Structural and Tribological Properties of Heat-Treated Stainless Steels against Abrasive and Lubricant Wear Conditions
Abstract
:1. Introduction
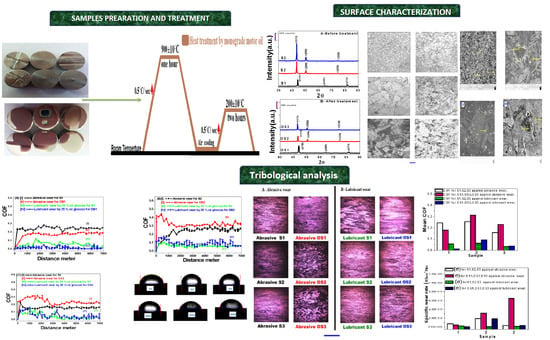
2. Materials and Methods
Surface Characterisation, Mechanical and Tribological Analysis
3. Results
3.1. XRD and Structural Analysis
3.2. Mechanical and Tribological Characterisation
4. Discussion
4.1. XRD and Structural Analysis
4.2. Mechanical and Tribological Characterization
4.2.1. Surface Microhardness
4.2.2. Surface Roughness and Wettability
4.2.3. Tribological Wear Properties against Abrasive and Lubricant Wear Conditions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| S1: | The first martensitic stainless steel grade. |
| S2: | The second austenite stainless steel grade. |
| S3: | The third austenite stainless steel grade. |
| OS1: | The first heat-treated martensitic stainless steel grade. |
| OS2: | The second heat-treated austenite stainless steel grade. |
| OS3: | The third heat-treated austenite stainless steel grade. |
| : | Specific wear rate. |
| COF: | Coefficient of friction. |
| COFS: | The COF behaviour for the untreated sample against abrasive wear conditions. |
| COFOS: | The COF behaviour for the heat-treated sample against abrasive wear conditions. |
| COFLS: | The COF behaviour for the untreated sample against lubricant wear conditions. |
| COFLOS: | The COF behaviour for the heat-treated sample against lubricant wear conditions. |
| ƠS: | The specific wear rate for the untreated sample against abrasive wear conditions. |
| ơOS: | The specific wear rate for the heat-treated sample against abrasive wear conditions. |
| ơLS: | The specific wear rate for the untreated sample against lubricant wear conditions. |
| ơLOS: | The specific wear rate for the heat-treated sample against lubricant wear conditions. |
References
- Singh, A.; Ansari, K.R.; Alanazi, A.K.; Quraishi, M.A.; Banerjee, P. Biological macromolecule as an eco-friendly high temperature corrosion inhibitor for P110 steel under sweet environment in NACE brine ID196: Experimental and computational approaches. J. Mol. Liq. 2021, 345, 117866. [Google Scholar] [CrossRef]
- Fajobi, M.A.; Loto, T.R.; Oluwole, O.O. Austenitic 316L Stainless Steel; Corrosion and Organic Inhibitor: A Review. In Key Engineering Materials; Trans Tech Publications Ltd.: Bäch SZ, Switzerland, 2021; Volume 886, pp. 133–142. [Google Scholar] [CrossRef]
- Burja, J.; Šuler, B.; Češnjaj, M.; Nagode, A. Effect of Intercritical Annealing on the Microstructure and Mechanical Properties of 0.1C-13Cr-3Ni Martensitic Stainless Steel. Metals 2021, 11, 392. [Google Scholar] [CrossRef]
- Monnot, M.; Nogueira, R.P.; Roche, V.; Berthomé, G.; Chauveau, E.; Estevez, R.; Mantel, M. Sulfide stress corrosion study of a super martensitic stainless steel in H2S sour environments: Metallic sulfides formation and hydrogen embrittlement. Appl. Surf. Sci. 2016, 394, 132–141. [Google Scholar] [CrossRef]
- Beliardouh, N.E.; Tlili, S.; Oulabbas, A.; Ramoul, C.E.; Meddah, S.; Kaleli, H. Investigation on dry sliding wear performance and corrosion resistance of 13Cr5Ni2Mo supermartensitic stainless steel. Tribol. Ind. 2021, 43, 107–116. [Google Scholar] [CrossRef]
- Ramoul, C.E.; Ghelloudj, O.; Gharbi, A.; Tlili, S.; Beliardouh, N.E.; Chouchane, T. Plastic deformation effect on wear and corrosion resistance of super martensitic stainless steel. J. Bio-Tribo-Corros. 2021, 7, 1–8. [Google Scholar] [CrossRef]
- de Oliveira, M.P.; Calderon-Hernandez, J.W.; Magnabosco, R.; Hincapie-Latino, D.; Alonso-Falleiros, N. Effect of niobium on phase transformations, mechanical properties and corrosion of supermartensitic stainless steel. J. Mater. Eng. Perfermance 2017, 26, 1664–1672. [Google Scholar] [CrossRef]
- Boer, D.; Ehl, T.; De Boer, G.N.; Hewson, R.W.; Thompson, H.M.; Gao, L.; Toropov, V.V. Two-scale EHL: Three-dimensional topography in tilted-pad bearings. Tribol. Int. 2014, 79, 111–125. [Google Scholar] [CrossRef]
- Sudeep, U.; Tandon, N.; Pandey, R.K. Performance of lubricated rolling/sliding concentrated contacts with surface textures: A review. J. Tribol. 2015, 137, 031501. [Google Scholar] [CrossRef]
- Von Recum, A.F.; Shannon, C.E.; Cannon, C.E.; Long, K.J.; van Kooten, T.G.; Meyle, J. Surface roughness, porosity, and texture as modifiers of cellular adhesion. Tissue Eng. 1996, 2, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Bizi-Bandoki, P.; Benayoun, S.; Valette, S.; Beaugiraud, B.; Audouard, E. Modifications of roughness and wettability properties of metals induced by femtosecond laser treatment. Appl. Surf. Sci. 2011, 257, 5213–5218. [Google Scholar] [CrossRef]
- Borgioli, F.; Galvanetto, E.; Bacci, T. Effects of surface modification by means of low-temperature plasma nitriding on wetting and corrosion behavior of austenitic stainless steel. Coatings 2020, 10, 98. [Google Scholar] [CrossRef] [Green Version]
- El-Kameesy, S.U.; El-Hossary, F.M.; Eissa, M.M.; Abd El-Moula, A.A.; Al-Shelkamy, S.A. Enhancing the capability of plasma treated austenite stainless steels as thermal reactor materials. Mater. Res. Express 2019, 6, 126589. [Google Scholar] [CrossRef]
- El-Kameesy, S.U.; El-Hossary, F.M.; Eissa, M.M.; Abd El-Moula, A.A.; Al-Shelkamy, S.A.; Saeed, A. Radiation shielding, mechanical and tribological properties of treated AISI304L using H2/N2 rf plasma. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2019; Volume 1253, p. 012034. [Google Scholar]
- El-Hossary, F.M.; El-Kameesy, S.U.; Eissa, M.M.; Abd El-Moula, A.A.; Al-Shelkamy, S.A. Influence of Rf plasma carbonitriding on AISI304L, SSMn6Ni and SSMn10Ni for nuclear applications. Mater. Res. Express 2019, 6, 096596. [Google Scholar] [CrossRef]
- Saeed, A.; Eissa, M.; El-Hossary, F.; EL-Kameesy, S.; Elmoula, A. Mechanical and gamma ray attenuation properties of N316L steel treated by rf plasma as a nuclear material. Arab. J. Nucl. Sci. Appl. 2019, 52, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Xi, Y.T.; Liu, D.X.; Han, D. Improvement of corrosion and wear resistances of AISI 420 martensitic stainless steel using plasma nitriding at low temperature. Surf. Coat. Technol. 2008, 202, 2577–2583. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Yuan, X.; Li, D.; Li, Y.Y.S. Microstructure evolution and orientation relationship of reverted austenite in 13Cr Supermartensitic Stainless Steel During the Tempering Process. Materials 2019, 12, 589. [Google Scholar] [CrossRef] [Green Version]
- Mosayebi, A.; Soleimani, M.; Mirzadeh, H.; Dehghanian, C. Tempering kinetics and corrosion resistance of quenched and tempered AISI 4130 medium carbon steel. Mater. Corros. 2021, 92, 1808–1812. [Google Scholar] [CrossRef]
- Hafeez, M.A.; Inam, A.; Farooq, A. Mechanical and corrosion properties of medium carbon low alloy steel after cyclic quenching and tempering heat–treatments. Mater. Res. Express 2020, 7, 016553. [Google Scholar] [CrossRef]
- Essa, F.A.; Elsheikh, A.H.; Yu, J.; Elkady, O.A.; Saleh, B. Studies on the effect of applied load, sliding speed and temperature on the wear behavior of M50 steel reinforced with Al2O3 and/or graphene nanoparticles. J. Mater. Res. Technol. 2021, 12, 283–303. [Google Scholar] [CrossRef]
- Soleimani, M.; Mirzadeh, H.; Dehghanian, C. Effects of spheroidization heat treatment and intercritical annealing on mechanical properties and corrosion resistance of medium carbon dual phase steel. Mater. Chem. Phys. 2021, 257, 123721. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Barakat, W.S.; YAMohamed, A.; AAlsaleh, N.; Elkady, O.A. The development of WC-based composite tools for friction stir welding of high-softening-temperature materials. Metals 2021, 11, 285. [Google Scholar] [CrossRef]
- Zinner, S.; Lenger, H.; Siller, I. M303 HIGH HARD—A corrosion resistant plastic mould steel with higher hardness. BHM Berg-und Hüttenmännische Monatshefte 2010, 155, 313–317. [Google Scholar] [CrossRef]
- Al-Hakkani, M.F.; Sedky; Hassan, H.A.; Saddik, M.S.; El-Mokhtar, M.A.; Al-Shelkamy, S.A. Bioengineering, characterization, and biological activities of C@Cu2O@Cu nanocomposite based-mediated the Vicia faba seeds aqueous extract. J. Mater. Res. Technol. 2021, 14, 1998–2016. [Google Scholar] [CrossRef]
- Nasiri, Z.; Mirzadeh, H. Spheroidization heat treatment and intercritical annealing of low carbon steel. J. Min. Metall. B Metall. 2019, 55, 405–411. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.T.; Li, Z.D.; Yang, C.F.; Xie, S.K.; Yong, Q.L. Cleavage fracture and microstructural effects on the toughness of a medium carbon pearlitic steel for high-speed railway wheel. Mater. Sci. Eng. A 2019, 761, 138036. [Google Scholar] [CrossRef]
- Essoussi, H.; Elmouhri, S.; Ettaqi, S.; Essadiqi, E. Heat treatment effect on mechanical properties of AISI 304 austenitic stainless steel. Procedia Manuf. 2019, 32, 883–888. [Google Scholar] [CrossRef]
- Tzeng, S.T.; Saibel, E. Surface roughness effect on slider bearing lubrication. ASLE Trans. 1967, 10, 334–348. [Google Scholar] [CrossRef]
- Choi, W.T.; Oh, K.; Singh, P.M.; Breedveld, V.; Hess, D.W. Wettability control of stainless steel surfaces via evolution of intrinsic grain structures. J. Mater. Sci. 2016, 51, 5196–5206. [Google Scholar] [CrossRef]
- Choi, W.T.; Oh, K.; Singh, P.M.; Breedveld, V.; Hess, D.W. Hydrophobicity and improved localized corrosion resistance of grain boundary etched stainless steel in chloride-containing environment. J. Electrochem. Soc. 2017, 164, C61. [Google Scholar] [CrossRef]
- Xie, Z.; Zhu, W. Theoretical and experimental exploration on the micro asperity contact load ratios and lubrication regimes transition for water-lubricated stern tube bearing. Tribol. Int. 2021, 164, 107105. [Google Scholar] [CrossRef]
- Xie, Z.; Zhu, W. An investigation on the lubrication characteristics of floatingring bearing with consideration of multi-coupling factors. Mech. Syst. Signal Process. 2022, 162, 108086. [Google Scholar] [CrossRef]
- Tabor, D. Friction and wear-developments over the last fifty years. IMechE 1987, 245, 157–172. [Google Scholar]
- Liu, R.; Li, D.Y. Experimental studies on tribological properties of pseudoelastic TiNi alloy with comparison to stainless steel 304. Metall. Mater. Trans. A 2000, 31, 2773–2783. [Google Scholar] [CrossRef]










| Chemical (wt.%) | S1 | S2 | S3 |
|---|---|---|---|
| C | 0.272 | 0.005 | 0.109 |
| Si | 0.260 | 0.351 | 0.561 |
| Mn | 0.688 | 1.478 | 8.85 |
| P | 0.016 | 0.018 | 0.064 |
| S | --- | 0.272 | 0.024 |
| Cu | 0.197 | 0.598 | 1.523 |
| Al | 0.025 | --- | --- |
| Cr | 14.86 | 17.66 | 13.23 |
| Mo | 0.904 | 0.315 | 0.117 |
| Ni | 0.853 | 9.51 | 0.430 |
| V | 0.091 | 0.036 | 0.027 |
| Ti | --- | 0.131 | 0.009 |
| Nb | 0.021 | 0.049 | 0.006 |
| Co | 0.047 | 0.064 | 0.105 |
| W | --- | --- | 0.184 |
| N | 0.074 | 0.013 | 0.152 |
| Fe | 81.692 | 69.455 | 74.609 |
| Density (g/cm3) | 7.62 | 7.71 | 7.92 |
| Sample Code | Texture Coefficient | FWHM for the Preferred Reflection Plane | Crystallite Size (A) |
|---|---|---|---|
| S1 | 0.67313 | 0.57044 | 282.13 |
| S2 | 0.67041 | 0.54529 | 344.57 |
| S3 | 0.69375 | 0.46892 | 241.28 |
| OS1 | 0.69643 | 0.55539 | 391.3 |
| OS2 | 0.52906 | 0.67757 | 145.57 |
| OS3 | 0.61853 | 0.55175 | 184.4 |
| Sample Code | Surface Microhardness (Hv. 10) | Average Surface Roughness Ra (µm) | Wettability | |
|---|---|---|---|---|
| Contact Angle (°) | Work of Adhesion [mN/m] | |||
| S1 | 351 ± 18 | 0.0695 ± 0.0034 | 81.037 ± 6.2 | 84.135 ± 4.20 |
| S2 | 276 ± 10 | 0.1304 ± 0.006 | 88.24 ± 4.5 | 75.025 ± 3.75 |
| S3 | 369 ± 15 | 0.2234 ± 0.009 | 92.97 ± 7.3 | 69.01 ± 4.02 |
| OS1 | 524 ± 25 | 0.3703 ± 0.018 | 89.115 ± 4.23 | 73.872 ± 3.69 |
| OS2 | 164 ± 19 | 0.0940 ± 0.0045 | 58.67 ± 9 | 110.60 ± 5.53 |
| OS3 | 221 ± 22 | 0.1084 ± 0.002 | 69.81 ± 5 | 97.91 ± 4.90 |
| Sample Code | Friction Coefficient against Abrasive Wear | Specific Abrasive Wear Rate (cm3/Nm) | ||
| Mean | Min | Max | ||
| S1 | 2.45 × 10−1 | 1.98 × 10−1 | 2.84 × 10−1 | 7.3978 × 10−11 ± 0.002729 × 10−11 |
| S2 | 2.56 × 10−1 | 8.45 × 10−2 | 2.93 × 10−1 | 1.92429 ×10−10 ± 0.003976 × 10−10 |
| S3 | 1.58 × 10−1 | 1.29 × 10−1 | 2.33 × 10−1 | 3.47039 × 10−11 ± 0.002205 × 10−11 |
| OS1 | 1.79 × 10−1 | 4.80 × 10−3 | 2.11 × 10−1 | 4.79294 × 10−11 ± 0.004352 × 10−11 |
| OS2 | 3.14 × 10−1 | 2.87 × 10−1 | 4.48 × 10−1 | 3.17628 × 10−10 ± 0.004311 × 10−10 |
| OS3 | 2.32 × 10−1 | 9.98 × 10−2 | 3.22 × 10−1 | 6.54368 × 10−10 ± 0.004535 × 10−10 |
| Sample Code | Friction Coefficient against Lubricant Wear | Specific Lubricant Wear Rate (cm3/Nm) | ||
| Mean | Min | Max | ||
| S1 | 5.70 × 10−2 | 5.60 × 10−3 | 1.25 × 10−1 | 2.4312 × 10−11 ± 0.002569 × 10−11 |
| S2 | 6.36 × 10−2 | 3.84 × 10−2 | 1.08 × 10−1 | 1.09763 × 10−11 ± 0.001264 × 10−11 |
| S3 | 3.27 × 10−2 | 1.02 × 10−4 | 1.50 × 10−1 | 1.33476 × 10−11 ± 0.003782 × 10−11 |
| OS1 | 1.30 × 10−2 | 8.52 × 10−6 | 5.80 × 10−2 | 6.94629 × 10−12 ± 0.001273 × 10−12 |
| OS2 | 9.38 × 10−2 | 4.31 × 10−1 | 1.76 × 10−1 | 1.88999 × 10−10 ± 0.002972 × 10−10 |
| OS3 | 3.90 × 10−2 | 5.11 × 10−6 | 1.62 × 10−1 | 3.70397 × 10−11 ± 0.00035 × 10−11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Shelkamy, S.A.; Abu Hashish, H.M.; Mahdy, A.A. Structural and Tribological Properties of Heat-Treated Stainless Steels against Abrasive and Lubricant Wear Conditions. Coatings 2021, 11, 1473. https://doi.org/10.3390/coatings11121473
Al-Shelkamy SA, Abu Hashish HM, Mahdy AA. Structural and Tribological Properties of Heat-Treated Stainless Steels against Abrasive and Lubricant Wear Conditions. Coatings. 2021; 11(12):1473. https://doi.org/10.3390/coatings11121473
Chicago/Turabian StyleAl-Shelkamy, Samah A., Hassan M. Abu Hashish, and Amir A. Mahdy. 2021. "Structural and Tribological Properties of Heat-Treated Stainless Steels against Abrasive and Lubricant Wear Conditions" Coatings 11, no. 12: 1473. https://doi.org/10.3390/coatings11121473