Corrosion Protection of 6061 Aluminum Alloys by Sol-Gel Coating Modified with ZnLaAl-LDHs
Abstract
:1. Introduction
2. Experimental
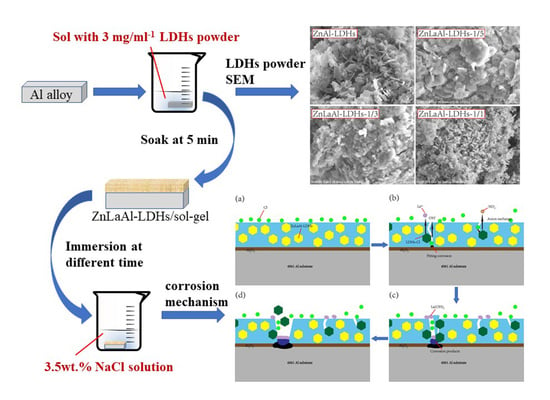
2.1. Materials and Preparation
2.2. Characterization
3. Results and Discussion
3.1. Preliminary Titrations
3.2. Characterization of LDHs Powders
3.3. Corrosion Behavior
3.4. Corrosion Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dursun, T.; Soutis, C. Recent developments in advanced aircraft aluminium alloys. Mater. Des. 2014, 56, 862–871. [Google Scholar] [CrossRef]
- Heinz, A.; Haszler, A.; Keidel, C.; Moldenhauer, S.; Benedictus, R.; Miller, W.S. Recent development in aluminium alloys for aerospace applications. Mater. Sci. Eng. A 2000, 280, 102–107. [Google Scholar] [CrossRef]
- Melchers, R.E. Time dependent development of aluminium pitting corrosion. Adv. Mater. Sci. Eng. 2015, 2015, 215712. [Google Scholar] [CrossRef] [Green Version]
- Guillaumin, V.; Mankowski, G. Localized corrosion of 2024 T351 aluminium alloy in chloride media. Corros. Sci. 1999, 41, 421–438. [Google Scholar] [CrossRef]
- Srinivasan, P.B.; Heitmann, V.; Dietzel, W. Stress corrosion cracking behaviour of chromated AA2024 T351 alloy in chloride solution. Corros. Eng. Sci. Technol. 2013, 39, 174–176. [Google Scholar] [CrossRef]
- Hakimizad, A.; Raeissi, K.; Ashrafizadeh, F. A comparative study of corrosion performance of sealed anodized layers of conventionally colored and interference-colored aluminium. Surf. Coat. Technol. 2012, 206, 4628–4633. [Google Scholar] [CrossRef]
- Knudsen, O.Ø.; Tanem, B.S.; Bjørgum, A.; Mårdalen, J.; Hallenstvet, M. Anodising as pre-treatment before organic coating of extruded and cast aluminium alloys. Corros. Sci. 2004, 46, 2081–2095. [Google Scholar] [CrossRef]
- Cui, X.; Zhu, G.; Pan, Y.; Shao, Q.; Zhao, C.; Dong, M.; Zhang, Y.; Guo, Z. Polydimethylsiloxane-titania nanocomposite coating: Fabrication and corrosion resistance. Polymer 2018, 138, 203–210. [Google Scholar] [CrossRef]
- Kumar, P.; Dehiya, B.S.; Sindhu, A.; Kumar, R.; Pruncu, C.I.; Yadav, A. Fabrication and characterization of silver nanorods incorporated calcium silicate scaffold using polymeric sponge replica technique. Mater. Des. 2020, 195, 109026. [Google Scholar] [CrossRef]
- Franquet, A.; le Pen, C.; Terryn, H.; Vereecken, J. Effect of bath concentration and curing time on the structure of non-functional thin organosilane layers on aluminium. Electrochim. Acta 2003, 48, 1245–1255. [Google Scholar] [CrossRef]
- Deflorian, F.; Rossi, S.; Fedrizzi, L. Silane pre-treatments on copper and aluminium. Electrochim. Acta 2006, 51, 6097–6103. [Google Scholar] [CrossRef]
- Subramanian, V.; van Ooij, W.J. Silane based metal pretreatments as alternatives to chromating: Shortlisted. Surf. Eng. 2013, 15, 168–172. [Google Scholar] [CrossRef]
- Campestrini, P.; Terryn, H.; Hovestad, A.; de Wit, J.H.W. Formation of a cerium-based conversion coating on AA2024: Relationship with the microstructure. Surf. Coat. Technol. 2004, 176, 365–381. [Google Scholar] [CrossRef]
- Paussa, L.; Rosero-Navarro, N.C.; Andreatta, F.; Castro, Y.; Duran, A.; Aparicio, M.; Fedrizzi, L. Inhibition effect of cerium in hybrid sol-gel films on aluminium alloy AA2024. Surf. Interface Anal. 2010, 42, 299–305. [Google Scholar] [CrossRef] [Green Version]
- Mills, S.J.; Christy, A.G.; Genin, J.M.R.; Kameda, T.; Colombo, F. Nomenclature of the hydrotalcite supergroup: Natural layered double hydroxides. Mineral. Mag. 2012, 76, 1289–1336. [Google Scholar] [CrossRef] [Green Version]
- Gu, Z.; Atherton, J.J.; Xu, Z.P. Hierarchical layered double hydroxide nanocomposites: Structure, synthesis and applications. Chem. Commun. 2015, 51, 3024–3036. [Google Scholar] [CrossRef] [Green Version]
- Bouali, A.C.; Serdechnova, M.; Blawert, C.; Tedim, J.; Ferreira, M.G.S.; Zheludkevich, M.L. Layered double hydroxides (LDHs) as functional materials for the corrosion protection of aluminum alloys: A review. Appl. Mater. Today 2020, 21, 100857. [Google Scholar] [CrossRef]
- Zhou, M.; Pang, X.L.; Wei, L.; Gao, K.W. Insitu grown superhydrophobic Zn-Al layered double hydroxides films on magnesium alloy to improve corrosion properties. Appl. Surf. Sci. 2015, 337, 172–177. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Zhang, Y.; Yu, M.; Liu, J. Enhanced protective Zn–Al layered double hydroxide film fabricated on anodized 2198 aluminum alloy. J. Alloys Compd. 2015, 630, 29–36. [Google Scholar] [CrossRef]
- Smalenskaite, A.; Vieira, D.E.L.; Salak, A.N.; Ferreira, M.G.S.; Katelnikovas, A.; Kareiva, A. A comparative study of co-precipitation and sol-gel synthetic approaches to fabricate cerium-substituted Mg-Al layered double hydroxides with luminescence properties. Appl. Clay Sci. 2017, 143, 175–183. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Liu, Z.-G.; Zeng, R.-C.; Li, S.-Q.; Cui, H.-Z.; Song, L.; Han, E.-H. Corrosion resistance of Mg-Al-LDH coating on magnesium alloy AZ31. Surf. Coat. Technol. 2014, 258, 1152–1158. [Google Scholar] [CrossRef]
- Gualandi, I.; Monti, M.; Scavetta, E.; Tonelli, D.; Prevot, V.; Mousty, C. Electrodeposition of layered double hydroxides on platinum: Insights into the reactions sequence. Electrochim. Acta 2015, 152, 75–83. [Google Scholar] [CrossRef]
- Syu, J.-H.; Uan, J.-Y.; Lin, M.-C.; Lin, Z.-Y. Optically transparent Li-Al-CO3 layered double hydroxide thin films on an AZ31 Mg alloy formed by electrochemical deposition and their corrosion resistance in a dilute chloride environment. Corros. Sci. 2013, 68, 238–248. [Google Scholar] [CrossRef]
- Vega, J.M.; Granizo, N.; de la Fuente, D.; Simancas, J.; Morcillo, M. Corrosion inhibition of aluminum by coatings formulated with Al–Zn–vanadate hydrotalcite. Prog. Org. Coat. 2011, 70, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, J.; Li, Y.; Yu, M.; Yin, X.; Li, S. Enhancement of active anticorrosion via Ce-doped Zn-Al layered double hydroxides embedded in sol-gel coatings on aluminum alloy. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2017, 32, 1199–1204. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, P.; Wu, J.; Chen, F.; Li, Y.; Zhang, Y.; Zuo, Y.; Qi, Y. Enhancement of anticorrosion protection via inhibitor-loaded ZnAlCe-LDH nanocontainers embedded in sol-gel coatings. J. Coat. Technol. Res. 2018, 15, 303–313. [Google Scholar] [CrossRef]
- Wu, L.; Ding, X.; Zheng, Z.; Tang, A.; Zhang, G.; Atrens, A.; Pan, F. Doublely-doped Mg-Al-Ce-V2O74−LDH composite film on magnesium alloy AZ31 for anticorrosion. J. Mater. Sci. Technol. 2021, 64, 66–72. [Google Scholar] [CrossRef]
- Zhou, B.; Wei, X.; Wang, Y.; Huang, Q.; Hong, B.; Wei, Y. Effect of lanthanum addition on microstructures and corrosion behavior of ZnAl-LDHs film of 6061 aluminum alloys. Surf. Coat. Technol. 2019, 379, 125056. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Yu, M.; Li, S.; Xue, B.; Yin, X. Influence of embedded ZnAlCe-NO3− layered double hydroxides on the anticorrosion properties of sol-gel coatings for aluminum alloy. Prog. Org. Coat. 2015, 81, 93–100. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Kuznetsova, A.; Kallip, S.; Starykevich, M.; Tedim, J.; Ferreira, M.G.S.; Zheludkevich, M.L. A novel bilayer system comprising LDH conversion layer and sol-gel coating for active corrosion protection of AA2024. Corros. Sci. 2018, 143, 299–313. [Google Scholar] [CrossRef]
- Tedim, J.; Kuznetsova, A.; Salak, A.N.; Montemor, F.; Snihirova, D.; Pilz, M.; Zheludkevich, M.L.; Ferreira, M.G.S. Zn-Al layered double hydroxides as chloride nanotraps in active protective coatings. Corros. Sci. 2012, 55, 1–4. [Google Scholar] [CrossRef]
- Velu, S.; Ramkumar, V.; Narayanan, A.; Swamy, C.S. Effect of interlayer anions on the physicochemical properties of zinc-aluminium hydrotalcite-like compounds. J. Mater. Sci. 1997, 32, 957–964. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Li, Y.; Yu, M.; Li, S.; Xue, B. Fabrication of inhibitor anion-intercalated layered double hydroxide host films on aluminum alloy 2024 and their anticorrosion properties. J. Coat. Technol. Res. 2015, 12, 293–302. [Google Scholar] [CrossRef]
- Duchoslav, J.; Steinberger, R.; Arndt, M.; Keppert, T.; Luckeneder, G.; Stellnberger, K.H.; Hagler, J.; Angeli, G.; Riener, C.K.; Stifter, D. Evolution of the surface chemistry of hot dip galvanized Zn-Mg-Al and Zn coatings on steel during short term exposure to sodium chloride containing environments. Corros. Sci. 2015, 91, 311–320. [Google Scholar] [CrossRef]
- Gao, Y.F.; Nagai, A.; Masuda, Y.; Sato, F.; Seo, W.S.; Koumoto, K. Surface precipitation of highly porous hydrotalcite-like film on Al from a zinc aqueous solution. Langmuir 2006, 22, 3521–3527. [Google Scholar] [CrossRef]
- Salak, A.N.; Tedim, J.; Kuznetsova, A.I.; Vieira, L.G.; Ribeiro, J.L.; Zheludkevich, M.L.; Ferreira, M.G.S. Thermal behavior of layered double hydroxide Zn-Al-pyrovanadate: Composition, structure transformations, and recovering Ability. J. Phys. Chem. C 2013, 117, 4152–4157. [Google Scholar] [CrossRef]
- Salak, A.N.; Lisenkov, A.D.; Zheludkevich, M.L.; Ferreira, M.G.S. Carbonate-free Zn-Al (1:1) layered double hydroxide film directly grown on zinc-aluminum alloy coating. ECS Electrochem. Lett. 2014, 3, C9–C11. [Google Scholar] [CrossRef]
- Serdechnova, M.; Salak, A.N.; Barbosa, F.S.; Vieira, D.E.L.; Tedim, J.; Zheludkevich, M.L.; Ferreira, M.G.S. Interlayer intercalation and arrangement of 2-mercaptobenzothiazolate and 1,2,3-benzotriazolate anions in layered double hydroxides: In situ X-ray diffraction study. J. Solid State Chem. 2016, 233, 158–165. [Google Scholar] [CrossRef] [Green Version]
- Ramezanzadeh, B.; Moghadam, M.H.M.; Shohani, N.; Mahdavian, M. Effects of highly crystalline and conductive polyaniline/graphene oxide composites on the corrosion protection performance of a zinc-rich epoxy coating. Chem. Eng. J. 2017, 320, 363–375. [Google Scholar] [CrossRef]
- Alibakhshi, E.; Ghasemi, E.; Mahdavian, M.; Ramezanzadeh, B. A comparative study on corrosion inhibitive effect of nitrate and phosphate intercalated Zn-Al- layered double hydroxides (LDHs) nanocontainers incorporated into a hybrid silane layer and their effect on cathodic delamination of epoxy topcoat. Corros. Sci. 2017, 115, 159–174. [Google Scholar] [CrossRef]











| Specimen | Time h | CPEox Ω−1·cm−2·Sn1 | RLDHs Ω·cm2 | CPEox Ω−1·cm−2·Sn2 | Rox Ω·cm2 | CPEdl Ω−1·cm−2·Sn3 | Rct Ω·cm2 | Χ2 10−3 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Y1 | n1 | Y2 | n2 | Y3 | n3 | ||||||
| 6061-Blank | 0 | 2.50 × 10−8 | 0.92 | 198.60 | 9.92 × 10−7 | 0.86 | 5.78 × 102 | 3.18 × 10−6 | 0.91 | 1.63 × 105 | 2.54 |
| 24 | 8.24 × 10−7 | 0.93 | 8.76 | 5.57 × 10−6 | 0.92 | 3.18 × 104 | 5.97 × 10−4 | 0.74 | 3.51 × 104 | 2.22 | |
| 72 | 6.09 × 10−7 | 0.86 | 16.14 | 8.23 × 10−6 | 0.92 | 2.02 × 104 | 2.68 × 10−4 | 0.62 | 2.96 × 104 | 1.25 | |
| 168 | 3.85 × 10−7 | 0.91 | 12.13 | 1.34 × 10−5 | 0.90 | 1.26 × 104 | 8.98 × 10−4 | 0.80 | 2.39 × 104 | 0.51 | |
| ZnAl- LDHs | 0 | 3.18 × 10−7 | 0.82 | 174.60 | 4.31 × 10−6 | 0.91 | 2.11 × 105 | 1.23 × 10−5 | 0.92 | 1.11 × 105 | 1.29 |
| 24 | 4.94 × 10−7 | 0.89 | 18.12 | 5.49 × 10−6 | 0.94 | 7.79 × 104 | 2.89 × 10−4 | 0.85 | 9.52 × 104 | 1.49 | |
| 72 | 4.68 × 10−7 | 0.92 | 13.51 | 6.18 × 10−6 | 0.93 | 4.39 × 104 | 1.36 × 10−3 | 0.75 | 2.95 × 104 | 1.69 | |
| 168 | 6.07 × 10−7 | 0.91 | 10.23 | 7.84 × 10−6 | 0.90 | 1.74 × 104 | 3.74 × 10−3 | 0.87 | 4.54 × 103 | 1.72 | |
| ZnLaAl- LDHs-1/5 | 0 | 3.38 × 10−8 | 0.97 | 123.20 | 4.38 × 10−6 | 0.93 | 7.05 × 105 | 8.66 × 10−5 | 0.75 | 1.04 × 106 | 0.60 |
| 24 | 3.93 × 10−7 | 0.93 | 10.68 | 4.95 × 10−6 | 0.94 | 2.87 × 105 | 4.36 × 10−5 | 0.75 | 5.24 × 105 | 0.78 | |
| 72 | 4.97 × 10−7 | 0.90 | 15.37 | 5.34 × 10−6 | 0.93 | 1.57 × 105 | 6.73 × 10−5 | 0.78 | 3.35 × 105 | 1.24 | |
| 168 | 6.61 × 10−7 | 0.93 | 12.57 | 5.86 × 10−6 | 0.93 | 1.05 × 105 | 9.24 × 10−5 | 0.99 | 2.27 × 105 | 1.30 | |
| ZnLaAl- LDHs-1/3 | 0 | 3.24 × 10−8 | 1.00 | 48.27 | 5.81 × 10−6 | 0.93 | 4.93 × 105 | 1.25 × 10−4 | 0.78 | 1.34 × 105 | 0.19 |
| 24 | 4.52 × 10−7 | 0.87 | 19.07 | 6.57 × 10−6 | 0.83 | 8.29 × 104 | 1.50 × 10−4 | 0.82 | 1.82 × 105 | 1.77 | |
| 72 | 9.69 × 10−7 | 0.92 | 3.93 | 8.14 × 10−6 | 0.92 | 2.88 × 104 | 9.99 × 10−5 | 0.80 | 8.97 × 104 | 1.75 | |
| 168 | 1.31 × 10−5 | 0.91 | 4.59 | 1.03 × 10−5 | 0.92 | 1.72 × 104 | 1.57 × 10−4 | 0.72 | 6.70 × 104 | 1.76 | |
| ZnLaAl- LDHs-1/1 | 0 | 2.09 × 10−8 | 0.98 | 102.90 | 2.92 × 10−6 | 0.90 | 3.87 × 104 | 2.74 × 10−6 | 0.93 | 5.07 × 104 | 0.28 |
| 24 | 5.44 × 10−7 | 0.88 | 12.72 | 6.04 × 10−6 | 0.92 | 5.57 × 104 | 1.53 × 10−5 | 0.77 | 1.02 × 105 | 1.28 | |
| 72 | 5.11 × 10−7 | 0.91 | 10.87 | 6.80 × 10−6 | 0.92 | 2.46 × 104 | 1.51 × 10−4 | 0.76 | 6.64 × 104 | 1.22 | |
| 168 | 8.40 × 10−7 | 0.91 | 8.49 | 9.13 × 10−6 | 0.91 | 1.49 × 104 | 4.27 × 10−4 | 0.97 | 3.15 × 104 | 1.39 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Huang, Q.; Zhou, B.; Yuan, Z.; Wei, Y.; Fujita, T. Corrosion Protection of 6061 Aluminum Alloys by Sol-Gel Coating Modified with ZnLaAl-LDHs. Coatings 2021, 11, 478. https://doi.org/10.3390/coatings11040478
Wang Y, Huang Q, Zhou B, Yuan Z, Wei Y, Fujita T. Corrosion Protection of 6061 Aluminum Alloys by Sol-Gel Coating Modified with ZnLaAl-LDHs. Coatings. 2021; 11(4):478. https://doi.org/10.3390/coatings11040478
Chicago/Turabian StyleWang, Youbin, Qiuyu Huang, Bingtao Zhou, Zengyin Yuan, Yuezhou Wei, and Toyohisa Fujita. 2021. "Corrosion Protection of 6061 Aluminum Alloys by Sol-Gel Coating Modified with ZnLaAl-LDHs" Coatings 11, no. 4: 478. https://doi.org/10.3390/coatings11040478