Bacterial Detection and Differentiation of Staphylococcus aureus and Escherichia coli Utilizing Long-Period Fiber Gratings Functionalized with Nanoporous Coated Structures
Abstract
:1. Introduction
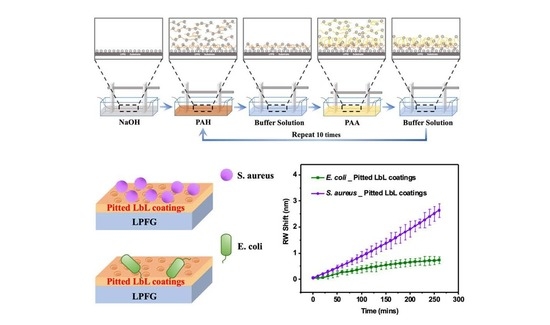
2. Results and Discussion
2.1. Characterization of Nanoporous [PAH/PAA]10-PAH Coatings
2.2. Rapid Detection of Bacteria Using LPFGs with Functional Coatings
2.3. Bacterial Differentiation Using Functional Coatings on LPFGs
3. Conclusions
4. Experimental Section
4.1. Chemical Reagents and Materials
4.2. LPFG Fabrication
4.3. Preparation of [PAH/PAA]10-PAH Coatings
4.4. Preparation of Nanoporous [PAH/PAA]10-PAH Coatings
4.5. Culture and Preparation of S. aureus and E. coli
4.6. Characterization and Analysis
4.7. Optofluidic Platform
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smietana, M.; Bock, W.J.; Mikulic, P.; Ng, A.; Chinnappan, R.; Zourob, M. Detection of bacteria using bacteriophages as recognition elements immobilized on long-period fiber gratings. Opt. Express 2011, 19, 7971. [Google Scholar] [CrossRef]
- Zourob, M.; Mohr, S.; Brown, B.J.T.; Fielden, P.R.; McDonnell, M.B.; Goddard, N.J. Bacteria detection using disposable optical leaky waveguide sensors. Biosens. Bioelectron. 2005, 21, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Shabani, A.; Zourob, M.; Allain, B.; Marquette, C.A.; Lawrence, M.F.; Mandeville, R. Bacteriophage-modified microarrays for the direct impedimetric detection of bacteria. Anal. Chem. 2008, 80, 9475–9482. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.D.; Yu, Q.; Chen, S.; Homola, J.; Jiang, S. Comparison of E. coli O157:H7 preparation methods used for detection with surface plasmon resonance sensor. Sens. Actuators B Chem. 2005, 107, 202–208. [Google Scholar] [CrossRef]
- Templier, V.; Roux, A.; Roupioz, Y.; Livache, T. Ligands for label-free detection of whole bacteria on biosensors: A review. TrAC-Trends Anal. Chem. 2016, 79, 71–79. [Google Scholar] [CrossRef]
- Wang, T.; Liu, J.; Gu, X.; Li, D.; Wang, J.; Wang, E. Label-free electrochemical aptasensor constructed by layer-by-layer technology for sensitive and selective detection of cancer cells. Anal. Chim. Acta 2015, 882, 32–37. [Google Scholar] [CrossRef]
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef]
- Bock, W.J.; Tripathi, S.M.; Smietana, M. Sensitive and Selective Lab-on-a-Fiber Sensor for Bacteria Detection in Water. Springer Ser. Surf. Sci. 2015, 56, 315–334. [Google Scholar]
- Baeumner, A.J.; Cohen, R.N.; Miksic, V.; Min, J. RNA biosensor for the rapid detection of viable Escherichia coli in drinking water. Biosens. Bioelectron. 2003, 18, 405–413. [Google Scholar] [CrossRef]
- Van Dorst, B.; Mehta, J.; Bekaert, K.; Rouah-Martin, E.; De Coen, W.; Dubruel, P.; Blust, R.; Robbens, J. Recent advances in recognition elements of food and environmental biosensors: A review. Biosens. Bioelectron. 2010, 26, 1178–1194. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Sorokulova, I.B.; Vodyanoy, V.J.; Simonian, A.L. Lytic phage as a specific and selective probe for detection of Staphylococcus aureus—A surface plasmon resonance spectroscopic study. Biosens. Bioelectron. 2007, 22, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Sindhu, A.; Dilbaghi, N.; Chaudhury, A. Biosensors as innovative tools for the detection of food borne pathogens. Biosens. Bioelectron. 2011, 28, 1–12. [Google Scholar] [CrossRef]
- Rijal, K.; Leung, A.; Shankar, P.M.; Mutharasan, R. Detection of pathogen Escherichia coli O157:H7 at 70 cells/mL using antibody-immobilized biconical tapered fiber sensors. Biosens. Bioelectron. 2005, 21, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Samuelson, D.R.; Xu, Y.; Zhang, H.; Wang, S.; Rasco, B.A.; Xu, J.; Konkel, M.E. Detecting and tracking nosocomial methicillin-resistant Staphylococcus aureus using a microfluidic SERS biosensor. Anal. Chem. 2013, 85, 2320–2327. [Google Scholar] [CrossRef]
- Yang, F.; Chang, T.-L.; Liu, T.; Wu, D.; Du, H.; Liang, J.; Tian, F. Label-free detection of Staphylococcus aureus bacteria using long-period fiber gratings with functional polyelectrolyte coatings. Biosens. Bioelectron. 2019, 133, 147–153. [Google Scholar] [CrossRef]
- Goodridge, L.; Chen, J.; Griffiths, M. Development and characterization of a fluorescent-bacteriophage assay for detection of Escherichia coli O157:H7. Appl. Environ. Microbiol. 1999, 65, 1397–1404. [Google Scholar] [CrossRef]
- Liu, K.; Chen, T.; He, S.; Robbins, J.P.; Podkolzin, S.G.; Tian, F. Observation and Identification of an Atomic Oxygen Structure on Catalytic Gold Nanoparticles. Angew. Chem.-Int. Ed. 2017, 56, 12952–12957. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; He, S.; Li, L.; Liu, Y.; Huang, Z.; Liu, T.; Wu, H.; Jiang, X.; Liu, K.; Tian, F. Spectroscopically clean Au nanoparticles for catalytic decomposition of hydrogen peroxide. Sci. Rep. 2021, 11, 9709. [Google Scholar] [CrossRef]
- He, S.; Wu, D.; Chen, S.; Liu, K.; Yang, E.-H.; Tian, F.; Du, H. Au-on-Ag nanostructure for in-situ SERS monitoring of catalytic reactions. Nanotechnology 2022, 33, 155701. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Chen, T.; Liu, K.; Du, H.; Podkolzin, S.G. Atomic, Molecular and Hybrid Oxygen Structures on Silver. Langmuir 2021, 37, 11603–11610. [Google Scholar] [CrossRef] [PubMed]
- Scoullos, E.V.; Hofman, M.S.; Zheng, Y.; Potapenko, D.V.; Tang, Z.; Podkolzin, S.G.; Koel, B.E. Guaiacol Adsorption and Decomposition on Platinum. J. Phys. Chem. C 2018, 122, 29180–29189. [Google Scholar] [CrossRef]
- Zheng, Y.; Tang, Z.; Podkolzin, S.G. Catalytic Platinum Nanoparticles Decorated with Subnanometer Molybdenum Clusters for Biomass Processing. Chem.-A Eur. J. 2020, 26, 5174–5179. [Google Scholar] [CrossRef]
- Robbins, J.P.; Ezeonu, L.; Tang, Z.; Yang, X.; Koel, B.E.; Podkolzin, S.G. Propane Dehydrogenation to Propylene and Propylene Adsorption on Ni and Ni-Sn Catalysts. ChemCatChem 2022, 14, e202101546. [Google Scholar] [CrossRef]
- Zheng, Y.; Qi, Y.; Tang, Z.; Hanke, F.; Podkolzin, S.G. Kinetics and Reaction Mechanisms of Acetic Acid Hydrodeoxygenation over Pt and Pt-Mo Catalysts. ACS Sustain. Chem. Eng. 2022, 10, 5212–5224. [Google Scholar] [CrossRef]
- Zheng, Y.; Qi, Y.; Tang, Z.; Tan, J.; Koel, B.E.; Podkolzin, S.G. Spectroscopic observation and structure-insensitivity of hydroxyls on gold. Chem. Commun. 2022, 58, 4036–4039. [Google Scholar] [CrossRef]
- Ezeonu, L.; Tang, Z.; Qi, Y.; Huo, F.; Zheng, Y.; Koel, B.E.; Podkolzin, S.G. Adsorption, surface reactions and hydrodeoxygenation of acetic acid on platinum and nickel catalysts. J. Catal. 2023, 418, 190–202. [Google Scholar] [CrossRef]
- Wolfbeis, O.S. Fiber-optic chemical sensors and biosensors. Anal. Chem. 2006, 78, 3859–3873. [Google Scholar] [CrossRef]
- Helmerhorst, E.; Chandler, D.J.; Nussio, M.; Mamotte, C.D. Real-time and label-free bio-sensing of molecular interactions by surface plasmon resonance: A laboratory medicine perspective. Clin. Biochem. Rev. 2012, 33, 161–173. [Google Scholar]
- Bandara, A.B.; Zuo, Z.; Ramachandran, S.; Ritter, A.; Heflin, J.R.; Inzana, T.J. Detection of methicillin-resistant staphylococci by biosensor assay consisting of nanoscale films on optical fiber long-period gratings. Biosens. Bioelectron. 2015, 70, 433–440. [Google Scholar] [CrossRef]
- Boudou, T.; Crouzier, T.; Ren, K.; Blin, G.; Picart, C. Multiple functionalities of polyelectrolyte multilayer films: New biomedical applications. Adv. Mater. 2010, 22, 441–467. [Google Scholar] [CrossRef]
- Saha, N.; Monge, C.; Dulong, V.; Picart, C.; Glinel, K. Influence of polyelectrolyte film stiffness on bacterial growth. Biomacromolecules 2013, 14, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Kanka, J.; Tian, F. Silica nanoparticle coated long-period grating for in situ monitoring of drug delivery thin films. Opt. Fibers Sens. Med. Diagn. Treat. Appl. XVII 2017, 10058, 1005809. [Google Scholar]
- Hammond, P.T. Building biomedical materials layer-by-layer. Mater. Today 2012, 15, 196–206. [Google Scholar] [CrossRef]
- Du, Y.; Chen, C.; Li, B.; Zhou, M.; Wang, E.; Dong, S. Layer-by-layer electrochemical biosensor with aptamer-appended active polyelectrolyte multilayer for sensitive protein determination. Biosens. Bioelectron. 2010, 25, 1902–1907. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, Y.; Podsiadlo, P.; Kotov, N.A. Biomedical applications of layer-by-layer assembly: From biomimetics to tissue engineering. Adv. Mater. 2006, 18, 3203–3224. [Google Scholar] [CrossRef]
- Chiavaioli, F.; Biswas, P.; Trono, C.; Bandyopadhyay, S.; Giannetti, A.; Tombelli, S.; Basumallick, N.; Dasgupta, K.; Baldini, F. Towards sensitive label-free immunosensing by means of turn-around point long period fiber gratings. Biosens. Bioelectron. 2014, 60, 305–310. [Google Scholar] [CrossRef]
- Mendelsohn, J.D.; Barrett, C.J.; Chan, V.V.; Pal, A.J.; Mayes, A.M.; Rubner, M.F. Fabrication of microporous thin films from polyelectrolyte multilayers. Langmuir 2000, 16, 5017–5023. [Google Scholar] [CrossRef]
- Sun, B.; Flessner, R.M.; Saurer, E.M.; Jewell, C.M.; Fredin, N.J.; Lynn, D.M. Characterization of pH-induced changes in the morphology of polyelectrolyte multilayers assembled from poly(allylamine) and low molecular weight poly(acrylic acid). J. Colloid Interface Sci. 2011, 355, 431–441. [Google Scholar] [CrossRef]
- Perera-Costa, D.; Bruque, J.M.; González-Martín, M.L.; Gómez-García, A.C.; Vadillo-Rodríguez, V. Studying the influence of surface topography on bacterial adhesion using spatially organized microtopographic surface patterns. Langmuir 2014, 30, 4633–4641. [Google Scholar] [CrossRef]
- Whitehead, K.A.; Colligon, J.; Verran, J. Retention of microbial cells in substratum surface features of micrometer and sub-micrometer dimensions. Colloids Surf. B Biointerfaces 2005, 41, 129–138. [Google Scholar] [CrossRef]
- Harris, J.J.; DeRose, P.M.; Bruening, M.L. Synthesis of passivating, nylon-like coatings through cross-linking of ultrathin polyelectrolyte films. J. Am. Chem. Soc. 1999, 121, 1978–1979. [Google Scholar] [CrossRef]
- Chang, T.L.; Zhou, X.; Liang, J. Synthesis and characterization of Ag-Cu alloy nanoparticles for antimicrobial applications: A polydopamine chemistry application. Mater. Sci. Eng. C 2019, 98, 675–684. [Google Scholar] [CrossRef]
- Vaamonde, G.; Chirife, J. Effect of phosphate buffer on Staphylococcus aureas growth at a reduced water activity. Int. J. Food Microbiol. 1986, 3, 51–55. [Google Scholar] [CrossRef]
- Guo, S.; Zhu, X.; Loh, X.J. Controlling cell adhesion using layer-by-layer approaches for biomedical applications. Mater. Sci. Eng. C 2017, 70, 1163–1175. [Google Scholar] [CrossRef] [PubMed]
- Lerebour, G.; Cupferman, S.; Bellon-Fontaine, M.N. Adhesion of Staphylococcus aureus and Staphylococcus epidermidis to the Episkin® reconstructed epidermis model and to an inert 304 stainless steel substrate. J. Appl. Microbiol. 2004, 97, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Trunk, T.; Khalil, H.S.; Leo, J.C. Bacterial autoaggregation. AIMS Microbiol. 2018, 4, 140–164. [Google Scholar] [CrossRef] [PubMed]
- Berne, C.; Ellison, C.K.; Ducret, A.; Brun, Y.V. Bacterial adhesion at the single-cell level. Nat. Rev. Microbiol. 2018, 16, 616–627. [Google Scholar] [CrossRef]
- Jacobson, S.H.; Tullus, K.; Brauner, A. Hydrophobic properties of Escherichia coli causing acute pyelonephritis. J. Infect. 1989, 19, 17–23. [Google Scholar] [CrossRef]
- Lichter, J.A.; Thompson, M.T.; Delgadillo, M.; Nishikawa, T.; Rubner, M.F.; Van Vliet, K.J. Substrata Mechanical Stiffness Can Regulate Adhesion of Viable Bacteria. Biomacromolecules 2008, 9, 1571–1578. [Google Scholar] [CrossRef]
- Lichter, J.A.; Rubner, M.F. Polyelectrolyte multilayers with intrinsic antimicrobial functionality: The importance of mobile polycations. Langmuir 2009, 25, 7686–7694. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, O.V.; Yang, F.; Tian, F.; Du, H. Thin-core fiber structures with overlays for sensing applications. Opt. Express 2017, 25, 31197. [Google Scholar] [CrossRef]
- Yang, F.; Sukhishvili, S.; Du, H.; Tian, F. Marine salinity sensing using long-period fiber gratings enabled by stimuli-responsive polyelectrolyte multilayers. Sens. Actuators B Chem. 2017, 253, 745–751. [Google Scholar] [CrossRef]
- Tian, F.; Kanka, J.; Zou, B.; Chiang, K.S.; Du, H. Long-period gratings inscribed in photonic crystal fiber by symmetric CO2 laser irradiation. Opt. Express 2013, 21, 13208. [Google Scholar] [CrossRef]
- Vengsarkar, A.M.; Lemaire, P.J.; Judkins, J.B.; Bhatia, V.; Erdogan, T.; Sipe, J.E. Long-period fiber gratings as band-rejection filters. J. Light. Technol. 1996, 14, 58–64. [Google Scholar] [CrossRef]
- Yang, F.; Hlushko, R.; Wu, D.; Sukhishvili, S.A.; Du, H.; Tian, F. Ocean Salinity Sensing Using Long-Period Fiber Gratings Functionalized with Layer-by-Layer Hydrogels. ACS Omega 2019, 4, 2134–2141. [Google Scholar] [CrossRef]
- Tian, F.; Kaňka, J.; Yang, F.; Min, J.; Hammond, P.T. Role of silica nanoparticles in monitoring and prolonging release of drug-eluting polyelectrolyte coatings using long-period fiber grating platform. Sens. Actuators B Chem. 2017, 252, 831–839. [Google Scholar] [CrossRef]






| Substrate | S. aureus Detection at 60 min | |
|---|---|---|
| RW Shift (nm) | Ratio of RW Shift between Various Platforms | |
| Bare LPFG | 0.07 ± 0.05 | 1 |
| [PAH-PAA]10-PAH LbL-coated LPFG | 0.31 ± 0.14 | 4.08 |
| Porous [PAH-PAA]10-PAH LbL-coated LPFG | 0.53 ± 0.21 | 7.09 |
| Substrate | RW Shift at 260 min (nm) | Ratio of RW Shift: S. aureus:E. coli | ||
|---|---|---|---|---|
| S. aureus | E. coli | 260 min | 60 min | |
| Bare LPFG | 0.31 ± 0.16 | 0.37 ± 0.20 | 0.83:1 | 0.31:1 |
| [PAH/PAA]10-PAH LbL-coated LPFG | 1.44 ± 0.33 | 0.21 ± 0.11 | 6.89:1 | 4.00:1 |
| Porous [PAH/PAA]10-PAH LbL-coated LPFG | 2.64 ± 0.17 | 0.74 ± 0.12 | 3.60:1 | 1.92:1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, S.; Wang, J.; Yang, F.; Chang, T.-L.; Tang, Z.; Liu, K.; Liu, S.; Tian, F.; Liang, J.-F.; Du, H.; et al. Bacterial Detection and Differentiation of Staphylococcus aureus and Escherichia coli Utilizing Long-Period Fiber Gratings Functionalized with Nanoporous Coated Structures. Coatings 2023, 13, 778. https://doi.org/10.3390/coatings13040778
He S, Wang J, Yang F, Chang T-L, Tang Z, Liu K, Liu S, Tian F, Liang J-F, Du H, et al. Bacterial Detection and Differentiation of Staphylococcus aureus and Escherichia coli Utilizing Long-Period Fiber Gratings Functionalized with Nanoporous Coated Structures. Coatings. 2023; 13(4):778. https://doi.org/10.3390/coatings13040778
Chicago/Turabian StyleHe, Shuyue, Jue Wang, Fan Yang, Tzu-Lan Chang, Ziyu Tang, Kai Liu, Shuli Liu, Fei Tian, Jun-Feng Liang, Henry Du, and et al. 2023. "Bacterial Detection and Differentiation of Staphylococcus aureus and Escherichia coli Utilizing Long-Period Fiber Gratings Functionalized with Nanoporous Coated Structures" Coatings 13, no. 4: 778. https://doi.org/10.3390/coatings13040778