Evaluation of the Durability of Slippery, Liquid-Infused Porous Surfaces in Different Aggressive Environments: Influence of the Chemical-Physical Properties of Lubricants
Abstract
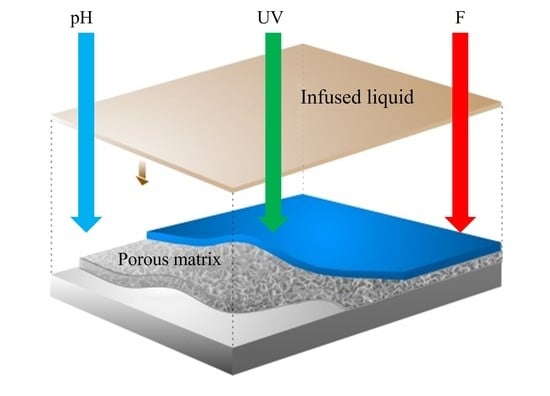
:1. Introduction
2. Materials and Methods
2.1. SLIPS Fabrication
2.2. SLIPS Characterization
2.3. Durability Tests
3. Results and Discussion
3.1. SLIPS Characterization
3.2. Durability Tests
3.2.1. Response to UV Irradiation
3.2.2. Response to Chemical Ageing
3.2.3. Response to Abrasion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhushan, B.; Jung, Y.C. Natural and Biomimetic Artificial Surfaces for Superhydrophobicity, Self-Cleaning, Low Adhesion, and Drag Reduction. Prog. Mater. Sci. 2011, 56, 1–108. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.Y.; Chuang, S.I.; Lo, Y.H.; Cheng, H.M.; Duh, J.G.; Chen, P.Y. Stalagmite-like Self-Cleaning Surfaces Prepared by Silanization of Plasma-Assisted Metal-Oxide Nanostructures. J. Mater. Chem. A 2016, 4, 3406–3414. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, P.; Liu, M.; Wang, S.; Jiang, L. Organogel-Based Thin Films for Self-Cleaning on Various Surfaces. Adv. Mater. 2013, 25, 4477–4481. [Google Scholar] [CrossRef]
- del Cerro, D.A.; Römer, G.R.B.E.; Huis In’t Veld, A.J. Erosion Resistant Anti-Ice Surfaces Generated by Ultra Short Laser Pulses. Phys. Procedia 2010, 5, 231–235. [Google Scholar] [CrossRef] [Green Version]
- Farhadi, S.; Farzaneh, M.; Kulinich, S.A. Anti-Icing Performance of Superhydrophobic Surfaces. App. Surf. Sci. 2011, 257, 6264–6269. [Google Scholar] [CrossRef]
- Ge, L.; Ding, G.; Wang, H.; Yao, J.; Cheng, P.; Wang, Y. Anti-Icing Property of Superhydrophobic Octadecyltrichlorosilane Film and Its Ice Adhesion Strength. J. Nanomater. 2013, 2013, 278936. [Google Scholar] [CrossRef] [Green Version]
- Golovin, K.B.; Gose, J.; Perlin, M.; Ceccio, S.L.; Tuteja, A. Bioinspired Surfaces for Turbulent Drag Reduction. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20160189. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, B.J.; van Buren, T.; Fu, M.K.; Smits, A.J. Turbulent Drag Reduction over Air- and Liquid-Impregnated Surfaces. Phys. Fluids 2016, 28, 015103. [Google Scholar] [CrossRef]
- Rizzo, G.; Massarotti, G.P.; Bonanno, A.; Paoluzzi, R.; Raimondom, M.; Blosi, M.; Veronesi, F.; Caldarelli, A.; Guarini, G. Axial Piston Pumps Slippers with Nanocoated Surfaces to Reduce Friction. Int. J. Fluid Power 2015, 16, 1–10. [Google Scholar] [CrossRef]
- Gittens, J.E.; Smith, T.J.; Suleiman, R.; Akid, R. Current and Emerging Environmentally-Friendly Systems for Fouling Control in the Marine Environment. Biotechnol. Adv. 2013, 31, 1738–1753. [Google Scholar] [CrossRef]
- Oldani, V.; del Negro, R.; Bianchi, C.L.; Suriano, R.; Turri, S.; Pirola, C.; Sacchi, B. Surface Properties and Anti-Fouling Assessment of Coatings Obtained from Perfluoropolyethers and Ceramic Oxides Nanopowders Deposited on Stainless Steel. J. Fluor. Chem. 2015, 180, 7–14. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the Sacred Lotus, or Escape from Contamination in Biological Surfaces. Planta 1997, 202, 1. [Google Scholar] [CrossRef]
- Zorba, V.; Stratakis, E.; Barberoglou, M.; Spanakis, E.; Tzanetakis, P.; Anastasiadis, S.H.; Fotakis, C. Biomimetic Artificial Surfaces Quantitatively Reproduce the Water Repellency of a Lotus Leaf. Adv. Mater. 2008, 20, 4049–4054. [Google Scholar] [CrossRef]
- Ebert, D.; Bhushan, B. Durable Lotus-Effect Surfaces with Hierarchical Structure Using Micro- and Nanosized Hydrophobic Silica Particles. J. Colloid Interface Sci. 2012, 368, 584–591. [Google Scholar] [CrossRef]
- Guo, M.; Ding, B.; Li, X.; Wang, X.; Yu, J.; Wang, M. Amphiphobic Nanofibrous Silica Mats with Flexible and High-Heat-Resistant Properties. J. Phys. Chem. C 2010, 114, 916–921. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, H.; Niu, H.; Gestos, A.; Lin, T. Robust, Self-Healing Superamphiphobic Fabrics Prepared by Two-Step Coating of Fluoro-Containing Polymer, Fluoroalkyl Silane, and Modified Silica Nanoparticles. Adv. Funct. Mater. 2013, 23, 1664–1670. [Google Scholar] [CrossRef]
- Ma, W.; Higaki, Y.; Otsuka, H.; Takahara, A. Perfluoropolyether-Infused Nano-Texture: A Versatile Approach to Omniphobic Coatings with Low Hysteresis and High Transparency. Chem. Commun. 2013, 49, 597–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Kato, K.; Blois, A.P.; Wong, T.S. Bioinspired Omniphobic Coatings with a Thermal Self-Repair Function on Industrial Materials. ACS Appl. Mater. Interfaces 2016, 8, 8265–8271. [Google Scholar] [CrossRef]
- Wong, T.-S.; Kang, S.H.; Tang, S.K.Y.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired Self-Repairing Slippery Surfaces with Pressure-Stable Omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef]
- Riedel, M.; Eichner, A.; Jetter, R. Slippery Surfaces of Carnivorous Plants: Composition of Epicuticular Wax Crystals in Nepenthes Alata Blanco Pitchers. Planta 2003, 218, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Niemelä-Anttonen, H.; Koivuluoto, H.; Tuominen, M.; Teisala, H.; Juuti, P.; Haapanen, J.; Harra, J.; Stenroos, C.; Lahti, J.; Kuusipalo, J.; et al. Icephobicity of Slippery Liquid Infused Porous Surfaces under Multiple Freeze–Thaw and Ice Accretion–Detachment Cycles. Adv. Mater. Interfaces 2018, 5, 1–8. [Google Scholar] [CrossRef]
- Yang, S.; Qiu, R.; Song, H.; Wang, P.; Shi, Z.; Wang, Y. Slippery Liquid-Infused Porous Surface Based on Perfluorinated Lubricant/Iron Tetradecanoate: Preparation and Corrosion Protection Application. Appl. Surf. Sci. 2015, 328, 491–500. [Google Scholar] [CrossRef]
- Preston, D.J.; Song, Y.; Lu, Z.; Antao, D.S.; Wang, E.N. Design of Lubricant Infused Surfaces. ACS Appl. Mater. Interfaces 2017, 9, 42383–42392. [Google Scholar] [CrossRef] [PubMed]
- Togasawa, R.; Tenjimbayashi, M.; Matsubayashi, T.; Moriya, T.; Manabe, K.; Shiratori, S. A Fluorine-Free Slippery Surface with Hot Water Repellency and Improved Stability against Boiling. ACS Appl. Mater. Interfaces 2018, 10, 4198–4205. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Tadanaga, K.; Matsuda, A.; Minami, T.; Tatsumisago, M. Formation of Anti-Reflective Alumina Films on Polymer Substrates by the Sol-Gel Process with Hot Water Treatment. Surf. Coat. Technol. 2006, 201, 3653–3657. [Google Scholar] [CrossRef]
- Raimondo, M.; Blosi, M.; Caldarelli, A.; Guarini, G.; Veronesi, F. Wetting Behavior and Remarkable Durability of Amphiphobic Aluminum Alloys Surfaces in a Wide Range of Environmental Conditions. Chem. Eng. J. 2014, 258, 101–109. [Google Scholar] [CrossRef]
- Kim, P.; Kreder, M.J.; Alvarenga, J.; Aizenberg, J. Hierarchical or Not? Effect of the Length Scale and Hierarchy of the Surface Roughness on Omniphobicity of Lubricant-Infused Substrates. Nano Lett. 2013, 13, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, M.; Kim, J.H.; Lee, J. Dynamic Contact Angle Measurements on Lubricant Infused Surfaces. J. Colloid Interface Sci. 2021, 586, 647–654. [Google Scholar] [CrossRef]
- Daniel, D.; Mankin, M.N.; Belisle, R.A.; Wong, T.-S.; Aizenberg, J. Lubricant-Infused Micro/Nano-Structured Surfaces with Tunable Dynamic Omniphobicity at High Temperatures. Appl. Phys. Lett. 2013, 102, 2–6. [Google Scholar] [CrossRef] [Green Version]
- Faucitano, A.; Buttafava, A.; Karolczak, S.; Guarda, P.A.; Marchionni, G. The Chemical Effects of Ionizing Radiations of Fluorinated Ethers. J. Fluor. Chem. 2004, 125, 221–241. [Google Scholar] [CrossRef]
- Bassi, M. Estimation of the Vapor Pressure of PFPEs by TGA. Thermochim. Acta 2011, 521, 197–201. [Google Scholar] [CrossRef]
- Parks, G.S.; Moore, G.E. Vapor Pressure and Other Thermodynamic Data for N-Hexadecane and n-Dodecylcyc1ohexane near Room Temperature. J. Chem. Phys. 1949, 17, 1151–1153. [Google Scholar] [CrossRef]
- Sawatari, C.; Kondo, T. Interchain Hydrogen Bonds in Blend Films of Poly(Vinyl Alcohol) and Its Derivatives with Poly(Ethylene Oxide). Macromolecules 1999, 32, 1949–1955. [Google Scholar] [CrossRef]
- Zheng, S.; Al, S.; Guo, Q. Poly(Hydroxyether of Phenolphthalein) and Its Blends with Poly(Ethylene Oxide). J. Polym. Sci. Part B Polym. Phys. 2003, 41, 466–475. [Google Scholar] [CrossRef]
- Wang, Y.; Dugan, M.; Urbaniak, B.; Li, L. Fabricating Nanometer-Thick Simultaneously Oleophobic/ Hydrophilic Polymer Coatings via a Photochemical Approach. Langmuir 2016, 32, 6723–6729. [Google Scholar] [CrossRef]
- Yang, Y.-W.; Hentschel, J.; Chen, Y.-C.; Lazari, M.; Zeng, H.; Michael Van Dam, R.; Guan, Z. “Clicked” Fluoropolymer Elastomers as Robust Materials for Potential Microfluidic Device Applications. J. Mater. Chem. 2012, 22, 1100. [Google Scholar] [CrossRef]
- Li, S.M.; Chen, X.H.; Gross, R.A.; Mccarthy, S.P. Hydrolytic Degradation of PCL/PEO Copolymers in Alkaline Media. J. Mater. Sci. Mater. Med. 2000, 11, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Shin, S.; Jiang, Y.; Jeong, C.; Stone, H.A.; Choi, C.H. Oil-Impregnated Nanoporous Oxide Layer for Corrosion Protection with Self-Healing. Adv. Funct. Mater. 2017, 27, 1606040. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, W.; Dong, G. Facile Fabrication of Biomimetic Liquid-Infused Slippery Surface on Carbon Steel and Its Self-Cleaning, Anti-Corrosion, Anti-Frosting and Tribological Properties. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 17–26. [Google Scholar] [CrossRef]
- Homola, A.M. Lubrication Issues in Magnetic Disk Storage Devices. IEEE Trans. Magn. 1996, 32, 1812–1818. [Google Scholar] [CrossRef]
- Tagawa, N.; Tani, H. Tribological Characteristics of Novel Branched Perfluoropolyether Lubricant Films in Hard Disk Drives. Microsyst. Technol. 2013, 19, 1513–1518. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, T.; Cheng, P.; Chao, J. Relationship between the Molecular Structures of Lubricants and Their Performance at the Head-Disk Interface of Hard Disk Drives. Wear 2003, 254, 321–331. [Google Scholar] [CrossRef]
- Tao, Z.; Bhushan, B. Bonding, Degradation, and Environmental Effects on Novel Perfluoropolyether Lubricants. Wear 2005, 259, 1352–1361. [Google Scholar] [CrossRef]
- Min, K.; Han, J.; Park, B.; Cho, E. Characterization of Mechanical Degradation in Perfluoropolyether Film for Its Application to Antifingerprint Coatings. ACS Appl. Mater. Interfaces 2018, 10, 37498–37506. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kawai, K.; Zhang, H.; Fukuzawa, K.; Koga, N.; Itoh, S.; Azuma, N. ReaxFF Reactive Molecular Dynamics Simulations of Mechano-Chemical Decomposition of Perfluoropolyether Lubricants in Heat-Assisted Magnetic Recording. J. Phys. Chem. C 2020, 124, 22496–22505. [Google Scholar] [CrossRef]










| Infused Liquid | Viscosity [mm2/s] | ACAW [°] | RCAW [°] | CAHW [°] | ACAH [°] | RCAH [°] | CAHH [°] |
|---|---|---|---|---|---|---|---|
| Krytox 100 | 7.8 | 124.2 ± 0.7 | 122.2 ± 0.4 | 1.9 ± 0.9 | 71.4 ± 0.4 | 69.5 ± 0.4 | 1.9 ± 0.5 |
| Krytox 103 | 30 | 121.8 ± 0.6 | 117.7 ± 1.3 | 4.2 ± 1.9 | 70.7 ± 0.4 | 68.1 ± 0.9 | 2.6 ± 0.6 |
| Krytox 105 | 160 | 121.5 ± 1.5 | 115.6 ± 0.9 | 5.9 ± 0.7 | 71.8 ± 1.1 | 65.5 ± 1.0 | 6.3 ± 0.8 |
| Krytox 107 | 450 | 120.9 ± 0.4 | 107.8 ± 1.0 | 13.1 ± 1.3 | 73.5 ± 0.9 | 61.1 ± 1.8 | 12.4 ± 1.3 |
| Hexadecane | 2.8 | 106.2 ± 2.5 | 103.2 ± 1.8 | 3.0 ± 0.8 | ≈0 | ≈0 | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veronesi, F.; Guarini, G.; Corozzi, A.; Raimondo, M. Evaluation of the Durability of Slippery, Liquid-Infused Porous Surfaces in Different Aggressive Environments: Influence of the Chemical-Physical Properties of Lubricants. Coatings 2021, 11, 1170. https://doi.org/10.3390/coatings11101170
Veronesi F, Guarini G, Corozzi A, Raimondo M. Evaluation of the Durability of Slippery, Liquid-Infused Porous Surfaces in Different Aggressive Environments: Influence of the Chemical-Physical Properties of Lubricants. Coatings. 2021; 11(10):1170. https://doi.org/10.3390/coatings11101170
Chicago/Turabian StyleVeronesi, Federico, Guia Guarini, Alessandro Corozzi, and Mariarosa Raimondo. 2021. "Evaluation of the Durability of Slippery, Liquid-Infused Porous Surfaces in Different Aggressive Environments: Influence of the Chemical-Physical Properties of Lubricants" Coatings 11, no. 10: 1170. https://doi.org/10.3390/coatings11101170