Strategies for Preventing and Treating Oral Mucosal Infections Associated with Removable Dentures: A Scoping Review
Abstract
:1. Introduction
2. Results
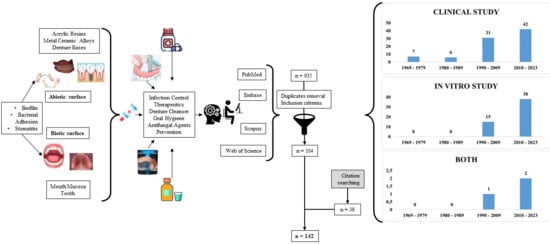
2.1. Search Results
2.2. Literature Review
2.2.1. Main Inflammations/Infections Related to the Use of removable Prostheses: Etiology and Diagnosis
2.2.2. Prevention and Treatment of Oral Diseases Related to the Use of Dentures
- Studies for topical and systemic treatments using antimicrobial agents, either alone or associated with local interventions (Table 1);
- Studies for prevention and local treatment using mechanical, chemical, physical, and associated hygiene methods (Table 2);
- Studies for prevention and local treatment using material modifications (Table 3).
3. Materials and Methods
3.1. Search Strategy
3.2. Eligibility Criteria
3.3. Study Selection
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Satishkumar, C.S.; Nair, S.J.; Joseph, A.M.; Suresh, S.; Muthupandian, I.; Kumaresan, S.; Ashekhi, A.; Nadeem, G. Relationship between perceived chewing ability, oral health related quality of life and depressive symptoms among completely edentulous individuals. Indian J. Dent. Res. 2021, 32, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Tarbet, W.J. Denture plaque: Quiet destroyer. J. Prosthet. Dent. 1982, 48, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Peltola, M.K.; Raustia, A.M.; Salonen, M.A. Effect of complete denture renewal on oral health—A survey of 42 patients. J. Oral Rehabil. 1997, 24, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Budtz-Jørgensen, E.; Mojon, P.; Rentsch, A.; Deslauriers, N. Effects of an oral health program on the occurrence of oral candidosis in a long-term care facility. Community Dent. Oral Epidemiol. 2000, 28, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Pires, F.R.; Santos, E.B.; Bonan, P.R.; De Almeida, O.P.; Lopes, M.A. Denture stomatitis and salivary Candida in Brazilian edentulous patients. J. Oral Rehabil. 2002, 29, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Jeganathan, S.; Payne, J.A.; Thean, H.P. Denture stomatitis in an elderly edentulous Asian population. J. Oral Rehabil. 1997, 24, 468–472. [Google Scholar] [CrossRef]
- Kagermeier-Callaway, A.S.; Willershausen, B.; Frank, T.; Stender, E. In vitro colonisation of acrylic resin denture base materials by Streptococcus oralis and Actinomyces viscosus. Int. Dent. J. 2000, 50, 79–85. [Google Scholar] [CrossRef]
- Paranhos, H.d.F.O.; da Silva, C.H.; Venezian, G.C.; Macedo, L.D.; de Souza, R.F. Distribution of biofilm on internal and external surfaces of upper complete dentures: The effect of hygiene instruction. Gerodontology 2007, 24, 162–168. [Google Scholar] [CrossRef]
- Ercalik-Yalcinkaya, S.; Özcan, M. Association between Oral Mucosal Lesions and Hygiene Habits in a Population of Removable Prosthesis Wearers. J. Prosthodont. 2015, 24, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Hoad-Reddick, G.; Grant, A.A.; Griffiths, C.S. Investigation into the cleanliness of dentures in an elderly population. J. Prosthet. Dent. 1990, 64, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Kulak-Ozkan, Y.; Kazazoglu, E.; Arikan, A. Oral hygiene habits, denture cleanliness, presence of yeasts and stomatitis in elderly people. J. Oral Rehabil. 2002, 29, 300–304. [Google Scholar] [CrossRef]
- Kanli, A.; Demirel, F.; Sezgin, Y. Oral candidosis, denture cleanliness and hygiene habits in an elderly population. Aging Clin. Exp. Res. 2005, 17, 502–507. [Google Scholar] [CrossRef]
- Kokubu, K.; Senpuku, H.; Tada, A.; Saotome, Y.; Uematsu, H. Impact of routine oral care on opportunistic pathogens in the institutionalized elderly. J. Med. Dent. Sci. 2008, 55, 7–13. [Google Scholar]
- Evren, B.A.; Uludamar, A.; Işeri, U.; Ozkan, Y.K. The association between socioeconomic status, oral hygiene practice, denture stomatitis and oral status in elderly people living different residential homes. Arch. Gerontol. Geriatr. 2011, 53, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Nicol, R.; Petrina Sweeney, M.; McHugh, S.; Bagg, J. Effectiveness of health care worker training on the oral health of elderly residents of nursing homes. Community Dent. Oral Epidemiol. 2005, 33, 115–124. [Google Scholar] [CrossRef]
- Nevalainen, M.J.; Närhi, T.O.; Ainamo, A. Oral mucosal lesions and oral hygiene habits in the home-living elderly. J. Oral Rehabil. 1997, 24, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Nikawa, H.; Hamada, T.; Yamamoto, T. Denture plaque—Past and recent concerns. J. Dent. 1998, 26, 299–304. [Google Scholar] [CrossRef]
- Nishi, Y.; Seto, K.; Kamashita, Y.; Take, C.; Kurono, A.; Nagaoka, E. Examination of denture-cleaning methods based on the quantity of microorganisms adhering to a denture. Gerodontology 2012, 29, e259–e266. [Google Scholar] [CrossRef]
- Andonissamy, L.; Karthigeyan, S.; Ali, S.A.; Felix, J.W. Effect of Chemical Denture Disinfectants and Tree Extracts on Biofilm-forming Staphylococcus aureus and Viridans Streptococcus Species Isolated from Complete Denture. J. Contemp. Dent. Pract. 2019, 20, 1307–1314. [Google Scholar] [PubMed]
- Leoney, A.; Karthigeyan, S.; Asharaf, A.S.; Felix, A.J.W. Detection and Categorization of Biofilm-forming Staphylococcus aureus, Viridans streptococcus, Klebsiella pneumoniae, and Escherichia coli Isolated from Complete Denture Patients and Visualization Using Scanning Electron Microscopy. J. Int. Soc. Prev. Community Dent. 2020, 10, 627–633. [Google Scholar] [CrossRef]
- Clemente, L.M.; Ribeiro, A.B.; Fortes, C.V.; Ribeiro, A.B.; Oliveira, V.C.; Macedo, A.P.; Salgado, H.C.; da Silva, C.H.L. Risk factors and immunological biomarkers in denture stomatitis: An observational cross-sectional study. Arch. Oral Biol. 2023, 155, 105799. [Google Scholar] [CrossRef]
- Cruz, P.C.; Andrade, I.M.; Peracini, A.; Souza-Gugelmin, M.C.; Silva-Lovato, C.H.; de Souza, R.F.; Paranhos, H.d.F.O. The effectiveness of chemical denture cleansers and ultrasonic device in biofilm removal from complete dentures. J. Appl. Oral Sci. 2011, 19, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Rossato, M.B.; Unfer, B.; May, L.G.; Braun, K.O. Analysis of the effectiveness of different hygiene procedures used in dental prostheses. Oral Health Prev. Dent. 2011, 9, 221–227. [Google Scholar]
- Duyck, J.; Vandamme, K.; Krausch-Hofmann, S.; Boon, L.; De Keersmaecker, K.; Jalon, E.; Twughels, W. Impact of Denture Cleaning Method and Overnight Storage Condition on Denture Biofilm Mass and Composition: A Cross-Over Randomized Clinical Trial. PLoS ONE 2016, 11, e0145837. [Google Scholar] [CrossRef] [PubMed]
- Andrucioli, M.C.D.; De Macedo, L.D.; Panzeri, H.; Lara, E.H.G.; Paranhos, H.F.O. Comparison of Two Cleansing Pastes for the Removal of Biofilm from Dentures and Palatal Lesions in Patients with Atrophic Chronic Candidiasis. Braz. Dent. J. 2004, 15, 220–224. [Google Scholar] [CrossRef]
- Boscato, N.; Radavelli, A.; Faccio, D.; Loguercio, A.D. Biofilm formation of Candida albicans on the surface of a soft denture-lining material. Gerodontology 2009, 26, 210–213. [Google Scholar] [CrossRef]
- Wiatrak, K.; Morawiec, T.; Rój, R.; Mertas, A.; Machorowska-Pieniażek, A.; Kownacki, P.; Tanasiewicz, M.; Skucha-Nowak, M.; Baron, S.; Piekarz, T.; et al. Oral Health of Patients Treated with Acrylic Partial Dentures Using a Toothpaste Containing Bee Product. Evid. Based Complement. Altern. Med. 2017, 2017, 4034179. [Google Scholar] [CrossRef] [PubMed]
- Panzeri, H.; Lara, E.H.; Paranhos, H.d.F.O.; Lovato da Silva, C.H.; de Souza, R.F.; de Souza Gugelmin, M.C.; Tirapelli, C.; Cruz, P.C.; de Andrade, I.M. In vitro and clinical evaluation of specific dentifrices for complete denture hygiene. Gerodontology 2009, 26, 26–33. [Google Scholar] [CrossRef]
- Berteretche, M.V.; Mastari, F.; Nicolas, E.; Hüe, O. The needs of denture-brushing in geriatrics: Clinical aspects and perspectives. Gerodontology 2012, 29, e768–e771. [Google Scholar] [CrossRef]
- Bloem, T.J.; Razzoog, M.E.; Chamberlain, B.B.; Lang, B. Efficacy of tissue brushing as measured by the prosthodontic tissue index. Spec. Care Dent. 1984, 4, 70–76. [Google Scholar] [CrossRef]
- Chamberlain, B.B.; Bernier, S.H.; Bloem, T.J.; Razzoog, M.E. Denture plaque control and inflammation in the edentulous patient. J. Prosthet. Dent. 1985, 54, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Kabawat, M.; de Souza, R.F.; Badaró, M.M.; de Koninck, L.; Barbeau, J.; Rompré, P.; Emami, E. Phase 1 clinical trial on the effect of palatal brushing on denture stomatitis. Int. J. Prosthodont. 2014, 27, 311–319. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.; Chaves, C.; Rohani, K.; Bouferguene, S.; Barbeau, J.; Borie, E.; Weber, B.; Fuentes, R.; Crizostomo, L.; Silva-Lovato, C.; et al. Palatal brushing for the treatment of denture stomatitis: A multicentre randomized controlled trial. J. Prosthodont. Res. 2023, 67, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Nikawa, H.; Yamamoto, T.; Hamada, T.; Rahardjo, M.B.; Murata, H. Commercial denture cleansers—Cleansing efficacy against Candida albicans biofilm and compatibility with soft denture-lining materials. Int. J. Prosthodont. 1995, 8, 434–444. [Google Scholar]
- Keng, S.B.; Lim, M. Denture plaque distribution and the effectiveness of a perborate-containing denture cleanser. Quintessence Int. 1996, 27, 341–345. [Google Scholar]
- Budtz-Jörgensen, E. Prevention of denture plaque formation by an enzyme denture cleanser. J. Biol. Buccale 1977, 5, 239–244. [Google Scholar]
- Paranhos, H.F.; Silva-Lovato, C.H.; Souza, R.F.; Cruz, P.C.; Freitas, K.M.; Peracini, A. Effects of mechanical and chemical methods on denture biofilm accumulation. J. Oral Rehabil. 2007, 34, 606–612. [Google Scholar] [CrossRef]
- Paranhos, H.D.F.O.; Davi, L.R.; Peracini, A.; Soares, R.B.; Lovato, C.H.D.S.; Souza, R.F.D. Comparison of physical and mechanical properties of microwave-polymerized acrylic resin after disinfection in sodium hypochlorite solutions. Braz. Dent. J. 2009, 20, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Hahnel, S.; Rosentritt, M.; Bürgers, R.; Handel, G.; Lang, R. Candida albicans biofilm formation on soft denture liners and efficacy of cleaning protocols. Gerodontology 2012, 29, e383–e391. [Google Scholar] [CrossRef]
- Duyck, J.; Vandamme, K.; Muller, P.; Teughels, W. Overnight storage of removable dentures in alkaline peroxide-based tablets affects biofilm mass and composition. J. Dent. 2013, 41, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, L.E.; Alalwan, H.K.A.; Kean, R.; Calvert, G.; Nile, C.J.; Lappin, D.F.; Robertson, D.; Williams, C.; Ramage, G.; Sherry, L. Candida albicans biofilm heterogeneity does not influence denture stomatitis but strongly influences denture cleansing capacity. J. Med. Microbiol. 2017, 66, 54–60. [Google Scholar] [CrossRef]
- Neppelenbroek, K.H. The importance of daily removal of the denture biofilm for oral and systemic diseases prevention. J. Appl. Oral Sci. 2015, 23, 547–548. [Google Scholar] [CrossRef]
- Hayran, Y.; Sarikaya, I.; Aydin, A.; Tekin, Y.H. Determination of the effective anticandidal concentration of denture cleanser tablets on some denture base resins. J. Appl. Oral Sci. 2018, 26, e20170077. [Google Scholar] [CrossRef] [PubMed]
- Beyari, M.M. Tissue inflammatory response and salivary Streptococcus mutans count with three different denture cleansers. Afr. J. Microbiol. Res. 2011, 5, 965–974. [Google Scholar] [CrossRef]
- Ghazal, A.R.A.; Idris, G.; Hajeer, M.Y.; Alawer, K.; Cannon, R.D. Efficacy of removing Candida albicans from orthodontic acrylic bases: An in vitro study. BMC Oral Health 2019, 19, 71. [Google Scholar] [CrossRef] [PubMed]
- Razzaq, T.A.; Shnan, A.J.D.; Mohammed, A.B. Sterilization of Surgical Tools: Removing Bacterial Endospores with a Combination of Povidone-iodine, Chlorhexidine Gluconate, Ethanol, and Methanol. J. Pure Appl. Microbiol. 2019, 13, 2499–2506. [Google Scholar] [CrossRef]
- Ramage, G.; O’Donnell, L.; Sherry, L.; Culshaw, S.; Bagg, J.; Czesnikiewicz-Guzik, M.; Brown, C.; McKenzie, D.; Cross, L.; MacInnes, A.; et al. Impact of frequency of denture cleaning on microbial and clinical parameters—A bench to chairside approach. J. Oral Microbiol. 2018, 11, 1538437. [Google Scholar] [CrossRef]
- Vasconcelos, G.L.L.; Curylofo, P.A.; Raile, P.N.; Macedo, A.P.; Paranhos, H.F.O.; Pagnano, V.O. Effect of Alkaline Peroxides on the Surface of Cobalt Chrome Alloy: An In Vitro Study. J. Prosthodont. 2019, 28, e337–e341. [Google Scholar] [CrossRef]
- Nishi, Y.; Seto, K.; Murakami, M.; Harada, K.; Ishii, M.; Kamashita, Y.; Kawamoto, S.; Hamano, T.; Yoshimura, T.; Kurono, A.; et al. Effects of Denture Cleaning Regimens on the Quantity of Candida on Dentures: A Cross-Sectional Survey on Nursing Home Residents. Int. J. Environ. Res. Public Health 2022, 19, 15805. [Google Scholar] [CrossRef]
- Kulak, Y.; Arikan, A.; Delibalta, N. Comparison of three different treatment methods for generalized denture stomatitis. J. Prosthet. Dent. 1994, 72, 283–288. [Google Scholar] [CrossRef]
- Olsen, I. Denture stomatitis. Effects of chlorhexidine and amphotericin B on the mycotic flora. Acta Odontol. Scand. 1975, 33, 41–46. [Google Scholar] [CrossRef]
- Lamfon, H.; Al-Karaawi, Z.; McCullough, M.; Porter, S.R.; Pratten, J. Composition of in vitro denture plaque biofilms and susceptibility to antifungals. FEMS Microbiol. Lett. 2005, 242, 345–351. [Google Scholar] [CrossRef]
- Silva, M.J.; de Oliveira, D.G.; Marcillo, O.O.; Neppelenbroek, K.H.; Lara, V.S.; Porto, V.C. Effect of denture-coating composite on Candida albicans biofilm and surface degradation after disinfection protocol. Int. Dent. J. 2016, 66, 86–92. [Google Scholar] [CrossRef]
- Budtz-Jörgensen, E.; Kaaber, S. Clinical effects of glazing denture acrylic resin bases using an ultraviolet curing method. Scand. J. Dent. Res. 1986, 94, 569–574. [Google Scholar] [CrossRef]
- Monsenego, P. Presence of microorganisms on the fitting denture complete surface: Study ‘in vivo’. J. Oral Rehabil. 2000, 27, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Scotti, R.; Zanini Kantorski, K.; Scotti, N.; Monaco, C.; Valandro, L.F.; Bottino, M.A. Early biofilm colonization on polished- and glazed-zirconium ceramic surface. Preliminary results. Minerva Stomatol. 2006, 55, 493–502. [Google Scholar]
- Boscato, N.; Delavi, J.D.; Muller, L.; Pereira-Cenci, T.; Imanishi, S.W. Influence of varnish application on a tissue conditioner: Analysis of biofilm adhesion. Gerodontology 2010, 27, 207–210. [Google Scholar] [CrossRef]
- Ikeya, K.; Iwasa, F.; Inoue, Y.; Fukunishi, M.; Takahashi, N.; Ishihara, K.; Baba, K. Inhibition of denture plaque deposition on complete dentures by 2-methacryloyloxyethyl phosphorylcholine polymer coating: A clinical study. J. Prosthet. Dent. 2018, 119, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Murakami, H.; Sakuma, S.; Nakamura, K.; Ito, Y.; Hattori, M.; Asai, A.; Noguchi, T.; Maeda, H.; Kameyama, Y.; Kimura, Y.; et al. Disinfection of removable dentures using ozone. Dent. Mater. J. 1996, 15, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Namangkalakul, W.; Benjavongkulchai, S.; Pochana, T.; Promchai, A.; Satitviboon, W.; Howattanapanich, S.; Phuprasong, R.; Ungvijanpunya, N.; Supakanjanakanti, D.; Chaitrakoonthong, T.; et al. Activity of chitosan antifungal denture adhesive against common Candida species and Candida albicans adherence on denture base acrylic resin. J. Prosthet. Dent. 2020, 123, 181.e1–181.e7. [Google Scholar] [CrossRef] [PubMed]
- Nawasrah, A.; AlNimr, A.; Ali, A.A. Antifungal Effect of Henna against Candida albicans Adhered to Acrylic Resin as a Possible Method for Prevention of Denture Stomatitis. Int. J. Environ. Res. Public Health 2016, 13, 520. [Google Scholar] [CrossRef]
- Kalivradzhiyan, E.; Lesnykh, N.; Kunin, V.; Mutafyan, M. Usage of low-intensity laser radiation for the treatment of the inflammatory processes of the oral cavity mucosa after applying removable plate dentures. Adv. Laser Dent. 1995, 1984, 225–230. [Google Scholar] [CrossRef]
- Bergendal, T.; Isacsson, G. Effect of nystatin in the treatment of denture stomatitis. Scand. J. Dent. Res. 1980, 88, 446–454. [Google Scholar] [CrossRef]
- Bergendal, T. Status and treatment of denture stomatitis patients: A 1-year follow-up study. Scand. J. Dent. Res. 1982, 90, 227–238. [Google Scholar] [CrossRef]
- Santarpia, R.P.; Pollock, J.J.; Renner, R.P.; Gwinnett, A.J. In vivo antifungal efficacy of salivary histidine-rich polypeptides: Preliminary findings in a denture stomatitis model system. J. Prosthet. Dent. 1991, 66, 693–699. [Google Scholar] [CrossRef]
- Blomgren, J.; Berggren, U.; Jontell, M. Fluconazole versus nystatin in the treatment of oral candidosis. Acta Odontol. Scand. 1998, 56, 202–205. [Google Scholar] [CrossRef]
- Parvinen, T.; Kokko, J.; Yli-Urpo, A. Miconazole lacquer compared with gel in treatment of denture stomatitis. Scand. J. Dent. Res. 1994, 102, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Khozeimeh, F.; Shahtalebi, M.A.; Noori, M.; Savabi, O. Comparative evaluation of ketoconazole tablet and topical ketoconazole 2% in orabase in treatment of Candida-infected denture stomatitis. J. Contemp. Dent. Pract. 2010, 11, 017–024. [Google Scholar] [CrossRef] [PubMed]
- Villar, C.C.; Lin, A.L.; Cao, Z.; Zhao, X.R.; Wu, L.A.; Chen, S.; Sun, Y.; Yeh, C.K. Anticandidal activity and biocompatibility of a rechargeable antifungal denture material. Oral Dis. 2013, 19, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Preissner, S.; Kastner, I.; Schutte, E.; Hartwig, S.; Schmidt-Westhausen, A.M.; Paris, S.; Preissner, R.; Hertel, M. Adjuvant antifungal therapy using tissue tolerable plasma on oral mucosa and removable dentures in oral candidiasis patients: A randomised double-blinded split-mouth pilot study. Mycoses 2016, 59, 467–475. [Google Scholar] [CrossRef] [PubMed]
- De Senna, A.M.; Vieira, M.M.F.; Machado-de-Sena, R.M.; Bertolin, A.O.; Núñez, S.C.; Ribeiro, M.S. Photodynamic inactivation of Candida ssp. on denture stomatitis. A clinical trial involving palatal mucosa and prosthesis disinfection. Photodiagnosis Photodyn. Ther. 2018, 22, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Alrabiah, M.; Alsahhaf, A.; Alofi, R.S.; Al-Aali, K.A.; Abduljabbar, T.; Vohra, F. Efficacy of photodynamic therapy versus local nystatin in the treatment of denture stomatitis: A randomized clinical study. Photodiagnosis Photodyn. Ther. 2019, 28, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, L.C.; Nascente, P.S.; Ribeiro, J.S.; Guimarães, V.B.S.; Etges, A.; Lund, R.G. Sensitivity to antifungals by Candida spp. samples isolated from cases of chronic atrophic candidiasis (CAC). Braz. J. Biol. 2020, 80, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Al-Aali, K.A.; Alqahtani, A.S.; AlZaid, A.A.; Almujel, S.H.; Alsaloum, M.; Alanazi, K.K. Efficacy of adjunct photodynamic therapy on Candida growth and oral health quality of life in denture stomatitis patients with type 2 diabetes mellitus wearing implant-retained overdentures: A randomized clinical study. Photodiagnosis Photodyn. Ther. 2023, 42, 103630. [Google Scholar] [CrossRef]
- Dvornyk, V.M.; Marchenko, A.V.; Ponomarenko, B.O.; Kovalenko, V.V.; Litovchenko, I.Y.; Teslenko, O.I.; Jerys, L.B. Pathogenic prevention of prosthetics stomatitis in persons with internal diseases. World Med. Biol. 2022, 1, 48–53. [Google Scholar] [CrossRef]
- Scher, E.A.; Ritchie, G.M.; Flowers, D.J. Antimycotic denture adhesive in treatment of denture stomatitis. J. Prosthet. Dent. 1978, 40, 622–627. [Google Scholar] [CrossRef]
- Mohammad, A.R.; Giannini, P.J.; Preshaw, P.M.; Alliger, H. Clinical and microbiological efficacy of chlorine dioxide in the management of chronic atrophic candidiasis: An open study. Int. Dent. J. 2004, 54, 154–158. [Google Scholar] [CrossRef]
- Amanlou, M.; Beitollahi, J.M.; Abdollahzadeh, S.; Tohidast-Ekrad, Z. Miconazole gel compared with Zataria multiflora Boiss. gel in the treatment of denture stomatitis. Phytother. Res. 2006, 20, 966–969. [Google Scholar] [CrossRef]
- Khan, S.T.; Ahamed, M.; Alhadlaq, H.A.; Musarrat, J.; Al-Khedhairy, A. Comparative effectiveness of NiCl2, Ni- and NiO-NPs in controlling oral bacterial growth and biofilm formation on oral surfaces. Arch. Oral Biol. 2013, 58, 1804–1811. [Google Scholar] [CrossRef]
- Segundo, A.d.L.; Pisani, M.X.; Nascimento, C.d; Souza, R.F.; Paranhos, H.d.F.O.; Silva-Lovato, C.H. Clinical trial of an experimental cleaning solution: Antibiofilm effect and integrity of a silicone-based denture liner. J. Contemp. Dent. Pract. 2014, 15, 534–542. [Google Scholar] [CrossRef]
- Rençber, S.; Karavana, S.Y.; Yılmaz, F.F.; Eraç, B.; Nenni, M.; Özbal, S.; Pekçetin, Ç.; Gurer-Orhan, H.; Hoşgör-Limoncu, M.; Güneri, P.; et al. Development, characterization, and in vivo assessment of mucoadhesive nanoparticles containing fluconazole for the local treatment of oral candidiasis. Int. J. Nanomed. 2016, 11, 2641–2653. [Google Scholar] [CrossRef]
- Tobouti, P.L.; Mussi, M.C.; Rossi, D.C.; Pigatti, F.M.; Taborda, C.P.; De Assis-Taveira, L.A.; De Souza, S.C. Influence of melaleuca and copaiba oils on Candida albicans adhesion. Gerodontology 2016, 33, 380–385. [Google Scholar] [CrossRef]
- Almeida, N.L.M.; Saldanha, L.L.; da Silva, R.A.; Pinke, K.H.; da Costa, E.F.; Porto, V.C.; Dokkedal, A.L.; Lara, V.S. Antimicrobial activity of denture adhesive associated with Equisetum giganteum- and Punica granatum-enriched fractions against Candida albicans biofilms on acrylic resin surfaces. Biofouling 2018, 34, 62–73. [Google Scholar] [CrossRef]
- Herman, J.L.; Wang, Y.; Lilly, E.A.; Lallier, T.E.; Peters, B.M.; Hamdan, S.; Xu, X.; Fidel, P.L., Jr.; Noverr, M.C. Synthesis, antifungal activity, and biocompatibility of novel 1,4-diazabicyclo[2.2.2]Octane (DABCO) compounds and DABCO-Containing denture base resins. Antimicrob. Agents Chemother. 2017, 61, e02575-16. [Google Scholar] [CrossRef]
- Jaworska-Zaremba, M.; Mierzwińska-Nastalskaal, E.; Burzyńska, B.; Swoboda-Kopeć, E.; Sikora, M. Clinical assessment of citrosept dental effectiveness in treatment of Candida-associated denture stomatitis, in patients using removable acrylic dentures. Protet. Stomatol. 2018, 68, 200–210. [Google Scholar] [CrossRef]
- Koseki, Y.; Tanaka, R.; Murata, H. Development of antibacterial denture cleaner for brushing containing tea tree and lemongrass essential oils. Dent. Mater. J. 2018, 37, 659–666. [Google Scholar] [CrossRef]
- Darwish, G.; Huang, S.; Knoernschild, K.; Sukotjo, C.; Campbell, S.; Bishal, A.K.; Barão, V.A.; Wu, C.D.; Taukodis, C.G.; Yang, B. Improving polymethyl methacrylate resin using a novel titanium dioxide coating. J. Prosthodont. 2019, 28, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, M.R.C.; Maciel, P.P.; Castellano, L.R.C.; Bonan, P.R.F.; Alves, D.D.N.; de Medeiros, A.C.D.; de Castro, R.D. Efficacy of essential oil of cinnamon for the treatment of oral candidiasis: A randomized trial. Spec. Care Dent. 2021, 41, 349–357. [Google Scholar] [CrossRef]
- Kaypetch, R.; Rudrakanjana, P.; Churnjitapirom, P.; Tua-Ngam, P.; Tonput, P.; Tantivitayakul, P. Geraniol and thymoquinone inhibit Candida spp. biofilm formation on acrylic denture resin without affecting surface roughness or color. J. Oral Sci. 2022, 64, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Ismaeil, M.A.A.; Ebrahim, M.I. Antifungal effect of acrylic resin denture base containing different types of nanomaterials: A comparative study. J. Int. Oral Health 2023, 15, 78–83. [Google Scholar] [CrossRef]
- Shankar, T.; Gowd, S.; Suresan, V.; Mantri, S.; Saxena, S.; Mishra, P.; Panday, P. Denture Hygiene Knowledge and Practices among Complete Denture Wearers attending a Postgraduate Dental Institute. J. Contemp. Dent. Pract. 2017, 18, 714–721. [Google Scholar] [CrossRef]
- Sesma, N.; Rocha, A.L.; Laganá, D.C.; Costa, B.; Morimoto, S. Effectiveness of denture cleanser associated with microwave disinfection and brushing of complete dentures: In vivo study. Braz. Dent. J. 2013, 24, 357–361. [Google Scholar] [CrossRef]
- Budtz-Jorgensen, E.; Bertram, U. Denture Stomatitis: II. The Effect of Antifungal and Prosthetic Treatment. Acta Odontol. Scand. 1970, 28, 283–304. [Google Scholar] [CrossRef]
- Andersson, L.; Persson, G. The treatment of stomatitis protetica granulomatosa with electrosurgery and two temporary reliners. Sven. Tandlak. Tidskr. 1973, 66, 453–460. [Google Scholar]
- Budtz-Jørgensen, E.; Milton Knudsen, A. Chlorhexidine gel and Steradent employed in cleaning dentures. Acta Odontol. Scand. 1978, 36, 83–87. [Google Scholar] [CrossRef]
- Salonen, M.A.; Raustia, A.M.; Oikarinen, K.S. Effect of treatment of palatal inflammatory papillary hyperplasia with local and systemic antifungal agents accompanied by renewal of complete dentures. Acta Odontol. Scand. 1996, 54, 87–91. [Google Scholar] [CrossRef]
- Lyon, J.P.; de Resende, M.A. Evaluation of adhesion to buccal epithelial cells in Candida species obtained from denture wearers after exposure to fluconazole. Mycoses 2007, 50, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Tawse-Smith, A.; Duncan, W.J.; Payne, A.G.; Thomson, W.M.; Wennström, J.L. Relative effectiveness of powered and manual toothbrushes in elderly patients with implant-supported mandibular overdentures. J. Clin. Periodontol. 2002, 29, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Paranhos, H.F.; Silva-Lovato, C.H.; de Souza, R.F.; Cruz, P.C.; de Freitas-Pontes, K.M.; Watanabe, E.; Ito, I.Y. Effect of three methods for cleaning dentures on biofilms formed in vitro on acrylic resin. J. Prosthodont. 2009, 18, 427–431. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.F.; Nascimento, C.; Regis, R.R.; Silva-Lovato, C.H.; Paranhos, H.F. Effects of the domestic use of a disclosing solution on the denture biofilm: A preliminary study. J. Oral Rehabil. 2009, 36, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Weitz, M.; Brownstein, C.; Deasy, M. Effect of a twice daily 0.12% chlorhexidine rinse on the oral health of a geriatric population. Clin. Prev. Dent. 1992, 14, 9–13. [Google Scholar] [PubMed]
- Budtz-Jörgensen, E.; Löe, H. Chlorhexidine as a denture disinfectant in the treatment of denture stomatitis. Scand. J. Dent. Res. 1972, 80, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.R.; Gomes, R.T.; de Mesquita, R.A.; de Moura, M.D.; França, E.C.; de Aguiar, E.G.; Naves, M.D.; Abreu, J.A.; Abreu, S.R. Efficacy of Brazilian propolis gel for the management of denture stomatitis: A pilot study. Phytother. Res. 2008, 22, 1544–1547. [Google Scholar] [CrossRef] [PubMed]
- Redding, S.; Bhatt, B.; Rawls, H.R.; Siegel, G.; Scott, K.; Lopez-Ribot, J. Inhibition of Candida albicans biofilm formation on denture material. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 107, 669–672. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, I.M.; Cruz, P.C.; Silva-Lovato, C.H.; de Souza, R.F.; Souza-Gugelmin, M.C.; Paranhos, H.d.F.O. Effect of chlorhexidine on denture biofilm accumulation. J. Prosthodont. 2012, 21, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Salles, M.M.; Oliveira, V.C.; Macedo, A.P.; do Nascimento, C.; Silva-Lovato, C.H.; Paranhos, H.F.O. Brushing associated with oral irrigation in maintaining implants and overdentures hygiene—A randomized clinical trial. Odontology 2021, 109, 284–294. [Google Scholar] [CrossRef]
- Salles, M.M.; de Cássia Oliveira, V.; Macedo, A.P.; Silva-Lovato, C.H.; de Freitas de Oliveira Paranhos, H. Effectiveness of Brushing Associated With Oral Irrigation in Maintenance of Peri-Implant Tissues and Overdentures: Clinical Parameters and Patient Satisfaction. J. Oral Implantol. 2021, 47, 117–123. [Google Scholar] [CrossRef]
- Ramage, G.; Zalewska, A.; Cameron, D.A.; Sherry, L.; Murray, C.; Finnegan, M.B.; Loewy, Z.G.; Jagger, D.C. A comparative in vitro study of two denture cleaning techniques as an effective strategy for inhibiting Candida albicans biofilms on denture surfaces and reducing inflammation. J. Prosthodont. 2012, 21, 516–522. [Google Scholar] [CrossRef]
- Ribeiro, D.G.; Pavarina, A.C.; Dovigo, L.N.; Mima, E.G.; Machado, A.L.; Bagnato, V.S.; Vergani, C.E. Photodynamic inactivation of microorganisms present on complete dentures. A clinical investigation. Photodynamic disinfection of complete dentures. Lasers Med. Sci. 2012, 27, 161–168. [Google Scholar] [CrossRef]
- Srinivasan, M.; Gulabani, M. A microbiological evaluation of the use of denture cleansers in combination with an oral rinse in complete denture patients. Indian J. Dent. Res. 2010, 21, 353–356. [Google Scholar] [CrossRef]
- Pirog, T.P.; Konon, A.D.; Beregovaya, K.A.; Shulyakova, M.A. Antiadhesive properties of the surfactants of Acinetobacter calcoaceticus IMB B-7241, Rhodococcus erythropolis IMB Ac-5017, and Nocardia vaccinii IMB B-7405. Mikrobiologiia 2014, 83, 631–639. [Google Scholar] [CrossRef]
- Valentini-Mioso, F.; Maske, T.T.; Cenci, M.S.; Boscato, N.; Pereira-Cenci, T. Chemical hygiene protocols for complete dentures: A crossover randomized clinical trial. J. Prosthet. Dent. 2019, 121, 83–89. [Google Scholar] [CrossRef]
- Gohlke-Wehrße, H.L.; Giese-Kraft, K.; Wöstmann, B. Clinical performance of a light-cured denture base material compared to polymethylmethacrylate—A randomized clinical study. Clin. Oral Investig. 2012, 16, 969–975. [Google Scholar] [CrossRef]
- Peralta, L.C.F.; Almeida, N.L.M.; Pontes, F.M.L.; Rinaldo, D.; Carneiro, C.A.; Neppelenbroek, K.H.; Lara, V.S.; Porto, V.C. Silver nanoparticles in denture adhesive: An antimicrobial approach against Candida albicans. J. Dent. 2023, 131, 104445. [Google Scholar] [CrossRef]
- Shuturminskiy, V.; Seredunko, I.; Bas, A. Evaluation of the efficacy of stomatitis prevention in prosthetics with complete dentures with additional fixation with the cream. J. Clin. Exp. Dent. 2023, 15, 142–148. [Google Scholar] [CrossRef]
- Lopes Vasconcelos, G.L.; Curylofo, P.A.; Targa Coimbra, F.C.; de Cássia Oliveira, V.; Macedo, A.P.; de Freitas Oliveira Paranhos, H.; Pagnano, V.O. In Vitro Antimicrobial Activity of Effervescent Denture Tablets on the Components of Removable Partial Dentures. Int. J. Prosthodont. 2020, 33, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.C.; Iugovaz, I.; Siboo, R.; Bilyk, M.; Barolet, R.; Amsel, R.; Wooley, C.; Klitorinos, A. Comparison of two popular methods for removal and killing of bacteria from dentures. J. Can. Dent. Assoc. 1991, 57, 937–939. [Google Scholar] [PubMed]
- Silva-Lovato, C.H.; Paranhos, H.d.F.O. Efficacy of biofilm disclosing agent and of three brushes in the control of complete denture cleansing. J. Appl. Oral Sci. 2006, 14, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, K.; Tamamoto, M.; Hamada, T. Evaluation of denture cleansers with and without enzymes against Candida albicans. J. Prosthet. Dent. 1991, 66, 792–795. [Google Scholar] [CrossRef]
- Handa, R.K.; Jagger, D.C.; Vowles, R.W. Denture cleansers, soft lining materials and water temperature: What is the effect? Prim. Dent. Care 2008, 15, 53–58. [Google Scholar] [CrossRef]
- Hong, G.; Murata, H.; Li, Y.; Sadamori, S.; Hamada, T. Influence of denture cleansers on the color stability of three types of denture base acrylic resin. J. Prosthet. Dent. 2009, 101, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Barnabé, W.; Mendonça Neto, T.; Pimenta, F.C.; Pegoraro, L.F.; Scolaro, J.M. Efficacy of sodium hypochlorite and coconut soap used as disinfecting agents in the reduction of denture stomatitis, Streptococcus mutans and Candida albicans. J. Oral Rehabil. 2004, 31, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Arruda, C.N.F.; Salles, M.M.; Badaró, M.M.; de Cássia Oliveira, V.; Macedo, A.P.; Silva-Lovato, C.H.; de Freitas Oliveira Paranhos, H. Effect of sodium hypochlorite and Ricinus communis solutions on control of denture biofilm: A randomized crossover clinical trial. J. Prosthet. Dent. 2017, 117, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Arruda, C.N.F.; Salles, M.M.; Oliveira, V.C.; Macedo, A.P.; Silva-Lovato, C.H.; Paranhos, H.F.O. Evaluation of biofilm removal and antimicrobial action of denture cleansers. Dent. Mater. 2018, 34, e83–e84. [Google Scholar] [CrossRef]
- Pinelli, L.A.; Montandon, A.A.; Corbi, S.C.; Moraes, T.A.; Fais, L.M. Ricinus communis treatment of denture stomatitis in institutionalised elderly. J. Oral Rehabil. 2013, 40, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Morelli, V.G.; Oliveira, V.C.; Vasconcelos, G.L.L.; Curylofo, P.A.; Monteiro, R.M.; Macedo, A.P.; Pagnano, V.O. Effect of effervescent tablets on removable partial denture hygiene. Am. J. Dent. 2023, 36, 75–80. [Google Scholar]
- Feldmann, A.; Alexandrino, L.D.; dos Santos, V.R.; Kapczinski, M.P.; Fraga, S.; da Silva, W.J.; Mengatto, C.M. Effect of a vinegar-hydrogen peroxide mixture on the surface properties of a cobalt-chromium alloy: A possible disinfectant for removable partial dentures. J. Prosthet. Dent. 2021, 127, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.F.D.; Terada, A.S.S.D.; Della Vecchia, M.P.; Regis, R.R.; Zanini, A.P.; Paranhos, H.D.F.O.; Silva-Lovato, C.H. Association between denture hygiene and oral health-related quality of life in edentulous patients. Rev. Odontol. UNESP 2013, 41, 48–53. [Google Scholar]
- Koujan, A.; Aggarwal, H.; Chen, P.H.; Li, Z.; Givan, D.A.; Zhang, P.; Fu, C.C. Evaluation of Candida albicans Adherence to CAD-CAM Milled, 3D-Printed, and Heat-Cured PMMA Resin and Efficacy of Different Disinfection Techniques: An In Vitro Study. J. Prosthodont. 2023, 32, 512–518. [Google Scholar] [CrossRef]
- Aati, S.; Aneja, S.; Kassar, M.; Leung, R.; Nguyen, A.; Tran, S.; Shrestha, B.; Fawzy, A. Silver-loaded mesoporous silica nanoparticles enhanced the mechanical and antimicrobial properties of 3D printed denture base resin. J. Mech. Behav. Biomed. Mater. 2022, 134, 105421. [Google Scholar] [CrossRef]
- Alfouzan, A.F.; Tuwaym, M.; Aldaghri, E.N.; Alojaymi, T.; Alotiabi, H.M.; Taweel, S.M.A.; Al-Otaibi, H.N.; Ali, R.; Alshehri, H.; Labban, N. Efficacy of Denture Cleansers on Microbial Adherence and Surface Topography of Conventional and CAD/CAM-Processed Denture Base Resins. Polymers 2023, 15, 460. [Google Scholar] [CrossRef]
- Nunes, T.S.B.S.; Silva, M.D.D.D.; Coelho, S.R.G.; Viotto, H.E.D.C.; Pero, A.C. Effectiveness of disinfectant solutions associated or not with brushing on the biofilm control of a 3D printed-denture base resin. J. Appl. Oral Sci. 2023, 17, e20230104. [Google Scholar] [CrossRef] [PubMed]
- Freitas-Pontes, K.M.; Silva-Lovato, C.H.; Paranhos, H.F. Mass loss of four commercially available heat-polymerized acrylic resins after toothbrushing with three different dentifrices. J. Appl. Oral Sci. 2009, 17, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.A.; Lovato-Silva, C.H.; Paranhos, H.d.F.O.; Ito, I.Y. Efficacy of three denture brushes on biofilm removal from complete dentures. J. Appl. Oral Sci. 2007, 15, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Pisani, M.X.; Silva, C.H.L.D.; Paranhos, H.D.F.O.; Souza, R.F.; Macedo, A.P. The effect of experimental denture cleanser solution Ricinus communis on acrylic resin properties. Mater. Res. 2010, 13, 369–373. [Google Scholar] [CrossRef]
- Davi, L.R.; Felipucci, D.N.B.; Souza, R.F.D.; Bezzon, O.L.; Lovato-Silva, C.H.; Pagnano, V.O.; Paranhos, H.D.F.O. Effect of denture cleansers on metal ion release and surface roughness of denture base materials. Braz. Dent. J. 2012, 23, 387–393. [Google Scholar] [CrossRef]
- Dos Santos, A.C.M.; Oliveira, V.C.; Macedo, A.P.; Bastos, J.K.; Ogasawara, M.S.; Watanabe, E.; Chaguri, I.M.; Silva-Lovato, C.H.; Paranhos, H.F.O. Effectiveness of Oil-Based Denture Dentifrices-Organoleptic Characteristics, Physicochemical Properties and Antimicrobial Action. Antibiotics 2021, 10, 803. [Google Scholar] [CrossRef]
- Paranhos, H.D.F.O.; Salles, A.E.S.; Macedo, L.D.D.; Silva-Lovato, C.H.D.; Pagnano, V.O.; Watanabe, E. Complete denture biofilm after brushing with specific denture paste, neutral soap and artificial saliva. Braz. Dent. J. 2013, 24, 47–52. [Google Scholar] [CrossRef]
- Curylofo, P.A.; Raile, P.N.; Vasconcellos, G.L.L.; Macedo, A.P.; Pagnano, V.O. Effect of Denture Cleansers on Cobalt-Chromium Alloy Surface: A Simulated Period of 5 Years’ Use. J. Prosthodont. 2020, 29, 142–150. [Google Scholar] [CrossRef]
- Paranhos, H.D.F.O.; Peracini, A.; Pisani, M.X.; Oliveira, V.D.C.; Souza, R.F.D.; Silva-Lovato, C.H. Color stability, surface roughness and flexural strength of an acrylic resin submitted to simulated overnight immersion in denture cleansers. Braz. Dent. J. 2013, 24, 152–156. [Google Scholar] [CrossRef]
- Felipucci, D.N.B.; Davi, L.R.; Paranhos, H.F.O.; Bezzon, O.L.; Silva, R.F.; Pagnano, V.O. Effect of different cleansers on the surface of removable partial denture. Braz. Dent. J. 2011, 22, 392–397. [Google Scholar] [CrossRef]
- Jeyapalan, K.; Kumar, J.K.; Azhagarasan, N.S. Comparative evaluation of the effect of denture cleansers on the surface topography of denture base materials: An in-vitro study. J. Pharm. Bioallied. Sci. 2015, 7, S548–S553. [Google Scholar] [CrossRef]
- Peracini, A.; Davi, L.R.; de Queiroz Ribeiro, N.; de Souza, R.F.; Lovato da Silva, C.H.; de Freitas Oliveira Paranhos, H. Effect of denture cleansers on physical properties of heat-polymerized acrylic resin. J. Prosthodont. Res. 2010, 54, 78–83. [Google Scholar] [CrossRef]
- Ribeiro, A.B.; Ribeiro, A.B.; de Araújo, C.B.; Fortes, C.V.; Clemente, L.M.; Paranhos, H.d.F.O.; Watanabe, E.; Salgado, H.C.; Silva-Lovato, C.H. Effect of a Hygiene Protocol on Denture-Related Stomatitis Remission, Local Inflammatory Factors, and Hemodynamic Responses by Arterial Pressure. Antibiotics 2022, 11, 1320. [Google Scholar] [CrossRef]
- Teixeira, A.B.V.; Valente, M.L.D.C.; Sessa, J.P.N.; Gubitoso, B.; Schiavon, M.A.; Dos Reis, A.C. Adhesion of biofilm, surface characteristics, and mechanical properties of antimicrobial denture base resin. J. Adv. Prosthodont. 2023, 15, 80–92. [Google Scholar] [CrossRef]
- Kreve, S.; Oliveira, V.C.; Bachmann, L.; Alves, O.L.; Reis, A.C.D. Influence of AgVO3 incorporation on antimicrobial properties, hardness, roughness and adhesion of a soft denture liner. Sci. Rep. 2019, 9, 11889. [Google Scholar] [CrossRef]
- De Castro, D.T.; Valente, M.L.; da Silva, C.H.; Watanabe, E.; Siqueira, R.L.; Schiavon, M.A.; Alves, O.L.; Dos Reis, A.C. Evaluation of antibiofilm and mechanical properties of new nanocomposites based on acrylic resins and silver vanadate nanoparticles. Arch. Oral Biol. 2016, 67, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.S.; Lovato da Silva, C.H.; de Cássia Oliveira, V.; Watanabe, E.; Dos Reis, A.; Lepri, C.P.; de Castro, D.T. Effects of Different Forms of Denture Adhesives on Biofilm Formation, Adhesive Strength and Hygiene of Complete Dentures. Int. J. Prosthodont. 2022, 35, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.B.; Tinelli, B.M.; Clemente, L.M.; Poker, B.d.C.; Oliveira, V.d.C.; Watanabe, E.; Silva-Lovato, C.H. Effect of Hygiene Protocols on the Mechanical and Physical Properties of Two 3D-Printed Denture Resins Characterized by Extrinsic Pigmentation as Well as the Mixed Biofilm Formed on the Surface. Antibiotics 2023, 12, 1630. [Google Scholar] [CrossRef]
- Mikkonen, M.; Nyyssönen, V.; Paunio, I.; Rajala, M. Prevalence of oral mucosal lesions associated with wearing removable dentures in Finnish adults. Community Dent. Oral Epidemiol. 1984, 12, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Jainkittivong, A.; Aneksuk, V.; Langlais, R.P. Oral mucosal lesions in denture wearers. Gerodontology 2010, 27, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Arya, N.R.; Rafiq, N.B. Candidiasis. In StatPearls; StatPearls Publishing: Treasure Island, CA, USA, 2023. [Google Scholar]
- Shulman, J.D.; Rivera-Hidalgo, F.; Beach, M.M. Risk factors associated with denture stomatitis in the United States. J. Oral Pathol. Med. 2005, 34, 340–346. [Google Scholar] [CrossRef]
- Al-Dwairi, Z.N. Prevalence and risk factors associated with denture-related stomatitis in healthy subjects attending a dental teaching hospital in North Jordan. J. Ir. Dent. Assoc. 2008, 54, 80–83. [Google Scholar] [PubMed]
- Gendreau, L.; Loewy, Z.G. Epidemiology and etiology of denture stomatitis. J. Prosthodont. 2011, 20, 251–260. [Google Scholar] [CrossRef]
- Perić, M.; Živković, R.; Milić Lemić, A.; Radunović, M.; Miličić, B.; Arsić Arsenijević, V. The severity of denture stomatitis as related to risk factors and different Candida spp. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 126, 41–47. [Google Scholar] [CrossRef]
- Newton, A.V. Denture sore mouth. A possible etiology. BDJ 1962, 112, 357–360. [Google Scholar]
- Neppelenbroek, K.H.; Falcão Procópio, A.L.; Gurgel Gomes, A.C.; Campos Sugio, C.Y.; Maia Neves Garcia, A.A.; Porto, V.C.; Urban, V.M. A modified Newton classification for denture stomatitis. Prim. Dent. J. 2022, 11, 55–58. [Google Scholar] [CrossRef]
- Jagger, D.C.; Harrison, A. Denture cleansing: The best approach. Br. Dent. J. 1995, 178, 413–417. [Google Scholar] [CrossRef]
- Barreiro, D.M.; Scheid, P.A.; May, L.G.; Unfer, B.; Braun, K.O. Evaluation of procedures employed for the maintenance of removable dentures in elderly individuals. Oral Health Prev. Dent. 2009, 7, 243–249. [Google Scholar]
- Ribeiro Rocha, G.D.S.; Neves Duarte, T.; de Oliveira Corrêa, G.; Nampo, F.K.; de Paula Ramos, S. Chemical cleaning methods for prostheses colonized by Candida spp.: A systematic review. J. Prosthet. Dent. 2020, 124, 653–658. [Google Scholar] [CrossRef]
- Zhe, G.C.S.; Green, A.; Fong, Y.T.; Lee, H.Y.; Ho, S.F. Rare case of type I hypersensitivity reaction to sodium hypochlorite solution in a healthcare setting. BMJ Case Rep. 2016, 2016, bcr2016217228. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]

| Antimicrobial Agents | Presentation Form | Frequency | |
|---|---|---|---|
| Antifungals | Nystatin | Mouthwash [66,88] Tablets (500,000 units each) [93] Ointment (100,000 IU—Nystaderm Mundgel) [70] Oral suspension (100,000 IU) [72] | 1 mL four times a day for 21 days [66] Four times a day on the surface of the prosthesis for 6 weeks [70] 10 mL for 1 min, three times a day for 15 days [88] Three times a day for 14 days [93] Four times a day for 15 days [72] |
| Amphotericin B | Tablets (10 mg) [52] | Four tablets, each taken at 4 h intervals throughout the day, for a period of 14 days [52] | |
| Miconazole | Varnish (55 mg/g) [67] Gel (2%) [67,71,74,96] | Gel (four times a day for 2 weeks) [67] Varnish (applied once to the mucosal surface of the prosthesis) [67] Three times a day for a month [71] Four times a day for two months [74] Three to four times a day for four weeks [96] | |
| Fluconazole | Capsule (50 mg) [66,96]. 2 μg mL−1 [97] | One capsule per day for 7 days [66] Once a day for 2 weeks [96] | |
| Tolerable tissue plasma (TTP) | TTP irradiation [70] | One time a week for six weeks [70] | |
| Ketoconazole | Orabase (2%) [68] Tablet (200 mg/day) [68] | Tablets (once a day for 14 days) [68] Orabase (twice a day for 14 days) [68] | |
| Chitosan | Solution (5 mg/mL) [139] | Does not contain this information | |
| Natural agents | Ricinus communis | Solution (2%) [80,135,139] | Three-year simulation with 20 min daily immersion [80,135,139] One-and-a-half-year simulation with daily immersion for 8 h (overnight) [135] |
| Tea tree | Essential oils (0.188%, 0.375%, 0.5%, 0.75%, 1%) [82,86] | Soak in the solutions for 1, 3, and 5 min [86] | |
| Copaiba | Oil (10%) [82] | Does not contain this information | |
| Herbal grapefruit seed extract | Citrosept Gel (1%) [85] | Three times a day for three weeks [85] | |
| Lemon grass | Essential oil (0.125%, 0.25%, 0.5%, 1.0%, 0.5%) [86] | Soak in the solutions for 1, 3, and 5 min [86] | |
| Cinnamomum zeylanicum Blume essential oil | Essential oil spray (0.5 mg/mL) [88] | Spray on dentures three times a day for 15 days [88] | |
| Propolis | Gel [103] | Four times a day for one week [103] | |
| Salivary polypeptides rich in histidine | Mouthrinse (histidine-rich polypeptides 3 or 4) [65] Denture soak (histidine-rich polypeptides 3 or 4) [65] | Mouthrinse twice a day for a period of 1 week [65] Overnight denture soak [65] | |
| Zataria multiflora | Gel (0.1%) [78] | Four times a day for 2 weeks [78] |
| Methods | Presentation Form | Frequency | |
|---|---|---|---|
| Chemical | Chlorhexidine Digluconate | Solution (0.02%, 0.12%, 0.15%, 0.2%, 0.3%, 1.25%, 2%, 2.5%) [36,51,52,101,102,131], Gel (1% gel) [95] Tablet (5 mg) [52] Mouthwash 0.12% [70,122,131] | Four times a day at 4 h intervals throughout the day for 14 days [51] 15 min in solution [52] Twice a day for 60 s after removing the prostheses for 6 weeks [70] Twice a day for 1 month [95] 15 s twice a day for 14 days [102] |
| * Sodium Hypochlorite | Solution (0.05%, 0.25% 0.45%, 0.5% and 1%) [23,38,44,82,120,121,122,131,135,136,140,141,144] | 10 min immersion over 15 days [122] 20 min soak daily for 180 days [38] 10 min for 6 weeks [23] 12 h a day for 365 days [121] Three-year simulation with daily immersion for 20 min [135] One-and-a-half-year simulation with daily immersion for 8 h (overnight) [135] 180 immersions of 10 min each [136,141] Continuous immersion for 182 days [140] 20 min a day for 10 days [144] 0.5% (20 min) and 1% (10 min) [131] | |
| * Peroxide solutions (Effervescent Tablets) | Bonyplus® [37] Efferdent® [117] Corega Tabs® [23,40,115,140] Steradent and Superdrug® [120] Steradent and Polident® [121] Polident® [129] Corega anti-bacteria denture cleanser tablets® [24] | Corega Tabs (5 min per day for 6 weeks) [23] Corega Tabs (30 min a day for 6 weeks) [23] Corega Tabs (overnight immersion for 14 days) [40] Corega Tabs (continuous immersion for 182 days) [140] Corega Tabs (6 months of use) [115] Bonyplus (5 min a day for 7 days, repeated three times) [37] Steradent and Superdrug (10 min soak cycles repeated at 0, 1, 3, 7, 14, 21, and 50 cycles) [120] Steradent and Polident (12 h of immersion per day for 365 days) [121] Polident (8 h of immersion) [129] | |
| Cetylpyridinium Chloride | Cepacol (0.500 mg) [136,141] | 180 immersions of 10 min each [136] 10 min for each immersion, resulting in 1800 min [141] | |
| Microbial surfactants | Acinetobacter calcoaceticus bacteria (0.003–0.036 mg/mL) [111] Rhodococcus erythropolis bacteria (0.03–0.12 mg/mL) [111] Nocardia vaccinii bacteria (0.005–0.05 mg/mL) [111]. | Does not contain this information | |
| Glutaraldehyde | Solution (2.5%) [129] | 90 min immersion [129] | |
| * Mechanical | * Brushing of complete dentures and mucosa | Dentifrice/brush [2,8,15,25,26,99,100] Brush [30,31,98] Biofilm-disclosing agent [8,100] | Brush twice a day for 2 min [2] Brush twice daily for 2 min for 60 days [30] Duration of 60 days [31] 2 min daily for 60 days [8] 3 times a day for 60 days [25] Daily for 6 weeks [26] Twice a day for 30 s for 6 weeks [98] 20 s [99] 2 min daily for 14 days [100] |
| Toothpaste formulations | Chloramine-T (1%) [28] Fluorosurfactant (0.01%—Zonyl R) [28] Resinous oil (0.5%—Copaifera officinalis) [137]; Resinous oil (0.5%—Pinus strobus) [137]; Essential oil (0.5%—Eucalyptus citriodora) [137]; Essential oil (0.5%—Melaleuca alternifolia) [137]; Artificial saliva (Oral Balance) [138] | 2 min daily for 21 days [28,138] 16.2 cycles of 3 min each [137] | |
| Brush | Conventional (Colgate [134] or Oral-B [118]); Denture-specific brushes (Bitufo; Medic Denture [134] or Condor or Johnson & Johnson [118]) | Three daily brushings within 10 weeks [134] Three daily brushings within 6 weeks [118] | |
| Low-pressure oral irrigation | Waterpik [106,107] | For 2 min, 3 times a day for 28 days, with a 7-day wash-out period after 14 days [107,108] | |
| Ultrasound | Ultrassonic Cleaner (modelo2840 D—Odontobrás) [22] Sonorex Bandelin RK100H [24] | Once at the end of a 21-day period (15 min) [22] Daily for 5 consecutive days [24] | |
| * Type of dentifrice | * Water [133,138,148]; * Soap (Protex, neutral) [138,148]; * Toothpaste (Colgate) [148]; * Denture specific toothpaste (Bony-plus, Dentu-creme, Corega Brite) [133,138] * Corega Tabs solution [148] | * Once a day (2 min) for 3 weeks [138] 2-year denture cleaning simulation [133] | |
| Physical | Photodynamic therapy | GaA1As diode laser [71,72] Suspension (50 and 100 mg/L—Photogem®) [110] | Twice a week, with an interval of at least 48 h between sessions, for four weeks. [71,72] 30 min (pre-irradiation solution) and 36 min during irradiation [109] |
| Microwave irradiation | Microwave Sterilizer (700 W) [92,129] | Once a day (3 min) for 14 days, with a wash-out period of 30 days break 7 days [92] 3 min irradiation [129] | |
| Associated | * Brushing and Sodium hypochlorite | 0.05%, 0.2%, 0.25%, 0.5% [23,26,112,122,123,144,149] | 5 and 30 min for 6 weeks [23] Soak 20 min once a week and brush daily for 6 weeks [26] Immersion for 10 min a day for 15 days [122] Brushing three times a day and soaking (20 min) for 14 days with a wash-out period on day 7 [123] Brushing for 1 min, three times a day, and immersion for 10 min, once a week for two weeks [112] Brushing the mucosa and prosthesis for 3 min, three times a day, and immersing the prosthesis for 20 min, once a day, for 10 days [144] 484 h of soaking and 60 months of brushing simulation [149] |
| * Brushing and Effervescent tablets (BonyfAG) | Bonyplus [37,99] Corega anti-bacteria denture cleanser tablets® [24] Corega Tabs® [22] Efferdent [117] | Brushing dentures three times a day and soaking once a day (20 min) for 21 days [22] Once a day for 5 days [24] Once a day for 5 min and three times a day (2 min) for 7 days; Cycle repeated three times [37] Soaking for 5 min and brushing 20 s [99] | |
| Chlorhexidine Digluconate and Alkaline peroxide effervescent tablets | Mouthwash (0.12%) [110] | 21-day period [110] | |
| Chlorhexidine Digluconate and local antifungals [54] | Miconazole (48 μg/mL) [52] Chlorhexidine Digluconate (0.2%) [52] | Does not contain this information | |
| Ultrasonic cleaning and Cleansing tablet | Corega anti-bacteria denture cleanser tablets® [24] Corega Tabs® [22] Ultrasonic Cleaner, modelo2840 D—Odontobrás [22] Sonorex Bandelin RK100H device® [24] | Immersion once a day (20 min) in an effervescent tablet and brushing three times a day for 21 days. At the end of the 21 days, 15 min of ultrasonic cleaning [22] Overnight for 5 consecutive days [24] | |
| * Denture Cleanser Associated with Microwave Disinfection and Brushing | Microwave steam sterilizer (700 W) [92] Ortoform [92] | Brushing three times a day and once a day microwave irradiation (3 min) for 14 days with a wash-out period on day 7 [92] Brushing three times a day, once a day microwave irradiation (3 min), and overnight soaking in enzymatic cleaner (8 h) for 14 days with a wash-out period on day 7 [92] | |
| Relining of prostheses and subsequent replacement | Associated with antifungals [93,152] Associated with surgical removal of hyperplastic tissue [64] | Two weeks for antifungal treatment and a pause from using the prosthesis [153] |
| Presentation Form | Frequency | |
|---|---|---|
| Coating for the surface of the prosthesis | Glaze (Biscover® LV) [53] Glaze (Surface Coat®) [53] Glaze (Perma Cure System) [54] Glaze (Permalink®) [55] Varnish (Sterngold) [57] | Applied once to rough and smooth surfaces [53] Applied once to fitting denture surface [54,55] Applied once to the tissue conditioner [57] |
| Incorporation of the agents in acrylic resin | Nystatin [104] Amphotericin B [104] Chlorhexidine [104] Miconazole (5%) [69] Titanium dioxide (TiO 2 NPs) nanoparticles (0.5% and 1%) [87,90] Nickel and nickel oxide nanoparticles (NiCl2, NiNPs, and NiONPs—50, 100 and 200 μg/mL) [79] Yamani henna powder (1%, 2.5%, 5%, 7.5%, 10%) [61] Silver (AgNPs—0.5% and 1%) [90,114] Nanostructured silver vanadate adorned with silver nanoparticles (AgVO3 and β- AgVO3—2.5%, 5%, 10%) [145] Incorporation of derivatives of the compound DABCO (DC11MAF and C2DC11MAF—1, 2, 4 wt%) [84] Mesoporous silica nanocarriers loaded with silver in varying proportions (0.0–2.0 wt%) (Ag/MSN) to 3D-printed denture base resin material [130] | Applied once to thin-film PMMA polymer [87,104] Atomic layer deposition (ALD) technique [87] Applied once to MAA-UDMA resin [69] Applied once to 3D-printed denture base resin material [61] Added to acrylic resin powder [90] Added to heat-cured acrylic [61] Adhesive applied to heat-cured acrylic resin [114] Added to heat-cured resin powder [145] Conjugation with methacrylate monomers [84] |
| Fluconazole and Chitosan | Adhesives with chitosan [60] Buccal mucoadhesive nanoparticle containing fluconazole coated with chitosan [81] | Heat-polymerized acrylic resin [60] Heat-polymerized acrylic resin disks [81] |
| Denture adhesive | Equisetum giganteum [83] Punica granatum [83] | Applied to heat-cured acrylic resin specimens [83] |
| Amphotericin B | Patches (2%) [76] | Three times a day for a maximum of 2 months [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, A.B.; Pizziolo, P.G.; Clemente, L.M.; Aguiar, H.C.; Poker, B.d.C.; Silva, A.A.M.e.; Makrakis, L.R.; Fifolato, M.A.; Souza, G.C.; Oliveira, V.d.C.; et al. Strategies for Preventing and Treating Oral Mucosal Infections Associated with Removable Dentures: A Scoping Review. Antibiotics 2024, 13, 273. https://doi.org/10.3390/antibiotics13030273
Ribeiro AB, Pizziolo PG, Clemente LM, Aguiar HC, Poker BdC, Silva AAMe, Makrakis LR, Fifolato MA, Souza GC, Oliveira VdC, et al. Strategies for Preventing and Treating Oral Mucosal Infections Associated with Removable Dentures: A Scoping Review. Antibiotics. 2024; 13(3):273. https://doi.org/10.3390/antibiotics13030273
Chicago/Turabian StyleRibeiro, Adriana Barbosa, Pillar Gonçalves Pizziolo, Lorena Mosconi Clemente, Helena Cristina Aguiar, Beatriz de Camargo Poker, Arthur Augusto Martins e Silva, Laís Ranieri Makrakis, Marco Aurelio Fifolato, Giulia Cristina Souza, Viviane de Cássia Oliveira, and et al. 2024. "Strategies for Preventing and Treating Oral Mucosal Infections Associated with Removable Dentures: A Scoping Review" Antibiotics 13, no. 3: 273. https://doi.org/10.3390/antibiotics13030273