Doxorubicin-Loaded Silica Nanocomposites for Cancer Treatment
Abstract
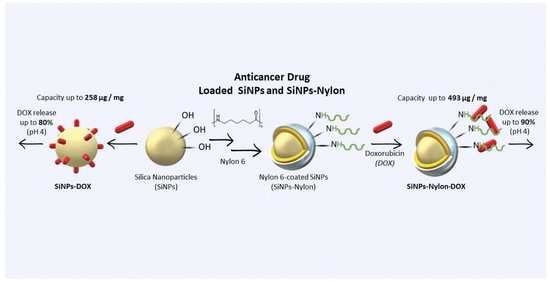
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of SiNPs, SiNPs-Nylon, SiNPs-DOX, and SiNPs-Nylon-DOX
2.3. SiNPs Synthesis
2.4. Nylon-Coated SiNPs Synthesis (SiNPs-Nylon)
2.5. SiNPs Stability
2.6. Doxorubicin-Loaded SiNPs
2.7. Doxorubicin Release from SiNPs
2.8. The Cytotoxicity Assay (MTT Test)
3. Results and Discussion
3.1. Synthesis and Characterization of SiNPs
3.2. SiNPs and Nylon 6 Nanocomposite Synthesis
3.3. Anticancer Drug Doxorubicin Loading
3.4. Doxorubicin Release
3.5. Cellular Toxicity Study of SiNPs-DOX
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Selvarajan, V.; Obuobi, S.; Ee, P.L.R. Silica Nanoparticles—A Versatile Tool for the Treatment of Bacterial Infections. Front. Chem. 2020, 8, 602. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Ghoran, S.H.; Niakan, M.H.; Jamali, K.; Moeini, Z.; Jangjou, A.; Izadpanah, P.; Amani, A.M. Mesoporous Silica Nanoparticle: Heralding a Brighter Future in Cancer Nanomedicine. Microporous Mesoporous Mater. 2021, 319, 110967. [Google Scholar] [CrossRef]
- Parra, M.; Gil, S.; Gaviña, P.; Costero, A.M. Mesoporous Silica Nanoparticles in Chemical Detection: From Small Species to Large Bio-Molecules. Sensors 2022, 22, 261. [Google Scholar] [CrossRef] [PubMed]
- Racles, C.; Zaltariov, M.; Peptanariu, D.; Vasiliu, T.; Cazacu, M. Functionalized Mesoporous Silica as Doxorubicin Carriers and Cytotoxicity Boosters. Nanomaterials 2022, 12, 1823. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Dai, X.; Wang, Z.; Chen, H.; Guo, B.; Huang, L. Recent Advances of Mesoporous Silica as a Platform for Cancer Immunotherapy. Biosensors 2022, 12, 109. [Google Scholar] [CrossRef]
- Slapak, E.J.; El Mandili, M.; Bijlsma, M.F.; Spek, C.A. Mesoporous Silica Nanoparticle-Based Drug Delivery Systems for the Treatment of Pancreatic Cancer: A Systematic Literature Overview. Pharmaceutics 2022, 14, 390. [Google Scholar] [CrossRef]
- Koohi, M.; Esfahani, M.; Alavi, S.E.; Cabot, P.J.; Islam, N.; Izake, E.L. Application of Mesoporous Silica Nanoparticles in Cancer Therapy and Delivery of Repurposed Anthelmintics for Cancer Therapy. Pharmaceutics 2022, 14, 1579. [Google Scholar] [CrossRef]
- Corma, A.; Botella, P.; Rivero-Buceta, E. Silica-Based Stimuli-Responsive Systems for Antitumor Drug Delivery and Controlled Release. Pharmaceutics 2022, 14, 110. [Google Scholar] [CrossRef]
- Trzeciak, K.; Chotera-ouda, A.; Bak-sypien, I.I.; Potrzebowski, M.J. Mesoporous Silica Particles as Drug Delivery Systems—The State of the Art in Loading Methods and the Recent Progress in Analytical Techniques for Monitoring These Processes. Pharmaceutics 2021, 13, 950. [Google Scholar] [CrossRef]
- Frickenstein, A.N.; Hagood, J.M.; Britten, C.N.; Abbott, B.S.; McNally, M.W.; Vopat, C.A.; Patterson, E.G.; Maccuaig, W.M.; Jain, A.; Walters, K.B.; et al. Mesoporous Silica Nanoparticles: Properties and Strategies for Enhancing Clinical Effect. Pharmaceutics 2021, 13, 570. [Google Scholar] [CrossRef] [PubMed]
- Isa, E.D.M.; Ahmad, H.; Rahman, M.B.A.; Gill, M.R. Progress in Mesoporous Silica Nanoparticles as Drug Delivery Agents for Cancer Treatment. Pharmaceutics 2021, 13, 152. [Google Scholar] [CrossRef]
- Abouaitah, K.; Lojkowski, W. Delivery of Natural Agents by Means of Mesoporous Silica Nanospheres as a Promising Anticancer Strategy. Pharmaceutics 2021, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Moodley, T.; Singh, M. Current Stimuli-Responsive Mesoporous Silica Nanoparticles for Cancer Therapy. Pharmaceutics 2021, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E.; Wahl, R. Nanoparticles: Promising Auxiliary Agents for Diagnosis and Therapy of Thyroid Cancers. Cancers 2021, 13, 4063. [Google Scholar] [CrossRef] [PubMed]
- Pontón, I.; del Rio, A.M.; Gómez, M.G.; Sánchez-García, D. Preparation and Applications of Organo-Silica Hybrid Mesoporous Silica Nanoparticles for the Co-Delivery of Drugs and Nucleic Acids. Nanomaterials 2020, 10, 2466. [Google Scholar] [CrossRef]
- Colilla, M.; Vallet-Regí, M. Targeted Stimuli-Responsive Mesoporous Silica Nanoparticles for Bacterial Infection Treatment. Int. J. Mol. Sci. 2020, 21, 8605. [Google Scholar] [CrossRef]
- Pal, N.; Lee, J.H.; Cho, E.B. Recent Trends in Morphology-Controlled Synthesis and Application of Mesoporous Silica Nanoparticles. Nanomaterials 2020, 10, 2122. [Google Scholar] [CrossRef]
- Baeza, A.; Vallet-Regí, M. Mesoporous Silica Nanoparticles as Theranostic Antitumoral Nanomedicines. Pharmaceutics 2020, 12, 957. [Google Scholar] [CrossRef]
- Chircov, C.; Spoială, A.; Păun, C.; Crăciun, L.; Ficai, D.; Ficai, A.; Andronescu, E.; Turculeƫ, S.C. Mesoporous Silica Platforms with Potential Applications in Release and Adsorption of Active Agents. Molecules 2020, 25, 3814. [Google Scholar] [CrossRef]
- Barui, S.; Cauda, V. Multimodal Decorations of Mesoporous Silica Nanoparticles for Improved Cancer Therapy. Pharmaceutics 2020, 12, 527. [Google Scholar] [CrossRef]
- Paris, J.L.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for Co-Delivery of Drugs and Nucleic Acids in Oncology: A Review. Pharmaceutics 2020, 12, 526. [Google Scholar] [CrossRef]
- Croissant, J.G.; Butler, K.S.; Zink, J.I.; Brinker, C.J. Synthetic Amorphous Silica Nanoparticles: Toxicity, Biomedical and Environmental Implications. Nat. Rev. Mater. 2020, 5, 886–909. [Google Scholar] [CrossRef]
- Kembuan, C.; Oliveira, H.; Graf, C. Effect of Different Silica Coatings on the Toxicity of Upconversion Nanoparticles on RAW 264.7 Macrophage Cells. Beilstein J. Nanotechnol. 2021, 12, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Popova, V.; Dmitrienko, E.; Chubarov, A. Magnetic Nanocomposites and Imprinted Polymers for Biomedical Applications of Nucleic Acids. Magnetochemistry 2023, 9, 12. [Google Scholar] [CrossRef]
- Osminkina, L.A.; Timoshenko, V.Y. Porous Silicon as a Sensitizer for Biomedical Applications. Open Mater. Sci. 2016, 3, 39–48. [Google Scholar] [CrossRef]
- Osminkina, L.A.; Nikolaev, A.L.; Sviridov, A.P.; Andronova, N.V.; Tamarov, K.P.; Gongalsky, M.B.; Kudryavtsev, A.A.; Treshalina, H.M.; Timoshenko, V.Y. Porous Silicon Nanoparticles as Efficient Sensitizers for Sonodynamic Therapy of Cancer. Microporous Mesoporous Mater. 2015, 210, 169–175. [Google Scholar] [CrossRef]
- Maximchik, P.V.; Tamarov, K.; Sheval, E.V.; Tolstik, E.; Kirchberger-Tolstik, T.; Yang, Z.; Sivakov, V.; Zhivotovsky, B.; Osminkina, L.A. Biodegradable Porous Silicon Nanocontainers as an Effective Drug Carrier for Regulation of the Tumor Cell Death Pathways. ACS Biomater. Sci. Eng. 2019, 5, 6063–6071. [Google Scholar] [CrossRef]
- Ceroni, P.; Chao, Y.; Crucho, C.; De Cola, L.; Fucikova, A.; Goyal, A.; Joo, J.; Kamali, A.R.; Osminkina, L.; Silvestrini, S.; et al. Silicon Nanostructures for Sensing and Bioimaging: General Discussion. Faraday Discuss. 2020, 222, 384–389. [Google Scholar] [CrossRef]
- Gongalsky, M.B.; Sviridov, A.P.; Bezsudnova, Y.I.; Osminkina, L.A. Biodegradation Model of Porous Silicon Nanoparticles. Colloids Surf. B Biointerfaces 2020, 190, 110946. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-Responsive Nanocarriers for Drug Delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, P.; Zhao, R.; Zhao, L.; Liu, J.; Peng, S.; Fu, X.; Wang, X.; Luo, R.; Wang, R.; et al. Silica Nanoparticles: Biomedical Applications and Toxicity. Biomed. Pharmacother. 2022, 151, 113053. [Google Scholar] [CrossRef]
- Sun, X.; Xue, Z.; Yasin, A.; He, Y.; Chai, Y.; Li, J.; Zhang, K. Colorectal Cancer and Adjacent Normal Mucosa Differ in Apoptotic and Inflammatory Protein Expression. Eng. Regen. 2021, 2, 279–287. [Google Scholar] [CrossRef]
- Sritharan, S.; Sivalingam, N. A Comprehensive Review on Time-Tested Anticancer Drug Doxorubicin. Life Sci. 2021, 278, 119527. [Google Scholar] [CrossRef]
- Christidi, E.; Brunham, L.R. Regulated Cell Death Pathways in Doxorubicin-Induced Cardiotoxicity. Cell Death Dis. 2021, 12, 339. [Google Scholar] [CrossRef]
- Curry, D.; Cameron, A.; MacDonald, B.; Nganou, C.; Scheller, H.; Marsh, J.; Beale, S.; Lu, M.; Shan, Z.; Kaliaperumal, R.; et al. Adsorption of Doxorubicin on Citrate-Capped Gold Nanoparticles: Insights into Engineering Potent Chemotherapeutic Delivery Systems. Nanoscale 2015, 7, 19611–19619. [Google Scholar] [CrossRef]
- Popova, V.; Poletaeva, Y.; Pyshnaya, I.; Pyshnyi, D.; Dmitrienko, E. Designing PH-Dependent Systems Based on Nanoscale Calcium Carbonate for the Delivery of an Antitumor Drug. Nanomaterials 2021, 11, 2794. [Google Scholar] [CrossRef]
- Yang, H.; Wang, N.; Yang, R.; Zhang, L.; Jiang, X. Folic Acid-Decorated β-Cyclodextrin-Based Poly(ε-Caprolactone)-Dextran Star Polymer with Disulfide Bond-Linker as Theranostic Nanoparticle for Tumor-Targeted Mri and Chemotherapy. Pharmaceutics 2022, 14, 52. [Google Scholar] [CrossRef]
- Caldera, F.; Nisticò, R.; Magnacca, G.; Matencio, A.; Khazaei Monfared, Y.; Trotta, F. Magnetic Composites of Dextrin-Based Carbonate Nanosponges and Iron Oxide Nanoparticles with Potential Application in Targeted Drug Delivery. Nanomaterials 2022, 12, 754. [Google Scholar] [CrossRef]
- Al-Musawi, S.; Albukhaty, S.; Al-Karagoly, H.; Almalki, F. Design and Synthesis of Multi-Functional Superparamagnetic Core-Gold Shell Coated with Chitosan and Folate Nanoparticles for Targeted Antitumor Therapy. Nanomaterials 2021, 11, 32. [Google Scholar] [CrossRef]
- Carvalho, S.M.; Leonel, A.G.; Mansur, A.A.P.; Carvalho, I.C.; Krambrock, K.; Mansur, H.S. Bifunctional Magnetopolymersomes of Iron Oxide Nanoparticles and Carboxymethylcellulose Conjugated with Doxorubicin for Hyperthermo-Chemotherapy of Brain Cancer Cells. Biomater. Sci. 2019, 7, 2102–2122. [Google Scholar] [CrossRef]
- Kovrigina, E.; Chubarov, A.; Dmitrienko, E. High Drug Capacity Doxorubicin-Loaded Iron Oxide Nanocomposites for Cancer Therapy. Magnetochemistry 2022, 8, 54. [Google Scholar] [CrossRef]
- Awan, U.A.; Raza, A.; Ali, S.; Saeed, R.F.; Akhtar, N. Doxorubicin-Loaded Gold Nanorods: A Multifunctional Chemo-Photothermal Nanoplatform for Cancer Management. Beilstein J. Nanotechnol. 2021, 12, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, S.; Shahzad, K.; Saeed, T.; Ul-Hamid, A.; Abbasi, B.H.; Ahmad, N.; Khalid, W.; Atif, M.; Ali, Z.; Abbasi, R. Biocompatibility and Cytotoxicity in Vitro of Surface-Functionalized Drug-Loaded Spinel Ferrite Nanoparticles. Beilstein J. Nanotechnol. 2021, 12, 1339–1364. [Google Scholar] [CrossRef] [PubMed]
- Khatami, F.; Matin, M.M.; Danesh, N.M.; Bahrami, A.R.; Abnous, K.; Taghdisi, S.M. Targeted Delivery System Using Silica Nanoparticles Coated with Chitosan and AS1411 for Combination Therapy of Doxorubicin and AntimiR-21. Carbohydr. Polym. 2021, 266, 118111. [Google Scholar] [CrossRef]
- Al-Nadaf, A.H.; Dahabiyeh, L.A.; Jawarneh, S.; Bardaweel, S.; Mahmoud, N.N. Folic Acid-Hydrophilic Polymer Coated Mesoporous Silica Nanoparticles Target Doxorubicin Delivery. Pharm. Dev. Technol. 2021, 26, 582–591. [Google Scholar] [CrossRef]
- Zhuang, J.; Chen, S.; Hu, Y.; Yang, F.; Huo, Q.; Xie, N. Tumour-Targeted and Redox-Responsive Mesoporous Silica Nanoparticles for Controlled Release of Doxorubicin and an SiRNA against Metastatic Breast Cancer. Int. J. Nanomed. 2021, 16, 1961–1976. [Google Scholar] [CrossRef]
- Xu, P.; Yao, J.; Li, Z.; Wang, M.; Zhou, L.; Zhong, G.; Zheng, Y.; Li, N.; Zhai, Z.; Yang, S.; et al. Therapeutic Effect of Doxorubicin-Chlorin E6-Loaded Mesoporous Silica Nanoparticles Combined with Ultrasound on Triple-Negative Breast Cancer. Int. J. Nanomed. 2020, 15, 2659–2668. [Google Scholar] [CrossRef]
- Luo, M.; Lewik, G.; Ratcliffe, J.C.; Choi, C.H.J.; Mäkilä, E.; Tong, W.Y.; Voelcker, N.H. Systematic Evaluation of Transferrin-Modified Porous Silicon Nanoparticles for Targeted Delivery of Doxorubicin to Glioblastoma. ACS Appl. Mater. Interfaces 2019, 11, 33637–33649. [Google Scholar] [CrossRef]
- Jiang, S.; Hua, L.; Guo, Z.; Sun, L. One-Pot Green Synthesis of Doxorubicin Loaded-Silica Nanoparticles for in Vivo Cancer Therapy. Mater. Sci. Eng. C 2018, 90, 257–263. [Google Scholar] [CrossRef]
- Hakeem, A.; Zahid, F.; Zhan, G.; Yi, P.; Yang, H.; Gan, L.; Yang, X. Polyaspartic Acid-Anchored Mesoporous Silica Nanoparticles for PH-Responsive Doxorubicin Release. Int. J. Nanomed. 2018, 13, 1029–1040. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Song, X.; Zhou, J.; Ouyang, X.; Li, J.; Deng, D. Virus-like Hollow Mesoporous Silica Nanoparticles for Cancer Combination Therapy. Colloids Surfaces B Biointerfaces 2021, 197, 111452. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Zhang, Y.; Li, Y.; Gao, Z.; Chen, F.; Sun, K.; An, P.; Sun, C.; Jiang, Y.; Sun, B. A Novel PH-Responsive Hollow Mesoporous Silica Nanoparticle (HMSN) System Encapsulating Doxorubicin (DOX) and Glucose Oxidase (GOX) for Potential Cancer Treatment. J. Mater. Chem. B 2019, 7, 3291–3302. [Google Scholar] [CrossRef]
- Miao, Y.; Feng, Y.; Bai, J.; Liu, Z.; Zhao, X. Optimized Mesoporous Silica Nanoparticle-Based Drug Delivery System with Removable Manganese Oxide Gatekeeper for Controlled Delivery of Doxorubicin. J. Colloid Interface Sci. 2021, 592, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Shan, X.; Han, Y.; Jin, H.; Gao, Y. Study of PH-Responsive and Polyethylene Glycol-Modified Doxorubicin-Loaded Mesoporous Silica Nanoparticles for Drug Delivery. J. Nanosci. Nanotechnol. 2020, 20, 5997–6006. [Google Scholar] [CrossRef]
- Moodley, T.; Singh, M. Sterically Stabilised Polymeric Mesoporous Silica Nanoparticles Improve Doxorubicin Efficiency: Tailored Cancer Therapy. Molecules 2020, 25, 742. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Z.; Ping, Y.; Miao, Y.; Xiao, Y.; Qu, L.; Zhang, L.; Hu, Y.; Wang, J. PEG/PEI-Functionalized Single-Walled Carbon Nanotubes as Delivery Carriers for Doxorubicin: Synthesis, Characterization, and in Vitro Evaluation. Beilstein J. Nanotechnol. 2020, 11, 1728–1741. [Google Scholar] [CrossRef]
- Hu, X.; Hao, X.; Wu, Y.; Zhang, J.; Zhang, X.; Wang, P.C.; Zou, G.; Liang, X.-J. Multifunctional hybrid silica nanoparticles for controlled doxorubicin loading and release with thermal and pH dual response. J. Mater. Chem. B 2013, 1, 1109–1118. [Google Scholar] [CrossRef]
- Bulgakova, A.; Chubarov, A.; Dmitrienko, E. Magnetic Nylon 6 Nanocomposites for the Microextraction of Nucleic Acids from Biological Samples. Magnetochemistry 2022, 8, 85. [Google Scholar] [CrossRef]
- Zhang, C.; Tjiu, W.W.; Liu, T.; Lui, W.Y.; Phang, I.Y.; Zhang, W.D. Dramatically Enhanced Mechanical Performance of Nylon-6 Magnetic Composites with Nanostructured Hybrid One-Dimensional Carbon Nanotube-Two-Dimensional Clay Nanoplatelet Heterostructures. J. Phys. Chem. B 2011, 115, 3392–3399. [Google Scholar] [CrossRef]
- Mohammadi, S.Z.; Safari, Z.; Madady, N. Synthesis of Co3O4@SiO2 Core/Shell–Nylon 6 Magnetic Nanocomposite as an Adsorbent for Removal of Congo Red from Wastewater. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3199–3212. [Google Scholar] [CrossRef]
- Choi, E.Y.; Kim, K.; Kim, C.K.; Kang, E. Reinforcement of Nylon 6,6/Nylon 6,6 Grafted Nanodiamond Composites by in Situ Reactive Extrusion. Sci. Rep. 2016, 6, 37010. [Google Scholar] [CrossRef] [PubMed]
- Ghambari, H.; Reyes-Gallardo, E.M.; Lucena, R.; Saraji, M.; Cárdenas, S. Magnetic Polyamide Nanocomposites for the Microextraction of Benzophenones from Water Samples. Molecules 2019, 24, 953. [Google Scholar] [CrossRef] [PubMed]
- Saeed, K.; Khan, I.; Ahad, M.; Shah, T.; Sadiq, M.; Zada, A.; Zada, N. Preparation of ZnO/Nylon 6/6 Nanocomposites, Their Characterization and Application in Dye Decolorization. Appl. Water Sci. 2021, 11, 105. [Google Scholar] [CrossRef]
- Dmitrienko, E.V.; Bulushev, R.D.; Haupt, K.; Kosolobov, S.S.; Latyshev, A.V.; Pyshnaya, I.A.; Pyshnyi, D.V. A Simple Approach to Prepare Molecularly Imprinted Polymers from Nylon-6. J. Mol. Recognit. 2013, 26, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Shakiba, M.; Rezvani Ghomi, E.; Khosravi, F.; Jouybar, S.; Bigham, A.; Zare, M.; Abdouss, M.; Moaref, R.; Ramakrishna, S. Nylon—A Material Introduction and Overview for Biomedical Applications. Polym. Adv. Technol. 2021, 32, 3368–3383. [Google Scholar] [CrossRef]
- Reyes-Gallardo, E.M.; Lucena, R.; Cárdenas, S. Silica Nanoparticles-Nylon 6 Composites: Synthesis, Characterization and Potential Use as Sorbent. RSC Adv. 2017, 7, 2308–2314. [Google Scholar] [CrossRef]
- Mahfuz, H.; Hasan, M.; Dhanak, V.; Beamson, G.; Stewart, J.; Rangari, V.; Wei, X.; Khabashesku, V.; Jeelani, S. Reinforcement of Nylon 6 with Functionalized Silica Nanoparticles for Enhanced Tensile Strength and Modulus. Nanotechnology 2008, 19, 445702. [Google Scholar] [CrossRef]
- Rao, K.S.; El-hami, K.; Kodaki, T.; Matsushige, K.; Makino, K. A Novel Method for Synthesis of Silica Nanoparticles. J. Colloid Interface 2005, 289, 125–131. [Google Scholar] [CrossRef]
- Masalov, V.M.; Sukhinina, N.S.; Emel’chenko, G.A. Synthesis of Monodisperse Silica Nanoparticles via Heterogeneous Tetraethoxysilane Hydrolysis Using L-Arginine as a Catalyst. Inorg. Mater. 2018, 54, 156–162. [Google Scholar] [CrossRef]
- Castro, Y.; Vazquez, N.I.; Gonzalez, Z. Synthesis of Mesoporous Silica Nanoparticles by Sol–Gel as Nanocontainer for Future Drug Delivery Applications. Bol. Soc. Esp. Ceram. 2017, 6, 139–145. [Google Scholar] [CrossRef]
- Can, K.; Ozmen, M.; Ersoz, M. Immobilization of Albumin on Aminosilane Modified Superparamagnetic Magnetite Nanoparticles and Its Characterization. Colloids Surf. B Biointerfaces 2009, 71, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, Y.; Wang, J.; Yang, Y.; Li, Y.; Yuan, Y.; Liu, C. Charge-Reversal APTES-Modified Mesoporous Silica Nanoparticles with High Drug Loading and Release Controllability. Appl. Mater. Interfaces 2016, 8, 17166–17175. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Acosta, J.R.; Silva, J.A.; Fernández-Izquierdo, L.; Díaz-Castañón, S.; Ortiz, M.; Zuaznabar-Gardona, J.C.; Díaz-García, A.M. Iron Oxide Nanoparticles (IONPs) with Potential Applications in Plasmid DNA Isolation. Colloids Surf. A Physicochem. Eng. Asp. 2018, 545, 167–178. [Google Scholar] [CrossRef]
- Ismail, A.F.; Goh, P.; Rezaei, M.; Arzhandi, D.; Ismail, N. Aptes and Teos Modified Binary Recyclable Hybrid Fe3O4@GO Nanocomposite for Photocatalytic Dye Removal. J. Teknol. 2018, 80, 157–164. [Google Scholar] [CrossRef]
- Hao, N.; Li, L.; Zhang, Q.; Huang, X.; Meng, X.; Zhang, Y.; Chen, D.; Tang, F.; Li, L. The Shape Effect of PEGylated Mesoporous Silica Nanoparticles on Cellular Uptake Pathway in Hela Cells. Microporous Mesoporous Mater. 2012, 162, 14–23. [Google Scholar] [CrossRef]
- Ferjaoui, Z.; Jamal Al Dine, E.; Kulmukhamedova, A.; Bezdetnaya, L.; Soon Chang, C.; Schneider, R.; Mutelet, F.; Mertz, D.; Begin-Colin, S.; Quilès, F.; et al. Doxorubicin-Loaded Thermoresponsive Superparamagnetic Nanocarriers for Controlled Drug Delivery and Magnetic Hyperthermia Applications. ACS Appl. Mater. Interfaces 2019, 11, 30610–30620. [Google Scholar] [CrossRef]
- Heggannavar, G.B.; Hiremath, C.G.; Achari, D.D.; Pangarkar, V.G.; Kariduraganavar, M.Y. Development of Doxorubicin-Loaded Magnetic Silica-Pluronic F-127 Nanocarriers Conjugated with Transferrin for Treating Glioblastoma across the Blood-Brain Barrier Using an in Vitro Model. ACS Omega 2018, 3, 8017–8026. [Google Scholar] [CrossRef]
- Hervault, A.; Dunn, A.E.; Lim, M.; Boyer, C.; Mott, D.; Maenosono, S.; Thanh, N.T.K. Doxorubicin Loaded Dual PH- and Thermo-Responsive Magnetic Nanocarrier for Combined Magnetic Hyperthermia and Targeted Controlled Drug Delivery Applications. Nanoscale 2016, 8, 12152–12161. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.; Nguyen, T.T.; Nghiem, T.H.L.; Nguyen, D.T.; Tran, T.T.H.; Vu, D.; Nguyen, T.B.N.; Nguyen, T.M.H.; Nguyen, V.T.; Nguyen, M.H. Optical Properties of Doxorubicin Hydrochloride Load and Release on Silica Nanoparticle Platform. Molecules 2021, 26, 3968. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]







| Method | Synthesis Variations | Hydrodynamic Diameter by DLS, nm | PDI | TEM Image, Figure 1 |
|---|---|---|---|---|
| Method 1 adapted from Vazquez et al. [70] | CTAB | 447 ± 9 | 0.15 ± 0.01 | A |
| HTAB | 376 ± 28 | 0.18 ± 0.01 | B | |
| Method 2 adapted from Masalov et al. [69] | organic fraction | 558 ± 3 | 0.19 ± 0.02 | - |
| intermediate fraction | 107 ± 10 | 0.32 ± 0.01 | C | |
| aquatic fraction | 118 ± 8 | 0.28 ± 0.01 | - | |
| Method 3 adapted from Rao et al. [68] | 0.010 M TEOS | 11.5 ± 0.3 | 0.25 ± 0.01 | - |
| 0.018 M TEOS | 63 ± 1 | 0.09 ± 0.01 | E, F | |
| 0.040 M TEOS | 65 ± 6 | 0.13 ± 0.01 | D | |
| 0.200 M TEOS | 166 ± 3 | 0.10 ± 0.02 | G, H |
| Sinps Type | Hydrodynamic Diameter, nm | PDI | ζ-Potential, mV | TEM Image, Figure |
|---|---|---|---|---|
| SiNPs | 63 ± 1 | 0.09 ± 0.01 | −28.6 ± 0.2 | 1 E, F |
| SiNPs-Nylon | 110 ± 2 | 0.16 ± 0.01 | 30.0 ± 0.4 | 3 |
| Initial Amount, mg | Initial DOX Amount, µg | DOX/SiNPs or SiNPs-Nylon, µg/mg | DOX Loading Efficiency, % | |
|---|---|---|---|---|
| SiNPs | SiNPs-Nylon | |||
| 1 | - | 300 | 145 ± 8 | 49 * |
| 1 | - | 200 | 127 ± 4 | 64 |
| 1 | - | 100 | 79 ± 7 | 79 |
| 0.8 | - | 100 | 83 ± 4 | 66 |
| 0.6 | - | 100 | 105 ± 3 | 63 |
| 0.4 | - | 100 | 122 ± 5 | 49 |
| 0.2 | - | 100 | 258 ± 6 | 52 |
| - | 1 | 200 | 160 ± 15 | 80 |
| - | 1 | 100 | 87 ± 10 | 87 |
| - | 0.8 | 100 | 112 ± 20 | 90 |
| - | 0.2 | 100 | 493 ± 38 | 98 |
| Sample | IC 50, μM | |
|---|---|---|
| μM (DOX Concentration) | µg/mL (NPs Concentration) | |
| SiNPs-DOX | 0.98 ± 0.09 | 3.8 ± 0.3 |
| SiNPs-Nylon-DOX | 1.12 ± 0.08 | 2.3 ± 0.2 |
| DOX | 1.47 ± 0.09 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popova, V.; Poletaeva, Y.; Chubarov, A.; Pyshnyi, D.; Dmitrienko, E. Doxorubicin-Loaded Silica Nanocomposites for Cancer Treatment. Coatings 2023, 13, 324. https://doi.org/10.3390/coatings13020324
Popova V, Poletaeva Y, Chubarov A, Pyshnyi D, Dmitrienko E. Doxorubicin-Loaded Silica Nanocomposites for Cancer Treatment. Coatings. 2023; 13(2):324. https://doi.org/10.3390/coatings13020324
Chicago/Turabian StylePopova, Victoriya, Yuliya Poletaeva, Alexey Chubarov, Dmitrii Pyshnyi, and Elena Dmitrienko. 2023. "Doxorubicin-Loaded Silica Nanocomposites for Cancer Treatment" Coatings 13, no. 2: 324. https://doi.org/10.3390/coatings13020324