google Scholar] [CrossRef] [Green Version]
- Molnár, Á. The Use of Chitosan-Based Metal Catalysts in Organic Transformations. Coord. Chem. Rev. 2019, 388, 126–171. [Google Scholar] [CrossRef]
- Khomthawee, K.; Nilada, N.; Homchuen, A.; Saejueng, P. Chitosan-Ethylenediaminetetraacetic Acid-Palladium Composite for the Suzuki–Miyaura Reaction. Appl. Organomet. Chem. 2023, 37, e6987. [Google Scholar] [CrossRef]
- Boominathan, T.; Sivaramakrishna, A. Room Temperature Catalytic Synthesis of Biaryl and Benzimidazole Derivatives—Triggered by Nano-Sized Pd-Loaded-Modified Chitosan Polyurethane Foams. J. Catal. 2023, 426, 6–20. [Google Scholar] [CrossRef]
- Luo, W.; Luo, K.; Yang, Y.; Lin, X.; Li, P.; Wen, Y. N-Maleyl Chitosan-Supported Palladium Catalyst for Heck Coupling Reaction and Reduction of 4-Nitrophenol. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129852. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.; Chen, Z.; Hu, J. Metal-Organic Framework Grown in Situ on Chitosan Microspheres as Robust Host of Palladium for Heterogeneous Catalysis: Suzuki Reaction and the p-Nitrophenol Reduction. Int. J. Biol. Macromol. 2022, 206, 232–241. [Google Scholar] [CrossRef]
- Wang, G.; Hu, Z.; Chen, Z.; Wang, J.; Hu, J.; Xu, X. Pd NPs Encapsulated by COF in Nitrogen-Doped Macroporous Chitosan Carbon Microspheres Act as an Efficient and Recyclable Multifunctional Catalyst. Appl. Surf. Sci. 2023, 631, 157538. [Google Scholar] [CrossRef]
- Schmidt, A.F.; Kurokhtina, A.A. Distinguishing between the Homogeneous and Heterogeneous Mechanisms of Catalysis in the Mizoroki-Heck and Suzuki-Miyaura Reactions: Problems and Prospects. Kinet. Catal. 2012, 53, 714–730. [Google Scholar] [CrossRef]
- Couty, S.; Liégault, B.; Meyer, C.; Cossy, J. Heck−Suzuki−Miyaura Domino Reactions Involving Ynamides. An Efficient Access to 3-(Arylmethylene)Isoindolinones. Org. Lett. 2004, 6, 2511–2514. [Google Scholar] [CrossRef] [PubMed]
- Phan, N.T.S.; Van Der Sluys, M.; Jones, C.W. On the Nature of the Active Species in Palladium Catalyzed Mizoroki–Heck and Suzuki–Miyaura Couplings—Homogeneous or Heterogeneous Catalysis, A Critical Review. Adv. Synth. Catal. 2006, 348, 609–679. [Google Scholar] [CrossRef]
- Albano, G.; Petri, A.; Aronica, L.A. Palladium Supported on Bioinspired Materials as Catalysts for C–C Coupling Reactions. Catalysts 2023, 13, 210. [Google Scholar] [CrossRef]
- Wang, G.; Lv, K.; Chen, T.; Chen, Z.; Hu, J. Immobilizing of Palladium on Melamine Functionalized Magnetic Chitosan Beads: A Versatile Catalyst for p-Nitrophenol Reduction and Suzuki Reaction in Aqueous Medium. Int. J. Biol. Macromol. 2021, 184, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Dadashi, J.; Ali Ghasemzadeh, M.; Salavati-Niasari, M. Recent Developments in Hydrogels Containing Copper and Palladium for the Catalytic Reduction/Degradation of Organic Pollutants. RSC Adv. 2022, 12, 23481–23502. [Google Scholar] [CrossRef]
- Molnar, A.; Papp, A. Catalyst Recycling—A Survey of Recent Progress and Current Status. Coord. Chem. Rev. 2017, 349, 1–65. [Google Scholar] [CrossRef]
- Baran, T.; Yılmaz Baran, N.; Menteş, A. Sustainable Chitosan/Starch Composite Material for Stabilization of Palladium Nanoparticles: Synthesis, Characterization and Investigation of Catalytic Behaviour of Pd@chitosan/Starch Nanocomposite in Suzuki–Miyaura Reaction. Appl. Organomet. Chem. 2018, 32, e4075. [Google Scholar] [CrossRef]
- Anuradha; Kumari, S.; Layek, S.; Pathak, D.D. Palladium Nanoparticles Immobilized on a Magnetic Chitosan-Anchored Schiff Base: Applications in Suzuki–Miyaura and Heck–Mizoroki Coupling Reactions. N. J. Chem. 2017, 41, 5595–5604. [Google Scholar] [CrossRef]
- Shahriari, M.; Ali Hosseini Sedigh, M.; Shahriari, M.; Stenzel, M.; Mahdi Zangeneh, M.; Zangeneh, A.; Mahdavi, B.; Asadnia, M.; Gholami, J.; Karmakar, B.; et al. Palladium Nanoparticles Decorated Chitosan-Pectin Modified Kaolin: It’s Catalytic Activity for Suzuki-Miyaura Coupling Reaction, Reduction of the 4-Nitrophenol, and Treatment of Lung Cancer. Inorg. Chem. Commun. 2022, 141, 109523. [Google Scholar] [CrossRef]
- Yılmaz Baran, N.; Baran, T.; Menteş, A. Production of Novel Palladium Nanocatalyst Stabilized with Sustainable Chitosan/Cellulose Composite and Its Catalytic Performance in Suzuki-Miyaura Coupling Reactions. Carbohydr. Polym. 2018, 181, 596–604. [Google Scholar] [CrossRef]
- Veisi, H.; Ozturk, T.; Karmakar, B.; Tamoradi, T.; Hemmati, S. In Situ Decorated Pd NPs on Chitosan-Encapsulated Fe3O4/SiO2-NH2 as Magnetic Catalyst in Suzuki-Miyaura Coupling and 4-Nitrophenol Reduction. Carbohydr. Polym. 2020, 235, 115966. [Google Scholar] [CrossRef] [PubMed]
- Hasan, K. Methyl Salicylate Functionalized Magnetic Chitosan Immobilized Palladium Nanoparticles: An Efficient Catalyst for the Suzuki and Heck Coupling Reactions in Water. ChemistrySelect 2020, 5, 7129–7140. [Google Scholar] [CrossRef]
- Sun, S.; Song, J.; Yuan, X.; Zhang, Y.; Shu, Z.; Xie, C.-X.; Jia, X. A PPh3 Modified-Chitosan Supported Pd Nanocatalyst for Heterogeneous Suzuki–Miyaura Cross Coupling Reactions. N. J. Chem. 2023, 47, 7410–7415. [Google Scholar] [CrossRef]
- Wang, G.; Chen, Z.; Yan, G.; Hu, J. Palladium Nanoparticles Immobilized on COF-Modified Honeycomb Chitosan Microcapsules as Catalysts for the Suzuki Reaction and p -Nitrophenol Reduction. N. J. Chem. 2023, 47, 297–306. [Google Scholar] [CrossRef]
- Lv, D.; Zhang, M. O-Carboxymethyl Chitosan Supported Heterogeneous Palladium and Ni Catalysts for Heck Reaction. Molecules 2017, 22, 150. [Google Scholar] [CrossRef]













| Entry | Catalyst | Base | Time (h) | Yield b (%) |
|---|---|---|---|---|
| 1 | Neat | K2CO3 (1 eq) + KF (2 eq) | 4 | 0 |
| 2 | Chitosan | K2CO3 (1 eq) + KF (2 eq) | 4 | 0 |
| 3 | Cs beads | K2CO3 (1 eq) + KF (2 eq) | 4 | 0 |
| 4 | PdCl2 (PPh3)2 | K2CO3 (3 eq) | 4 | 28 |
| 5 | PdCl2 (PPh3)2 | KF (3 eq) | 6 | 26 |
| 6 | PdCl2 (PPh3)2 | K2CO3 (1 eq) + KF (2 eq) | 4 | 34 |
| 7 | Pd NPs-CS | KF (3 eq) | 6 | 66 |
| 8 | Pd NPs-CS | K2CO3 (1 eq) + KF (2 eq) | 4 | 82 |
| 9 | Pd NPs-CS | K2CO3 (1 eq) + KF (2 eq) | 4 | 91 |
| Entry | Catalyst | Solvent | Yield b (%) |
|---|---|---|---|
| 1 | Neat | Ethanol/Water | 0 |
| 2 | Chitosan (CS) | Ethanol/Water | 0 |
| 3 | CS beads | Ethanol/Water | 0 |
| 4 | Pd NPs-CS | Ethanol/Water | 82 |
| 5 | Pd NPs-CS | Et3N | 27 |
| Catalyst | Conditions | Time (h) | Yields (%) | Ref. | |
|---|---|---|---|---|---|
| Suzuki–Miyaura coupling reaction | Pd@chitosan/starch | Microwave irradiation (400 W); Neat; 50 °C | 0.1 | 35–99 | [29] |
| Pd@CS-SB @Fe3O4 | PEG-200:H2O (1:1); 50 °C | 0.5 | 72–99 | [30] | |
| Pd@CS-Pectine@Kaolin | H2O/EtOH (1:1); 40 °C | 0.1–3 | 50–98 | [31] | |
| Pd@chitosan/cellulose | Microwave irradiation; Neat; 50 °C | 0.08 | 55–100 | [32] | |
| Pd NPs@CS/Fe3O4/SiO2-NH2 | H2O/EtOH (1:1); 40 °C | 0.25–12 | 50–98 | [33] | |
| Fe3O4@CS@MS@Pd | H2O/EtOH(4:1); 80 °C | 8 | 25–98 | [34] | |
| Pd@PPh3-CS | H2O/EtOH(3:1); 50 °C | 4 | 83–99 | [35] | |
| Pd@CS/ZIF-8@COF | H2O/EtOH (1:1); 80 °C | 4 | 39–99 | [36] | |
| PdNPs-CS beads | H2O/EtOH (1:1); 40 °C | 4 | 44–91 | This work | |
| Heck coupling reaction | Pd@MMT/CS | DMSO/ethylene glycol (30:2); 110 °C | 4 | 47–96 | [37] |
| Pd@CS-SB @Fe3O4 | PEG-200:H2O (1:1); 110 °C | 0.67 | 62–98 | [25] | |
| Pd/MAAC | DMF (3 mL)/glycol (0.2 g); 110 °C | 3–12 | 67–99 | [26] | |
| Pd@CPS | DMAc (3.0 g); 110 °C | 24 | 0–90 | [28] | |
| PdNPs-CS beads | Et3N; H2O/EtOH (1:1); 70 °C | 7 | 76–90 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oudghiri, K.; Bahsis, L.; Eddarir, S.; Anane, H.; Taourirte, M. In Situ Decorated Palladium Nanoparticles on Chitosan Beads as a Catalyst for Coupling Reactions. Coatings 2023, 13, 1367. https://doi.org/10.3390/coatings13081367
Oudghiri K, Bahsis L, Eddarir S, Anane H, Taourirte M. In Situ Decorated Palladium Nanoparticles on Chitosan Beads as a Catalyst for Coupling Reactions. Coatings. 2023; 13(8):1367. https://doi.org/10.3390/coatings13081367
Chicago/Turabian StyleOudghiri, Khaoula, Lahoucine Bahsis, Said Eddarir, Hafid Anane, and Moha Taourirte. 2023. "In Situ Decorated Palladium Nanoparticles on Chitosan Beads as a Catalyst for Coupling Reactions" Coatings 13, no. 8: 1367. https://doi.org/10.3390/coatings13081367