Listeria monocytogenes Biofilms Are Planktonic Cell Factories despite Peracetic Acid Exposure under Continuous Flow Conditions
Abstract
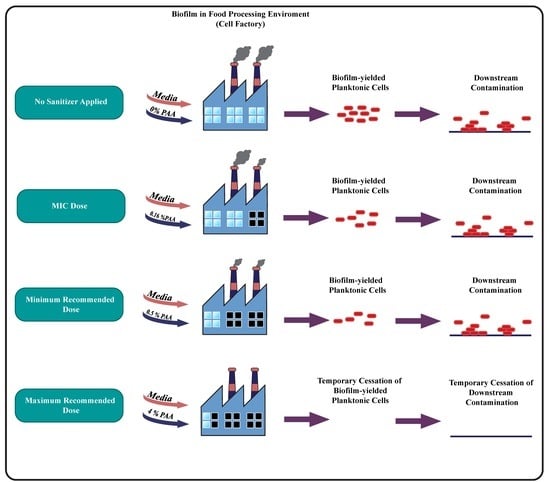
:1. Introduction
2. Results
2.1. Minimum Inhibitory Concentration (MIC) Determination
2.2. Effect of PAA on Cell Release, In-Situ Biofilm Biomass and Metabolic Activity under Continuous-Flow Conditions at Different Temperatures
2.2.1. Biofilms Cultivated at 10 °C
2.2.2. Comparison to Biofilms Cultivated at Higher Temperatures
3. Discussion
4. Materials and Methods
4.1. Microorganisms and Cultivation Conditions
4.2. Food-Grade Peracetic Acid (PAA) Sanitizer Formulation and Usage
4.3. Minimum Inhibitory Concentration (MIC) Determination
4.4. In-Situ Biofilm Biomass and Metabolic Activity Monitoring under Continuous-Flow Conditions
4.5. Abiotic Controls for Continuous-Flow Biofilm Cultivation
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siderakou, D.; Zilelidou, E.; Poimenidou, S.; Paramithiotis, S.; Mavrogonatou, E.; Zoumpopoulou, G.; Tsipra, I.; Kletsas, D.; Tsakalidou, E.; Skandamis, P.N. In Vitro Virulence Potential, Surface Attachment, and Transcriptional Response of Sublethally Injured Listeria Monocytogenes Following Exposure to Peracetic Acid. Appl. Environ. Microbiol. 2022, 88, e01582-21. [Google Scholar] [CrossRef]
- Skowron, K.; Hulisz, K.; Gryń, G.; Olszewska, H.; Wiktorczyk, N.; Paluszak, Z. Comparison of Selected Disinfectants Efficiency against Listeria Monocytogenes Biofilm Formed on Various Surfaces. Int. Microbiol. 2018, 21, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.I.; Cappato, L.P.; Corassin, C.H.; Cruz, A.G.; Oliveira, C.A.F. Effect of Peracetic Acid on Biofilms Formed by Staphylococcus Aureus and Listeria Monocytogenes Isolated from Dairy Plants. J. Dairy Sci. 2016, 99, 2384–2390. [Google Scholar] [CrossRef]
- Akinbobola, A.B.; Amaeze, N.J.; Mackay, W.G.; Ramage, G.; Williams, C. ‘Secondary Biofilms’ Could Cause Failure of Peracetic Acid High-Level Disinfection of Endoscopes. J. Hosp. Infect. 2021, 107, 67–75. [Google Scholar] [CrossRef]
- Kathariou, S. Listeria Monocytogenes Virulence and Pathogenicity, a Food Safety Perspective. J. Food Prot. 2002, 65, 1811–1829. [Google Scholar] [CrossRef]
- Rodríguez-López, P.; José Rodríguez-Herrera, J.; Vázquez-Sánchez, D.; López Cabo, M. Current Knowledge on Listeria Monocytogenes Biofilms in Food-Related Environments: Incidence, Resistance to Biocides, Ecology and Biocontrol. Foods 2018, 7, 85. [Google Scholar] [CrossRef] [Green Version]
- Leggett, L.N.; Tomasula, P.M.; Van Hekken, D.L.; Porto-Fett, A.C.S.; Shoyer, B.; Renye, J.A.; Luchansky, J.B.; Farkye, N. Effect of Storage At 4 And 10c On The Growth Of Listeria Monocytogenes In And On Queso Fresco*. J. Food Saf. 2012, 32, 236–245. [Google Scholar] [CrossRef]
- Gründling, A.; Burrack, L.S.; Bouwer, H.G.A.; Higgins, D.E. Listeria Monocytogenes Regulates Flagellar Motility Gene Expression through MogR, a Transcriptional Repressor Required for Virulence. Proc. Natl. Acad. Sci. USA 2004, 101, 12318–12323. [Google Scholar] [CrossRef] [Green Version]
- Goetz, C.; Larouche, J.; Aristizabal, M.V.; Niboucha, N.; Jean, J. Efficacy of Organic Peroxyacids for Eliminating Biofilm Preformed by Microorganisms Isolated from Dairy Processing Plants. Appl. Environ. Microbiol. 2022, 88, e01889-21. [Google Scholar] [CrossRef]
- Pan, Y.; Breidt, F.; Kathariou, S. Resistance of Listeria Monocytogenes Biofilms to Sanitizing Agents in a Simulated Food Processing Environment. Appl. Environ. Microbiol. 2006, 72, 7711. [Google Scholar] [CrossRef]
- Bester, E.; Wolfaardt, G.M.; Aznaveh, N.B.; Greener, J. Biofilms’ Role in Planktonic Cell Proliferation. Int. J. Mol. Sci. 2013, 14, 21965–21982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slavkin Biofilms, Microbial Ecology and Antoni van Leeuwenhoek. J. Am. Dent. Assoc. 1997, 128, 492–495. [CrossRef] [PubMed]
- Widder, S.; Allen, R.J.; Pfeiffer, T.; Curtis, T.P.; Wiuf, C.; Sloan, W.T.; Cordero, O.X.; Brown, S.P.; Momeni, B.; Shou, W.; et al. Challenges in Microbial Ecology: Building Predictive Understanding of Community Function and Dynamics. ISME J. 2016, 10, 2557–2568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, J.; Niharika, N.; Lata, P.; Lal, R. Biofilms: United We Stand, Divided We Fall. Indian J. Microbiol. 2014, 54, 246. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial Life on Surfaces. Emerg. Infect. Dis. 2002, 8, 881. [Google Scholar] [CrossRef]
- Dobosz, K.M.; Kolewe, K.W.; Schiffman, J.D. Green Materials Science and Engineering Reduces Biofouling: Approaches for Medical and Membrane-Based Technologies. Front. Microbiol. 2015, 6, 196. [Google Scholar] [CrossRef]
- Bester, E.; Wolfaardt, G.; Joubert, L.; Garny, K.; Saftic, S. Planktonic-Cell Yield of a Pseudomonad Biofilm. Appl. Environ. Microbiol. 2005, 71, 7792. [Google Scholar] [CrossRef] [Green Version]
- Bester, E.; Edwards, E.A.; Wolfaardt, G.M. Planktonic Cell Yield Is Linked to Biofilm Development. Can. J. Microbiol. 2009, 55, 1195–1206. [Google Scholar] [CrossRef]
- Bester, E.; Kroukamp, O.; Wolfaardt, G.M.; Boonzaaier, L.; Liss, S.N. Metabolic Differentiation in Biofilms as Indicated by Carbon Dioxide Production Rates. Appl. Environ. Microbiol. 2010, 76, 1189–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klopper, K.B.; de Witt, R.N.; Bester, E.; Dicks, L.M.T.; Wolfaardt, G.M. Biofilm Dynamics: Linking in Situ Biofilm Biomass and Metabolic Activity Measurements in Real-Time under Continuous Flow Conditions. NPJ Biofilms Microbiomes 2020, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Kroukamp, O.; Wolfaardt, G.M. CO2 Production as an Indicator of Biofilm Metabolism. Appl. Environ. Microbiol. 2009, 75, 4391–4397. [Google Scholar] [CrossRef] [Green Version]
- Kroukamp, O.; Dumitrache, R.G.; Wolfaardt, G.M. Pronounced Effect of the Nature of the Inoculum on Biofilm Development in Flow Systems. Appl. Environ. Microbiol. 2010, 76, 6025–6031. [Google Scholar] [CrossRef] [Green Version]
- Bucur, F.I.; Grigore-Gurgu, L.; Crauwels, P.; Riedel, C.U.; Nicolau, A.I. Resistance of Listeria Monocytogenes to Stress Conditions Encountered in Food and Food Processing Environments. Front. Microbiol. 2018, 9, 2700. [Google Scholar] [CrossRef] [Green Version]
- Rieu, A.; Briandet, R.; Habimana, O.; Garmyn, D.; Guzzo, J.; Piveteau, P. Listeria Monocytogenes EGD-e Biofilms: No Mushrooms but a Network of Knitted Chains. Appl. Environ. Microbiol. 2008, 74, 4491. [Google Scholar] [CrossRef] [Green Version]
- Bester, E.; Kroukamp, O.; Hausner, M.; Edwards, E.A.; Wolfaardt, G.M. Biofilm Form and Function: Carbon Availability Affects Biofilm Architecture, Metabolic Activity and Planktonic Cell Yield. J. Appl. Microbiol. 2011, 110, 387–398. [Google Scholar] [CrossRef]
- Hota, S.; Hirji, Z.; Stockton, K.; Lemieux, C.; Dedier, H.; Wolfaardt, G.; Gardam, M.A. Outbreak of Multidrug-Resistant Pseudomonas Aeruginosa Colonization and Infection Secondary to Imperfect Intensive Care Unit Room Design. Infect. Control Hosp. Epidemiol. 2009, 30, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Reardon, C.P.; Read, N.; Thorpe, S.; Evans, A.; Todd, N.; Van Der Woude, M.; Krauss, T.F. Attachment and Antibiotic Response of Early-Stage Biofilms Studied Using Resonant Hyperspectral Imaging. NPJ Biofilms Microbiomes 2020, 6, 57. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, Y.; Lin, S.; Zhang, W.; Shu, G.; Lin, J.; Li, H.; Xu, F.; Tang, H.; Peng, G.; et al. Strategies for Interfering with Bacterial Early Stage Biofilms. Front. Microbiol. 2021, 12, 1339. [Google Scholar] [CrossRef]
- Flores-Vargas, G.; Bergsveinson, J.; Lawrence, J.R.; Korber, D.R. Environmental Biofilms as Reservoirs for Antimicrobial Resistance. Front. Microbiol. 2021, 12, 3880. [Google Scholar] [CrossRef]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Determination of Minimum Inhibitory Concentrations (MICs) of Antibacterial Agents by Broth Dilution. Clin. Microbiol. Infect. 2003, 9, 509–515. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klopper, K.B.; Bester, E.; Wolfaardt, G.M. Listeria monocytogenes Biofilms Are Planktonic Cell Factories despite Peracetic Acid Exposure under Continuous Flow Conditions. Antibiotics 2023, 12, 209. https://doi.org/10.3390/antibiotics12020209
Klopper KB, Bester E, Wolfaardt GM. Listeria monocytogenes Biofilms Are Planktonic Cell Factories despite Peracetic Acid Exposure under Continuous Flow Conditions. Antibiotics. 2023; 12(2):209. https://doi.org/10.3390/antibiotics12020209
Chicago/Turabian StyleKlopper, Kyle B., Elanna Bester, and Gideon M. Wolfaardt. 2023. "Listeria monocytogenes Biofilms Are Planktonic Cell Factories despite Peracetic Acid Exposure under Continuous Flow Conditions" Antibiotics 12, no. 2: 209. https://doi.org/10.3390/antibiotics12020209