2.1. Chemistry
1H- and
13C-NMR spectra were recorded on an Avance III nanobay-300 MHz instrument (Bruker, Bremen, Germany, 300 MHz for
1H, 75 MHz for
13C). Chemical shifts are reported in ppm relative to the solvent in which the spectrum was recorded [
1H: δ (d
6-DMSO) = 2.50 ppm, δ (CDCl
3) = 7.27 ppm;
13C: δ (d
6-DMSO) = 39.52 ppm, δ (CDCl
3) = 77.16 ppm]. Combustion analyses were performed at the analysis facilities of Spectropole (
https://fr-chimie.univ-amu.fr/spectropole) with a Thermo Finnigan (San Jose, CA, USA) EA 1112 apparatus; all compounds had purity higher than 95%. Microwave-assisted reactions were performed in a CEM Discover microwave reactor with a focused field (CEM Corporation, Matthews, NC, USA). Silica gel F-254 plates (0.25 mm; Merck, Darmstadt, Germany) were used for thin-layer chromatography (TLC), and silica gel 60 (200–400 mesh; Merck) was used for flash chromatography. Unless otherwise stated, reagents were obtained from commercial sources and were used without further purification. Dihydroberberine (compound
1), 13-hydroxyberberine (compound
2), 8-acetonyl dihydroberberine (compound
3) (yield: 85,40 and 85%) were synthesized by a procedure described in the literature [
14].
2.1.1. General Procedure for the Synthesis of Compounds 4–20
Berberine chloride (3.71 g, 10 mmol) was dissolved in 5N NaOH (20 mL) under stirring at room temperature. Acetone (5 mL) was added dropwise at that temperature and stirred for 1 h, precipitation occurred during that time and the reaction mixture was filtered and washed with 80% MeOH to give the desired acetonylberberberine (compound 3) (3.34 g, 85%). Compound 3 (1 g, 2.5 mmol) was used without further purification, dissolved in acetonitrile, NaI (0.5 g, 3.3 mmol) was added with various methylbromide (3 mmol) at 80 °C for 4 h. The reaction mixture was concentrated under vaccuo and chromatographed on silica gel (CH2Cl2/CH3OH, 90/10 v/v) to give compound 4–20.
13-(Acetic Acid Ethylester) Berberine (Compound 4)
Yellow solid; yield: 26%; mp: 216 °C [
12];
1H-NMR (CDCl
3) δ ppm: 10.56 (1H, s, H-8), 7.86 (1H, d,
J = 9.2 Hz, H-12), 7.73 (1H, d,
J = 9.2 Hz, H-11), 7.24 (1H, s, H-1), 6.90 (1H, s, H-4), 6.10 (2H, s, -OCH
2O-), 5.20 (2H, brs, H-6), 4.37 (3H, s, OCH
3-9), 4.35 (2H, q,
J = 7.1 Hz, H-3′), 4.28 (2H, s, H-1′), 4.07 (3H, s, OCH
3-10), 3.26 (2H, t,
J = 5.1 Hz, H-5), 1.37 (3H, t,
J = 7.1 Hz, H-4′).
13C-RMN (CDCl
3) δ ppm: 170.6 (C-2′), 150.7 (C-10), 150.2 (C-3), 147.5 (C-2), 147.2 (C-8), 146.5 (C-9), 137.6 (C-13a), 134.11 (C-4a), 133.42 (C-12a), 125.93 (C-12), 125.68 (C-13), 121.79 (C-8a), 119.79 (C-13b), 119.34 (C-11), 109.12 (C-4), 108.66 (C-1), 102.13 (-OCH
2O-), 63.12 (OCH
3-C9), 62.29 (C-3′), 57.41 (C-6), 56.97 (OCH
3-C10), 37.15 (C-1′), 28.50 (C-5), 14.2 (C-4′). Anal. calcd. for C
24H
24BrNO
6: C, 57.38; H, 4.82; N, 2.79. Found: C, 57.44; H, 4.87; N, 2.63.
13-(Acetic Acid) Berberine (Compound 5)
Yield 53%; 1H-RMN (CDCl3) δ ppm: 10.56 (1H, s, H-8), 7.86 (1H, d, J = 9.2 Hz, H-12), 7.73 (1H, d, J = 9.2 Hz, H-11), 7.24 (1H, s, H-1), 6.90 (1H, s, H-4), 6.10 (2H, s, -OCH2O-), 5.20 (2H, brs, H-6), 4.37 (3H, s, OCH3-9), 4.35 (2H, q, J = 7.1 Hz, H-3′), 4.28 (2H, s, H-1′), 4.07 (3H, s, OCH3-10), 3.26 (2H, t, J = 5.1 Hz, H-5), 1.37 (3H, t, J = 7.1 Hz, H-4′). 13C-RMN (CDCl3) δ ppm: 168.89 (COOH’), 150.66 (C-10), 149.21 (C-3), 147.46 (C-2), 147.21 (C-8), 145.31 (C-9), 137.60 (C-13a), 134.11 (C-4a), 133.11 (C-12a), 130.24 (C-13), 125.93 (C-12), 121.79 (C-8a), 119.79 (C-13b), 119.34 (C-11), 109.12 (C-4), 108.66 (C-1), 102.01 (-OCH2O-), 63.12 (OCH3-C9), 57.41 (C-6), 56.97 (OCH3-C10), 40.03 (C-1′), 28.50 (C-5). Anal. calcd. for C22H20BrNO6: C, 55.71; H, 4.25; N, 2.95. Found: C, 55.73; H, 4.32; N, 2.89.
13-(4-Fluorobenzyl)Berberine (Compound 6)
Yellow solid; yield: 55%; mp: 238 °C; 1H-RMN (CDCl3) δ ppm: 10.45 (1H, s, H-8), 7.73 (1H, d, J = 9.3 Hz, H-11), 7.60 (1H, d, J = 9.3 Hz, H-12), 7.08 (2H, m, H-4′), 7.08 (2H, m, H-3′), 6.92 (1H, s, H-1), 6.89 (1H, s, H-4), 6.02 (2H, s, -OCH2O-), 5.20 (2H, brs, H-6), 4.65 (2H, s, H-1′), 4.39 (3H, s, OCH3-9), 4.04 (3H, s, OCH3-10), 3.29 (2H, t, J = 5.7 Hz, H-5). 13C-RMN (CDCl3) δ ppm: 161.72 (d, JCF = 246.5 Hz, 1C, C-5′), 150.52 (C-10), 149.93 (C-3), 147.25 (C-8), 147.10 (C-2), 146.3 (C-9), 137.37 (C-13a), 133.81 (d, JCF = 3.3 Hz, 1C, C-2′), 133.81 (C-4a), 133.30 (C-12a), 129.61 (C-13), 129.40 (d, JCF = 7.8 Hz, 1C, C-3′), 125.63 (C-12), 121.91 (C-8a), 120.74 (C-11), 119.86 (C-13b), 116.35 (d, JCF = 21.5 Hz, 1C, C-4′), 108.64 (C-1), 108.64 (C-4), 101.96 (-OCH2O-), 63.07 (OCH3-C9), 57.50 (C-6), 56.86 (OCH3-C10), 35.68 (C-1′), 28.53 (C-5). Anal. calcd. for C27H23BrFNO4: C, 61.84; H, 4.42; N, 2.67. Found: C, 61.75; H, 4.46; N, 2.61.
13-(4-Cyanobenzyl)Berberine (Compound 7)
Dark green solid; yield: 60%; 1H-RMN (300 MHz, CDCl3) δ ppm: 10.49 (1H, s, H-8), 7.73 (1H, d, J = 9.2 Hz, H-11), 7.67 (1H, d, J = 8.4 Hz, H-4′), 7.51 (1H, d, J = 9.2 Hz, H-12), 7.31 (1H, d, J = 8.4 Hz, H-3′), 6.90 (1H, s, H-4), 6.77 (1H, s, H-1), 6.02 (2H, s, -OCH2O-), 5.20 (2H, brs, H-6), 4.65 (2H, s, H-1′), 4.35 (3H, s, OCH3-9), 4.04 (3H, s, OCH3-10), 3.30 (2H, t, J = 5.7 Hz, H-5). 13C-RMN (CDCl3) δ ppm: 150.64 (C-10), 150.20 (C-3), 147.29 (C-2), 147.20 (C-8), 146.38 (C-9), 143.77 (C-2′), 137.74 (C-13a), 133.76 (C-4a), 133.21 (C-12a), 133.21 (C-4′), 128.88 (C-3′), 128.46 (C-13), 125.87 (C-12), 121.96 (C-8a), 120.47 (C-11), 119.71 (C-13b), 118.29 (C-CN), 111.43 (C-5′), 108.76 * (C-4), 108.44 * (C-1),102.12 (-OCH2O-), 63.13 (OCH3-C-9), 57.86 (C-6), 56.89 (OCH3-C-10), 36.81 (C-1′), 28.48 (C-5). Anal. calcd. for C28H23BrN2O4: C, 63.29; H, 4.36; N, 5.27. Found: C, 63.02; H, 4.41; N, 5.22.
13-(4-Iodomethylbenzyl)Berberine (Compound 8)
Yellow solid; yield: 28%; 1H-RMN (300 MHz, CDCl3) δ ppm: 10.41 (1H, s, H-8), 7.72 (1H, d, J = 9.3 Hz, H-11), 7.59 (1H, d, J = 9.3 Hz, H-12), 7.38 (2H, d, J = 8.3 Hz, H-4′), 7.06 (2H, d, J = 8.3 Hz, H-3′), 6.91 (1H, s, H-1), 6.88 (1H, s, H-4), 6.00 (2H, s, -OCH2O), 5.20 (2H, brs, H-6), 4.64 (2H, s, H-1′), 4.45 (2H, s, H-6′), 4.41 (3H, s, OCH3-9), 4.03 (3H, s, OCH3-10), 3.30 (2H, t, J = 5.8 Hz, H-5). 13C-RMN (300 MHz, CDCl3) δ ppm: 150.46 (C-10), 150.03 (C-3), 147.19 (C-2), 146.49 (C-8), 146.30 (C-9), 138.34 (C-5′), 137.85 (C-2′), 137.58 (C-13a), 133.65 * (C-4a), 133.55 * (C-12a), 129.83 (C-4′), 129.81 (C-13), 128.38 (C-3′), 125.76 (C-12), 121.80(C-8a), 111.82 (C-11), 119.92 (C-13b), 108.79 (C-1), 108.57 (C-4), 63.21 (-OCH2O-), 63.21 (OCH3-C9), 58.13 (C-6), 56.83 (OCH3-C10), 36.36 (C-1′), 28.37 (C-5), 4.73 (C-6′). * may be reverse. Anal. calcd. for C28H25BrINO4: C, 52.03; H, 3.90; N, 2.17. Found: C, 51.93; H, 3.98; N, 2.09.
13-(4-Ethenylbenzyl)Berberine (Compound 9)
Yellow solid; yield: 18%;
1H-RMN (DMSO-d
6) δ ppm: 10.02 (1H, s, H-8), 8.10 (1H, d,
J = 9.4 Hz, H-11), 7.81 (1H, d,
J = 9.4 Hz, H-12), 7.17 (1H, s, H-4), 7.03 (1H,s, H-1), 6.88 (1H, d,
J = 7.9, H-7’), 6.83 (1H, brs, H-3′), 6.56 (1H, brd,
J = 7.9, H-6′), 6.10 (2H, s, -OCH2O- [
2,
3]),), 6.02 (2H, s, -OCH2O- [4′,5′]), 4.88 (2H, brs, H-6), 4.64 (2H, s, H-1′), 4.12 (3H, s, OCH3-9), 4.03 (3H, s, OCH3-10), 3.16 (3H, t,
J = 5.1 Hz, H-5).
13C-RMN (DMSO-d
6) δ ppm: 150.17 (C-10), 149.17 (C-3), 147.90 (C-4′), 146.40 (C-2), 146.02 (C-5′), 145.40 (C8), 144.18 (C-9), 137.14 (C-13a),), 134.01 (C-4a), 132.83 (C-2′), 132.76 (C-12a), 130.11 (C-13), 126.12 (C-11), 121.67 (C-12), 121.29 (C-8a), 121.05 (C-7′), 120.02 (C-13b), 108.65 * (C-3′), 108.58 * (C-6′), 108.47 (C-4), 108.15 (C-1), 102.08 (-OCH2O- [
2,
3]), 101.17 (-OCH2O- [4′,5′]), 62.21 (OCH3-C-9), 56.98 (C-6), 56.93 (OCH3-C-10), 35.14 (C-1′), 27.24(C-5). * may be reverse. Anal. calcd. for C
29H
26BrNO
4: C, 65.42; H, 4.92; N, 2.63. Found: C, 65.36; H, 5.01; N, 2.58.
13-(4-Sulfamoylbenzyl)Berberine (Compound 10)
Green solid; yield: 73%; 1H-RMN (DMSO-d6) δ ppm: 10.05 (1H, s, H-8), 8.08 (1H, d, J = 9.3 Hz, H-11), 7.80 (2H, d, J = 8.0Hz, H-4’), 7.73 (1H, d, J = 9.3 Hz, H-12), 7.38 (2H, d, J = 8.0Hz, H3’), 7.17 (1H, s, H-4), 6.89 (1H, s, H-1), 6.08 (2H, s, -OCH2O-), 4.89 (2H, s, H-6), 4.84 (2H, brs, H-1′), 4.13 (3H, s, OCH3-9), 4.02 (3H, s, OCH3-10), 3.18 (3H, t, J = 5.1 Hz, H-5). 13C-RMN (DMSO-d6) δ ppm: 150.22 (C-10), 149.25 (C-3), 146.40 (C-2), 145.61 (C8), 144.36 (C-9), 143.20 * (C-2′), 142.59 * (C-5′), 137.34 (C-13a), 134.10 (C4a), 132.57 (C12a), 128.57 (C-3′), 126.29 (C-11), 126.29 (C-4′), 121.47 (C-12), 121.30 (C-8a), 119.89 (C-13b), 108.51 (C-4), 108.00 (C-1), 102.07 (-OCH2O-), 62.06 (OCH3-C-9), 56.95 (C-6), 56.95 (OCH3-C-10), 35.41 (C-1′), 27.23 (C-5). * (C13) et (C-4″) not observed. Anal. calcd. for C27H25BrN2O6S: C, 55.39; H, 4.30; N, 4.78. Found: C, 55.33; H, 4.38; N, 4.88.
13-(4-Aminomethylbenzyl)Berberine (Compound 11)
Reddish solid; yield: 10%; 1H-RMN (CDCl3) δ ppm: 10.05 (1H, s, H-8), 8.17 (2H,brs, NH2), 8.06 (1H, d, J = 9.4 Hz, H-11), 7.72 (1H, d, J = 9.4 Hz, H-12), 7.45 (2H, d, J = 8.4 Hz, H-4′), 7.24 (2H, d, J = 8.4 Hz, H-3′), 6.93 (1H, s, H-1), 7.18 (1H, s, H-4), 6.08 (2H, s, -OCH2O), 4.88 (2H, brs, H-6), 4.76 (2H, brs, H-1′), 4.14 (3H, s, OCH3-9), 4.03 (2H, sl, H-6′), 4.03 (3H, s, OCH3-10), 3.17(2H, t, J = 5.8 Hz, H-5). 13C-RMN (CDCl3) δ ppm: 150.16 (C-10), 149.19 (C-3), 147.26 (C-2), 146.97 (C-8), 146.30 (C-9), 145.12 (C-2′), 137.69 (C-13a), 134.08 (C-5′), 133.69 (C-4a), 133.37 (C-12a), 129.53 (C-4′), 128.93 (C-13), 128.26 (C-3′), 125.74 (C-12), 121.90 (C-8a), 120.53 (C-11), 119.78 (C-13b), 108.69 * (C-4), 108.56 * (C-1), 102.06 (-OCH2O-), 62.05 (OCH3-C-9), 57.02 (C-6), 56.99 (OCH3-C-10), 41.85 (C-6′), 40.35 (C-1′), 27.21 (C-5). Anal. calcd. for C28H27BrN2O4: C, 62.81; H, 5.08; N, 5.23. Found: C, 62.78; H, 5.12; N, 5.24.
13-(4-Formylbenzyl)Berberine (Compound 12)
Red solid; yield: 75%; 1H-RMN (CDCl3) δ ppm: 10.02 (1H, s, H-CHO), 10.46 (1H, s, H-8), 7.90 (2H, d, J = 8.3 Hz, H-4′), 7.71 (1H, d, J = 9.3 Hz, H-11), 7.54 (1H, d, J = 9.3 Hz, H-12), 7.36 (2H, d, J = 8.3 Hz, H-3′), 6.90 (1H, s, H-4), 6.83 (1H, s, H-1), 6.01 (2H, s, -OCH2O-), 5.20 (2H, brs, H-6), 4.79 (2H, s, H-1′), 4.41 (3H, s, OCH3-9), 4.03 (3H, s, OCH3-10), 3.32 (2H, t, J = 5.5 Hz, H-5). 13C-RMN (CDCl3) δ ppm: 191.44 (C-CHO), 150.58 (C-10), 150.14 (C-3), 147.26 (C-2), 146.97 (C-8), 146.30 (C-9), 145.20 (C-2′), 137.69 (C-13a), 135.45 (C-5′), 133.69 (C-4a), 133.37 (C-12a), 130.78 (C-4′), 128.93 (C-13), 128.71 (C-3′), 125.74 (C-12), 121.90 (C-8a), 120.53 (C-11), 119.78 (C-13b), 108.69 * (C-4), 108.56 * (C-1), 102.06 (-OCH2O-), 63.16 (OCH3-C-9), 57.98 (C-6), 56.86 (OCH3-C-10), 36.91 (C-1′), 28.40 (C-5). Anal. calcd. for C28H24BrNO5: C, 62.93; H, 4.53; N, 2.62. Found: C, 62.84; H, 4.67; N, 2.60.
13-[(2H-1,3-Benzodioxol-5-yl)methyl]Berberine (Compound 13)
Yellow solid; yield: 40%;
1H-RMN (DMSO-d
6) δ ppm: 10.02 (1H, s, H-8), 8.10 (1H, d,
J = 9.4 Hz, H-11), 7.81 (1H, d,
J = 9.4 Hz, H-12), 7.17 (1H, s, H-4), 7.03 (1H,s, H-1), 6.88 (1H, d,
J = 7.9, H-7’), 6.83 (1H, brs, H-3′), 6.56 (1H, brd,
J = 7.9, H-6′), 6.10 (2H, s, -OCH
2O- [
2,
3]), 6.02 (2H, s, -OCH
2O- [4′,5′]), 4.88 (2H, brs, H-6), 4.64 (2H, s, H-1′), 4.12 (3H, s, OCH
3-9), 4.03 (3H, s, OCH
3-10), 3.16 (3H, t, J = 5.1 Hz, H-5).
13C-RMN (DMSO-d
6) δ ppm: 150.17 (C-10), 149.17 (C-3), 147.90 (C-4′), 146.40 (C-2), 146.02 (C-5′), 145.40 (C8), 144.18 (C-9), 137.14 (C-13a), 134.01 (C-4a), 132.83 (C-2′), 132.76 (C-12a), 130.11 (C-13), 126.12 (C-11), 121.67 (C-12), 121.29 (C-8a), 121.05 (C-7′), 120.02 (C-13b), 108.65 * (C-3′), 108.58 * (C-6′), 108.47 (C-4), 108.15 (C-1), 102.08 (-OCH
2O- [
2,
3]), 101.17 (-OCH
2O- [4′,5′]), 62.21 (OCH
3-C-9), 56.98 (C-6), 56.93 (OCH
3-C-10), 35.14 (C-1′), 27.24(C-5). * may be reverse. Anal. calcd. for C
29H
26BrNO
4: C, 61.10; H, 4.40; N, 2.54. Found: C, 61.02; H, 4.48; N, 2.51.
13[(2,3,4-Trimethoxyphenyl)methyl]Berberine (Compound 14)
Brown solid; yield: 51%; 1H-RMN (DMSO-d6) δ ppm: 10.02 (1H, s, H-8), 8.12 (1H, d, J = 9.3 Hz, H-11), 7.77 (1H, d, J = 9.3 Hz, H-12), 7.16 (1H, s, H-4), 6.92 (1H, s, H-1), 6.65 (1H, d, J = 8.6 Hz, H-7’), 6.34 (1H, d, J = 8.6 Hz, H-6′), 6.08 (2H, s, -OCH2O-),4.88 (2H, brs, H-6), 4.51 (2H, brs, H-1′), 4.13 (3H, s, OCH3-9), 4.04 (3H, s, OCH3-10), 3.91 (3H, s, OCH3-5′), 3.76 * (3H, s, OCH3-3′), 3.75 * (3H, s, OCH3-4′), 3.16 (3H, t, J = 5.7 Hz, H-5). 13C-RMN (DMSO-d6) δ ppm: 152.64 (C-3′), 150.61 (C-5′), 150.12 (C-10), 149.13 (C-3), 146.38 (C-2), 145.21 (C8), 144.14 (C-9), 142.04 (C-4′), 137.03 (C-13a), 133.93 (C-4a), 132.94 (C-12a), 130.11 (C-13), 126.22 (C-11), 124.42 (C-2′), 122.72 (C-7′), 121.46 (C-12), 121.11 (C-8a), 119.98 (C-13b), 108.40 (C-1), 108.04 (C-4), 107.84 (C-6′), 102.02 (-OCH2O-),62.04 (OCH3-C-9), 60.43 * (OCH3-C-3′), 60.30 * (OCH3-C-4′), 56.91 (C-6), 56.91 (OCH3-C-10), 55.76 (OCH3-C-5′), 30.51 (C-1′), 27.19(C-5). * may be reverse. Anal. calcd. for C30H30BrNO7: C, 60.41; H, 5.07; N, 2.35. Found: C, 60.36; H, 5.10; N, 2.35.
13[(3,4,5-Trimethoxyphenyl)methyl]Berberine (Compound 15)
Brown solid; yield: 50%; 1H-RMN (DMSO-d6) δ ppm: 10.03 (1H, s, H-8), 8.13 (1H, d, J = 9.3 Hz, H-11), 7.88 (1H, d, J = 9.3 Hz, H-12), 7.17 * (1H, brs, H-4), 7.15 * (1H,brs, H-1), 6.47 (2H, s, H-3′, H-7’), 6.11 (2H, s, -OCH2O-),4.89 (2H, brs, H-6), 4.69 (2H, s, H-1′), 4.14 (3H, s, OCH3-9), 4.04 (3H, s, OCH3-10), 3.65 (9H, brs, OCH3-4′, OCH3-5′, OCH3-6′), 3.17 (2H, brt, J = 4.8 Hz, H-5). 13C-RMN (DMSO-d6) δ ppm: 153.22 (C-4′), 153.22 (C-6′), 150.05 (C-10), 149.12 (C-3), 146.38 (C-2), 145.35 (C8), 144.08 (C-9), 137.14 (C-13a), 136.27 (C-5′), 134.68 (C-2′), 133.91 (C-4a), 132.86 (C-12a), 130.07 (C-13), 126.05 (C-11), 121.54 (C-12), 121.22 (C-8a), 120.08 (C-13b), 108.35 * (C-1), 108.33 * (C-4), 105.69 (C-3′), 105.69 (C-7′), 101.99 (-OCH2O-),61.97 (OCH3-C-9), 59.96 (OCH3-C-5′), 56.93 (C-6), 56.87 (OCH3-C-10), 56.10 (OCH3-C-4′), 56.10 (OCH3-C-6′), 35.55 (C-1′), 27.23(C-5).). * may be reverse. Anal. calcd. for C30H30BrNO7: C, 60.41; H, 5.07; N, 2.35. Found: C, 60.21; H, 5.02; N, 2.33.
4-[2-(Berberin-13-yl) acetyl]-2,6-dimethoxyphenyl Benzoate (Compound 16)
Yellow solid; yield: 16%; 1H-RMN (CDCl3) δ ppm: 10.03 (1H, s, H-8), 8.19(1H, d, J = 9.5 Hz, H-11), 8.15 (2H, m, H-3″), 7.82 (1H, d, J = 9.5 Hz, H-12), 7.80 (1H, m, H-5″), 7.65 (2H, m, H-4 ″), 7.64 (2H, s, H-4’), 7.19 (1H, s, H-4), 6.89 (1H, s, H-1), 6.11 (2H, s, -OCH2O-), 5.37 (2H, brs, H-6), 4.90 (2H, br s, H-1′), 4.14 (3H, s, OCH3-9), 4.07 (3H, s, OCH3-10), 3.90 (3H, s, OCH3-5’), 3.15 (3H, t, J = 5.6 Hz, H-5). 13C-RMN (CDCl3) δ ppm: 196.91 (C-2’), 163.24 (C-1″), 152.24 (C-5′), 153.31 (C-10), 149.44 (C-3), 146.79 (C-2), 145.69 (C-8), 144.20 (C-9), 134.35 (C-4a), 133.99 (C-5″), 133.59 (C-12a), 132.86 * (C-6′), 132.84 * (C-3′), 129.90 (C-3″), 129.14 (C-4″), 128.02 (C-13), 127.66 (C-2″), 126.28 (C-11), 121.52 (C-12), 120.90 (C-8a), 119.93 (C-13b), 108.52 (C-4), 107.91 (C-1), 105.74 (C-4’), 102.13 (-OCH2O-), 62.07 (OCH3-C-9), 57.02 (OCH3-C-10), 56.78 (C-6), 56.50 (OCH3-C-5’), 27.24 (C-5). * may be reverse. Anal. calcd. for C37H32BrNO9: C, 62.19; H, 4.51; N, 1.96. Found: C, 62.20; H, 4.48; N, 2.00.
4-[2-(Berberin-13-yl)acetyl]-3-(benzoyloxy)phenyl Benzoate (Compound 17)
Yellow solid; yield: 55%; 1H-RMN (DMSO-d6) δ ppm: 9.97 (1H, s, H-8), 8.52 (1H, d, J = 9.4 Hz, H-4’), 8.03 (4H, m, H-3″), 8.20 (4H, m, H-3″), 8.13(1H, d, J = 9.4 Hz, H-11), 7.89 (1H, d, J = 9.4 Hz, H-12), 7.80 (2H, m, H-5″), 7.66 (4H, m, H-4″), 7.62 (1H, brs, H-7′), 7.61 (1H, dd, J = 9.4 Hz, J = 2.0 Hz, H-5′), 7.15 (1H, s, H-4), 6.86 (1H, s, H-1), 6.16 (2H, s, -OCH2O-), 5.25 (2H, s, H-1′), 4.82 (2H, brs, H-6), 4.10 (3H, s, OCH3-9), 4.06 (3H, s, OCH3-10), 3.08 (3H, t, J = 4.9 Hz, H-5). 13C-RMN (DMSO-d6) δ ppm: 196.23 (C-2’), 164.18, 163.98 (C-1″), 154.74 (C-6′), 150.27 (C-10), 149.44 (C-3), 146.91 (C-2), 145.56 (C-8), 144.13 (C-9), 137.06 (C-13a), 134.43 (C-4a), 134.10, 134.07 (C-5″), 132.77 (C-12a), 132.19 (C-4′), 129.98,129.76 (C-4″), 129.05, 128.85 (C-3″), 128.42, 128.34 (C-2″), 127.08 (C-13), 126.97 (C-3′), 126.09 (C-11), 121.52 (C-12), 120.87 (C-8a), 120.40 (C-5′), 119.78 (C13b), 118.62 (C-7′), 108.50 (C-1), 107.89 (C-4), 102.20 (-OCH2O-), 62.02 (OCH3-C-9), 57.04(OCH3-C-10), 56.67 (C-6), 43.88 (C-1′), 27.24 (C-5). Anal. calcd. for C42H32BrNO9: C, 65.12; H, 4.16; N, 1.81. Found: C, 65.10; H, 4.21; N, 1.80.
8-{4-[(Berberin-13-yl)methyl]phenoxy}-2H-chromen-2-one (Compound 18)
Yellow solid; yield: 12%; 1H-RMN (DMSO-d6) δ ppm: 10.06 (1H, s, H-8), 8.09 (1H, d, J = 9.5 Hz, H-12), 8.00 (1H, d, J = 9.5Hz, H3″), 7.78(1H, d, J = 9.5 Hz, H-11), 7.65 (1H, d, J = 8.8Hz, H-4″), 7.47 (2H, d, J = 8.3Hz, H3’), 7.21 (2H, d, J = 8.3Hz, H-4’), 7.17 (1H, s, H-4), 7.08 (1H, d, J = 2.5, H-7″), 7.02 (1H, dd, J = 2.5Hz, J = 8.8Hz, H-5″), 6.95 (1H, s, H-1), 6.30 (1H, d, J = 9.5, H-2″), 6.07 (2H, s, -OCH2O-), 5.20 (1H, s, H-6’), 4.88 (2H, brs, H-6), 4.76 (2H, s, H-1′), 4.12 (3H, s, OCH3-9), 4.02 (3H, s, OCH3-10), 3.16 (3H, t, J = 5.5 Hz, H-5). 13C-RMN (DMSO-d6) δ ppm: 161.44 (C-6″), 160.26 (C-1″), 155.32 (C-8″), 152.22 (C-10), 149.21 (C-3), 146.39 (C-2), 145.50 (C-8), 144.31 (C-3″),144.23 (C-9), 139.12 (C-2’), 137.17 (C-13a), 137.76 (C-5′), 134.06 (C-4a), 132.69 (C-12a), 129.89 (C-13), 129.89 (C-4″), 129.54 (C-3’), 128.84 (C-4’), 126.17 (C-11), 121.67 (C-12), 121.26 (C-8a), 119.98 (C-13b), 112.96 (C-5″), 112.63 (C-2″), 112.53 (C-9″), 108.51 (C-4), 108.08 (C-1), 102.06 (-OCH2O-),101.56 (C-7″), 69.62 (C-6’), 62.07 (OCH3-C-9), 56.95 (C-6), 56.90 (OCH3-C-10), 35.28 (C-1’), 27,26 (C-5). Anal. calcd. for C37H30BrNO7: C, 65.30; H, 4.44; N, 2.06. Found: C, 65.25; H, 4.48; N, 2.02.
9-{[4-(1,3-Benzothiazol-2-yl)phenyl]methyl}berberine (Compound 19)
Red solid; yield: 20%; 1H-RMN (DMSO-d6) δ ppm: 10.06 (1H, s, H-8), 8.13 (1H, brd, J = 7.8, H-7″), 8.11 (1H, d, J = 9.4 Hz, H-11), 8.09 (2H, d, J = 8.1 Hz, H-4’), 8.04 (1H, brd, J = 7.8 Hz, H-4″), 7.81 (1H, d, J = 9.4 Hz, H-12), 7.55 (1H, brt, J = 7.8 Hz, H-5″), 7.47(1H, brt, J = 7.8Hz, H-6″), 7.39 (2H, d, J = 8.1 Hz, H3’), 7.18 (1H, s, H-4), 6.99 (1H, s, H-1), 6.08 (2H, s, -OCH2O-), 4.90 (2H, brs, H-6), 4.86 (2H, s, H-1′), 4.14 (3H, s, OCH3-9), 4.03 (3H, s, OCH3-10), 3.19 (3H, t, J = 5.3 Hz, H-5). 13C-RMN (DMSO-d6) δ ppm: 166.70 (C-2″), 153.50 (C-3a″), 150.19 (C-10), 149.23 (C-3), 146.40 (C-2), 145.54 (C8), 144.29 (C-9), 143.73 (C-2′), 137.27 (C-13a), 134.40 (C-7a″), 134.10 (C4a), 132.63 (C12a), 131.43 (C-5′), 129.45 (C-13), 129.01 (C-3′), 127.81 (C-4′),126.62(C-5″), 126.25 (C-11), 125.51 (C-6″), 122.77 (C-4″), 122.29 (C-7″), 121.54 (C-12), 121.22 (C-8a), 119.88 (C-13b), 108.46 (C-1), 108.11 (C-4), 102.02 (-OCH2O-),62.01 (OCH3-C-9), 56.89 (C-6), 56.89 (OCH3-C-10), 35.49 (C-1′), 27.22 (C-5). Anal. calcd. for C34H27BrN2O4S: C, 63.85; H, 4.26; N, 4.38. Found: C, 63.75; H, 4.12; N, 4.40.
9-{[4-(1,3,4-Oxadiazol-2-yl)phenyl]methyl}berberine (Compound 20)
Yellow solid; yield: 57%;
1H-RMN (DMSO-d
6) δ ppm: 10.06 (1H, s, H-8), 9.33 (1H, s, H-2″), 8.09 (1H, d,
J = 9.5 Hz, H-11), 8.02(2H, d,
J = 8.3 Hz, H4′), 7.79 (1H, d,
J =9.5 Hz, H-12), 7.42(2H,
J = 8.3 Hz, H3′), 7.18 (1H, s, H-4), 6.94 (1H,s, H-1), 6.08 (2H, s, -OCH
2O- [
2,
3]), 4.90 (2H, brs, H-6), 4.87 (2H, brs, H-1′), 4.14 (3H, s, OCH
3-9), 4.03 (3H, s, OCH
3-10), 3.18 (2H, t,
J = 5.2 Hz, H-5).
13C-RMN (DMSO-d
6) δ ppm: 163.35 (C-1″), 154.47 (C-2″), 150.22 (C-10), 149.3 (C-3), 146.5 (C-2), 145.61 (C8), 144.35 (C-9), 143.52 (C-2′), 137.92 (C-5′), 137.34 (C-13a), 134.11 (C-4a), 132.63 (C-12a), 129.34 (C-13), 129.11 (C-3′), 127.40 (C-4′), 126.28 (C-11), 121.79 (C-12), 121.51 (C-8a), 119.89 (C-13b), 108.50 (C-4), 108.11 (C-1), 102.07 (-OCH
2O- [
2,
3]), 62.55 (OCH
3-C-9), 57.44 (C-6), 57.44 (OCH
3-C-10), 36.08 (C-1′), 27.27(C-5). Anal. calcd. for C
29H
24BrN
3O
5: C, 60.64; H, 4.21; N, 7.32. Found: C, 60.51; H, 4.25; N, 7.29.
N-({4-[(Berberin-13-yl)methyl]phenyl}methylidene)hydroxylamine (Compound 21)
Compound 12 (0.4 g, 0.74 mmol), hydroxylamine hydrochloride (0.16 g, 2.2 mmol, 3 eq) in EtOH/H2O (10/2 mL) was added in sodium acetate (0.24 g, 3 mmol, 4 eq). The mixture was stirred at 60 °C for 1 h, poured onto water (50 mL). The solution was extracted with ether (3 × 20 mL) and the organic phase was removed under vacuum and reprecipitate in CHCl3. The yellow precipitate was collected washed with CHCl3 to give pure compound 21. Yellow solid; yield: 10%; 1H-RMN (DMSO-d6) δ ppm: 11.21 (1H, s, OH), 10.02 (1H, s, H-8), 8.09 (1H, d, J = 9.4 Hz, H-11), 7.78 (1H, d, J = 9.4 Hz, H-12), 8.12 (1H, s, H-6′), 7.57 (2H, d, J = 8.1 Hz, H-4′),7.21 (2H, d, J = 8.1 Hz, H-3′), 7.16 (1H, s, H-1), 6.96 (1H, s, H-4), 6.08 (2H, s, -OCH2O-), 4.88 (2H, brs, H-6), 4.76 (2H, s, H-1′), 4.13 (3H, s, OCH3-9), 4.02 (3H, s, OCH3-10), 3.17 (2H, t, J = 5.7 Hz, H-5). 13C-RMN (DMSO-d6) δ ppm: 150.19 (C-10), 149.22 (C-3), 147.65 (C-6′), 146.40 (C-2), 145.49 (C-8), 144.30(C-9), 140.32 (C-2′), 137.22 (C-13a), 134.04 (C-5′), 133.12 (C-4a), 131.67 (C-12a), 129.68 (C-13), 128.41 (C-4′), 127.04 (C-3′), 126.21 (C-12), 121.59 (C-11), 121.27 (C-8a), 119.97 (C-13b), 108.49 (C-4), 108.15 (C-1), 102.05 (-OCH2O-), 62.05 (OCH3-C-9), 57.00 (OCH3-C-10) 56.93 (C-6), 35.43 (C-1′), 27.25 (C-5). Anal. calcd. for C27H25BrN2O5: C, 60.34; H, 4.69; N, 5.21. Found: C, 60.29; H, 4.71; N, 5.23.
(2E)-3-{4-[(Berberin-13-yl)methyl]phenyl}prop-2-enoic Acid (Compound 22)
Compound 12 (1 g, 1.8 mmol), malonic acid (0.4 g, 4.1 mmol, 2.2 eq) in MeOH/ethyl acetate (5/2 mL) was added in acetic acid (15 mL). Then was added piperidine (0.5 mL, 4 mmol, 2.7 eq) and the reaction mixture was submitted to microwave irradiation in a CEM microwave apparatus (Power time, Acetic acid, Tmax: 110 °C, 30 min). The solution was poured onto ice/water (200 mL) and the precipitate was collected by sucion and washed with excess water to give 22 as a reddish powder. Red solid; yield: 50%; 1H-RMN (DMSO-d6) δ ppm: 10.04 (1H, s, H-8), 8.09 (1H, d, J = 9.4 Hz, H-11), 7.76 (1H, d, J = 9.4 Hz, H-12), 7.68 (2H, d, J = 8.2 Hz, H-4′), 7.58 (1H, d, J = 16.0 Hz, H-6′), 7.22 (2H, d, J = 8.2 Hz, H-3′), 7.17 (1H, s, H-1), 6.93 (1H, s, H-4), 6.53 (1H, d, J = 16.0 Hz, H-7′), 6.08 (2H, s, -OCH2O-), 4.87 (2H, brs, H-6), 4.77 (2H, s, H-1′), 4.12 (3H, s, OCH3-9), 4.02 (3H, s, OCH3-10), 3.16 (2H, t, J = 5.7 Hz, H-5). 13C-RMN (DMSO-d6) δ ppm: 167.78 (C-8′), 150.18 (C-10), 149.19 (C-3), 146.38 (C-2), 145.49 (C-8), 144.26 (C-9), 142.39 (C-6′), 141.13 (C-2′), 137.20 (C-13a), 134.03 (C-5′), 133.12 (C-4a), 132.65 (C-12a), 129.68 (C-13), 128.71 (C-4′), 128.55 (C-3′), 126.18 (C-12), 121.58 (C-11), 121.24 (C-8a), 120.40 (C-7′), 119.95 (C-13b),), 108.47 (C-4), 108.12 (C-1), 102.04 (-OCH2O-), 62.04 (OCH3-C-9), 56.95 (C-6), 56.90 (OCH3-C-10), 27.24(C-5). Anal. calcd. for C30H26BrNO6: C, 62.51; H, 4.55; N, 2.43. Found: C, 62.41; H, 4.58; N, 2.47.
(2E)-3-{4-[(Berberin-13-yl)methyl]phenyl}-N-[2-(4-sulfamoylphenyl)ethyl]prop-2-enamide (Compound 23)
Compound 22 (0.2 g, 0.36 mmol), 1-(3-dimethyl-aminopropyl)-3-ethylcarbodiimide hydrochloride: EDCI (0.06 g, 0,4 mmol, 1,1 eq) and 1-hydroxybenzotriazole: HOBT (0.05 g, 0.4 mmol, 1.1 eq) in DMF (10 mL) were stirred for 2.5 h at r.t., then was added 4-(2-aminoethyl) benzene-sulfonamide (0.1 g, 0.45 mmol, 1.3 eq) under stirring for 12 h. The reaction mixture was poured onto ice/water (100 mL), the precipitate was filtered off and washed with excess water to give 23 as a dark green powder. Green solid; yield: 16%; 1H-RMN (DMSO-d6) δ ppm: 10.04 (1H, s, H-8), 8.21 (1H, t, J = 5.7 Hz, NH), 8.09 (1H, d, J = 9.3 Hz, H-11), 7.77 (1H, d, J = 9.3 Hz, H-12), 7.73 (2H, d, J = 8.2 Hz, H5″), 7.55 (2H, d, J = 8.0 Hz, H-4′), 7.41 (2H, d, J = 8.2 Hz, H-4″), 7.40 (1H, d, J = 15.8 Hz, H-6′), 7.30 (2H, s, NH2), 7.21 (2H, d, J = 8.0 Hz, H-3′), 7.17 (1H, s, H-4), 6.94 (1H, s, H-1), 6.57 (1H, d, J = 15.8 Hz, H-7′), 6.08 (2H, s, -OCH2O-), 4.87 (2H, brs, H-6), 4.76 (2H, s, H-1′), 4.12 (3H, s, OCH3-9), 4.02 (3H, s, OCH3-10), 3.44 (2H, m, H-1″), 3.16 (2H, t, J = 5.7 Hz, H-5), 2.85 (2H,t, J = 7.0 Hz, H-2″). 13C-RMN (DMSO-d6) δ ppm: 164.9 (C-8′), 150.2 (C-10), 149.2 (C-3), 146.4 (C-2), 145.5 (C-8), 144.2 (C-9), 143.6 (C-6′’), 142.0 (C-2′), 140.54 (C-6′), 138.0 (C-3″), 137.2 (C-13a), 134.0 (C-4a), 133.5 (C5′), 132.7 (C-12a), 129.7 (C-13), 129.1 (C-4″), 128.6 (C-3′), 128.2 (C-4′), 126.2 (C-11), 125.6 (C5″), 122.2 (C-7′), 121.6 (C-12), 121.2 (C-8a), 119.9 (C-13b), 108.5 (C-4), 108.1 (C-1), 102.3 (-OCH2O-), 62.0 (OCH3-C-9), 56.9 (C-6), 56.9 (OCH3-C-10), 35.4 (C-1′), 34.7 (C-2″), 27.2 (C-5). Anal. calcd. for C38H36BrN3O7S: C, 60.16; H, 4.78; N, 5.54. Found: C, 60.21; H, 4.81; N, 5.44.
N-{3-[(2E)-3-{4-[(Berberin-13-yl)methyl]phenyl}prop-2-enoyl]-4-hydroxyphenyl}acetamide (Compound 24)
Compound 12 (0.44 g, 0.8 mmol, 0.7 eq) and N-(3-acetyl-4-hydroxyphenyl)acetamide (0.2 g, 1.1 mmol, 1.2 eq) were solubilized in methanol (20 mL). To this solution was added LiOH (0.15 g, 6 mmol, 8 eq.) and the solution was submitted to microwave irradiation using a CEM apparatus (mode open vessel, new method, mode standard, hold time: 2 min., power: 300 W, run time: 20 min.). At the end of the irradiation the solvent was removed under vacuum to give a red mixture. Addition of 1N HCl (50 mL) under ice give a yellow precipitate that was filtered with ether to give a yellow solid of 24. Yellow solid; yield: 40%; 1H-RMN (DMSO-d6) δ ppm: 11,62 (1H, s, OH-2″), 10.05 (1H, s, H-8), 9.92 (1H, s, NH), 8.18 (1H, d, J = 2.5 Hz, H-6″), 8.10 (1H, d, J = 9.4 Hz, H-11), 7.80 (2H, d, J = 7.2 Hz, H-3′), 7.79 (1H, d, J = 9.4 Hz, H-12), 7.77 (1H, d, J = 15.6 Hz, H-6′), 7.73 (1H, d, J = 15.6 Hz, H-7′), 7.65 (1H, dd, J = 8.8 Hz, J = 2.5 Hz, H-4″), 7.28 (2H, d, J = 7.2 Hz, H-4′), 7.18 (1H, s, H-4), 6.96 (1H, s, H-1), 6.96 (1H, d, J = 8.8 Hz, H-3″), 6.09 (2H, s, -OCH2O-), 4.88 (2H, brs, H-6), 4.81 (2H, s, H-1′), 4.13 (3H, s, OCH3-9), 4.03 (3H, s, OCH3-10), 3.17 (2H, t, J = 5.4 Hz, H-5), 2.02 (3H,s, CH3-NHCOCH3). 13C-RMN (DMSO-d6) δ ppm: 193.2 (C-8′),168.2 (CO-NHCOCH3), 156.9 (C-2″), 150.6 (C-10), 149.5 (C-3), 146.2 (C-2), 145.2 (C-8), 144.5 (C-9), 143.6 (C-6′), 142.5 (C-2′), 142.4 (C-5′), 137.5 (C-13a), 134.3 (C-4a), 132.9 (C-12a), 129.9 (C-13), 129.1 (C-3′), 128.4 (C-4′), 127.6 (C-4″), 125.9 (C-11), 122.7 (C-7′), 121.6 (C-8a),121.6 (C-1″), 121.4 (C-5″), 121.3 (C-12), 120.6 (C-6″), 120.2 (C-13b), 117.4 (C-3″), 108.2 (C-4), 107.9 (C-1), 101.8 (-OCH2O-), 61.7 (OCH3-C-9), 56.6 (C-6), 56.6 (OCH3-C-10), 35.2 (C-1′), 27.0 (C-5), 23.4 (C-CH3 NHCOCH3). Anal. calcd. for C38H33BrN2O7: C, 64.32; H, 4.69; N, 3.95. Found: C, 64.30; H, 4.671; N, 4.00.
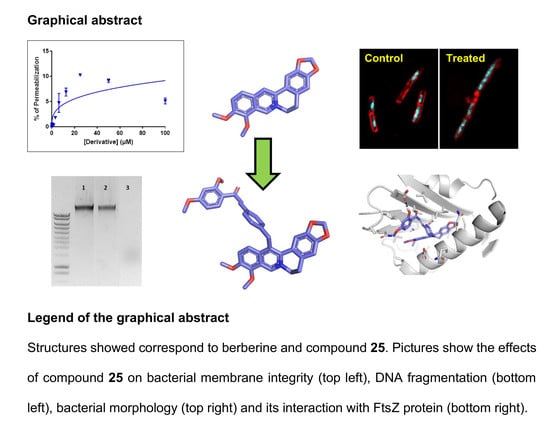
(2E)-3-{4-[(Berberin-13-yl)methyl]phenyl}-1-(2-hydroxy-4-methoxyphenyl)prop-2-en-1-one (Compound 25)
Using the same protocol as for 24 with 2-hydroxy-4-methoxyacetophenone gives 25. Green solid; yield: 78%; 1H-RMN (DMSO-d6) δ ppm: 10.08 (1H, s, H-8), 8.27 (1H, d, J = 9.1 Hz, H-6″), 8.10 (1H, d, J = 9.4 Hz, H-11), 8.01 (2H, d, J = 15.5 Hz, H-a),7.92 (2H, d, J = 8.3Hz, H-4’), 7.80 (2H, d, J = 15.5 Hz, H-b), 7.78 (1H, d, J = 9.4 Hz, H-12), 7.28 (2H, d, J = 8.3Hz, H3’), 7.18 (1H, s, H-4), 6.96 (1H, s, H-1), 6.56 (1H, dd, J = 2.5Hz, J = 9.1Hz, H-5″), 6.53 (1H, d, J =2.5, H-3″), 6.08 (2H, s, -OCH2O-), 4.90 (2H, brs, H-1′), 4.81 (2H, brs, H-6), 4.13 (3H, s, OCH3-9), 4.02 (3H, s, OCH3-10), 3.85 (3H, s, OCH3-4″),3.17 (3H, t, J =5.7 Hz, H-5). 13C-RMN (DMSO-d6) δ ppm: 191.82 (C-b’), 166.04 (C-2″), 165.68 (C-4″), 150.24 (C-10), 149.28 (C-3), 146.46 (C-2), 145.59 (C-8), 144.30 (C-9), 143.52 (C-b, 142.19 (C-2’), 137.25 (C-13a), 134.10 (C-4a), 133.59 (C-5′), 132.78 (C-6″), 132,66 (C-12a), 129.83 (C-4’), 129.63 (C-13), 128.67 (C-3’), 126.23 (C-11), 121.64 (C-12), 121,29 (C-8a), 121.26 (C-a), 119,97 (C-13b), 113.91 (C-1″), 108.54 (C-4), 108.14 (C-1), 107.49 (C-5″), 102.11 (-OCH2O-), 100.95 (C-3″),62.10 (OCH3-C-9), 56,98 (C-6), 56,93 (OCH3-C-10), 55.81 (OCH3-4″), 35.59 (C-1’), 27,29 (C-5). Anal. calcd. for C37H32BrNO7: C, 65.11; H, 4.73; N, 2.05. Found: C, 65.02; H, 4.76; N, 2.01.
2-{4-[(Berberin-13-yl)methyl]phenyl}-7-methoxy-4H-chromen-4-one (Compound 26)
Compound 25 (0.17 g, 0.24 mmol) was solubilized in a solution of DMSO (20 mL) and Iodine (17 mg, 10% weight). The solution was submitted to microwave irradiation (CEM, Power time, Tmax: 140 °C, 30 min). The solution was poured on ice/1N HCl (100 mL) to give a yellow precipitate of 26 which was washed with excess water. Yellow solid; yield: 18%; 1H-RMN (DMSO-d6) δ ppm: 10.06 (1H, s, H-8), 8.10 (1H, d, J = 9.3 Hz, H-11), 8.06 (2H, d, J = 8.3Hz, H-4’), 7.95 (1H, d, J = 8.5Hz, H-5″), 7.79 (1H, d, J = 9.3 Hz, H-12), 7.38 (2H, d, J = 8.3Hz, H3’), 7.29 (1H, d, J = 2.2Hz, H8″), 7.18 (1H, s, H-4), 7.08 (1H, dd, J = 8.5Hz, J = 2.2Hz, H-6″), 6.97 (1H, s, H3″), 6.95 (1H, s, H-1), 6.08 (2H, s, -OCH2O-), 4.90 (2H, brs, H-6), 4.85 (2H, s, H-1′), 4.12 (3H, s, OCH3-9), 4.02 (3H, s, OCH3-10), 3.92 (3H, s, OCH3-7″), 3.18 (3H, t, J = 5.1 Hz, H-5). 13C-RMN (DMSO-d6) δ ppm: 163.8 (C-7″), 161.7 (C-2″), 157.5 (C-9″), 150.3 (C-10), 149.3 (C-3), 146.5 (C-2), 145.6 (C8), 144.2 (C-9), 143.5 (C-2′), 137.4 (C-13a), 134.2 (C4a), 132.6 (C12a), 129.5 (C-5′), 128.8 (C-3′), 127.1 (C-4′), 126.4 (C-5″), 126.1 (C-11), 121.6 (C-12), 121.3 (C-8a), 119.9 (C-13b), 117.1 (C-10″), 114.7 (C-6″), 108.6 (C-4), 107.9 (C-1), 107.6 (C-3″), 102.1 (-OCH2O-), 101.1 (C-8″), 62.2 (OCH3-C-9), 57.1 (C-6), 56.9 (OCH3-C-10), 56.2 (OCH3-C-7″), 35.7 (C-1′), 27.5 (C-5). * (C13) et (C-4″) not observed. Anal. calcd. for C37H30BrNO7: C, 65.30; H, 4.44; N, 2.06. Found: C, 65.20; H, 4.49; N, 1.99.