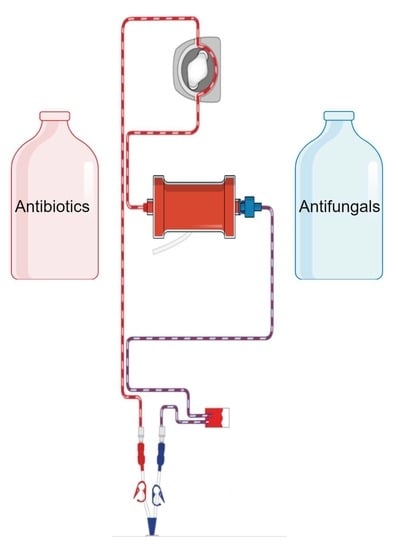
The Effects of Hemoadsorption on the Kinetics of Antibacterial and Antifungal Agents
Abstract
:1. Introduction
2. Principles of Hemoadsorption and Available Devices
3. Experimental Studies
4. Clinical Studies
5. Practical Considerations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Chousterman, B.G.; Swirski, F.K.; Weber, G.F. Cytokine Storm and Sepsis Disease Pathogenesis. Semin. Immunopathol. 2017, 39, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, e1063–e1143. [Google Scholar] [CrossRef] [PubMed]
- Ankawi, G.; Neri, M.; Zhang, J.; Breglia, A.; Ricci, Z.; Ronco, C. Extracorporeal Techniques for the Treatment of Critically Ill Patients with Sepsis beyond Conventional Blood Purification Therapy: The Promises and the Pitfalls. Crit. Care 2018, 22, 262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monard, C.; Rimmelé, T.; Ronco, C. Extracorporeal Blood Purification Therapies for Sepsis. Blood Purif. 2019, 47 (Suppl. S3), 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rimmelé, T.; Kellum, J.A. Clinical Review: Blood Purification for Sepsis. Crit. Care 2011, 15, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joannes-Boyau, O.; Honoré, P.M.; Perez, P.; Bagshaw, S.M.; Grand, H.; Canivet, J.-L.; Dewitte, A.; Flamens, C.; Pujol, W.; Grandoulier, A.-S.; et al. High-Volume versus Standard-Volume Haemofiltration for Septic Shock Patients with Acute Kidney Injury (IVOIRE Study): A Multicentre Randomized Controlled Trial. Intensive Care Med. 2013, 39, 1535–1546. [Google Scholar] [CrossRef]
- Breilh, D.; Honore, P.M.; De Bels, D.; Roberts, J.A.; Gordien, J.B.; Fleureau, C.; Dewitte, A.; Coquin, J.; Rozé, H.; Perez, P.; et al. Pharmacokinetics and Pharmacodynamics of Anti-Infective Agents during Continuous Veno-Venous Hemofiltration in Critically Ill Patients: Lessons Learned from an Ancillary Study of the IVOIRE Trial. J. Transl. Int. Med. 2019, 7, 155–169. [Google Scholar] [CrossRef] [Green Version]
- Hoff, B.M.; Maker, J.H.; Dager, W.E.; Heintz, B.H. Antibiotic Dosing for Critically Ill Adult Patients Receiving Intermittent Hemodialysis, Prolonged Intermittent Renal Replacement Therapy, and Continuous Renal Replacement Therapy: An Update. Ann. Pharmacother. 2020, 54, 43–55. [Google Scholar] [CrossRef]
- Brouwer, W.P.; Duran, S.; Ince, C. Improved Survival beyond 28 Days up to 1 Year after Cytosorb® Treatment for Refractory Septic Shock: A Propensity-Weighted Retrospective Survival Analysis. Blood Purif. 2021, 50, 539–545. [Google Scholar] [CrossRef]
- Brouwer, W.P.; Duran, S.; Kuijper, M.; Ince, C. Hemoadsorption with Cytosorb® Shows a Decreased Observed versus Expected 28-Day All-Cause Mortality in ICU Patients with Septic Shock: A Propensity-Score-Weighted Retrospective Study. Crit. Care 2019, 23, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Rugg, C.; Klose, R.; Hornung, R.; Innerhofer, N.; Bachler, M.; Schmid, S.; Fries, D.; Ströhle, M. Hemoadsorption with Cytosorb® in Septic Shock Reduces Catecholamine Requirements and In-Hospital Mortality: A Single-Center Retrospective “Genetic” Matched Analysis. Biomedicines 2020, 8, 539. [Google Scholar] [CrossRef] [PubMed]
- Hawchar, F.; Rao, C.; Akil, A.; Mehta, Y.; Rugg, C.; Scheier, J.; Adamson, H.; Deliargyris, E.; Molnar, Z. The Potential Role of Extracorporeal Cytokine Removal in Hemodynamic Stabilization in Hyperinflammatory Shock. Biomedicines 2021, 9, 768. [Google Scholar] [CrossRef] [PubMed]
- Schultz, P.; Schwier, E.; Eickmeyer, C.; Henzler, D.; Köhler, T. High-Dose Cytosorb® Hemoadsorption Is Associated with Improved Survival in Patients with Septic Shock: A Retrospective Cohort Study. J. Crit. Care 2021, 64, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Wendel Garcia, P.D.; Hilty, M.P.; Held, U.; Kleinert, E.-M.; Maggiorini, M. Cytokine Adsorption in Severe, Refractory Septic Shock. Intensive Care Med. 2021, 47, 1334–1336. [Google Scholar] [CrossRef]
- Zuccari, S.; Damiani, E.; Domizi, R.; Scorcella, C.; D’Arezzo, M.; Carsetti, A.; Pantanetti, S.; Vannicola, S.; Casarotta, E.; Ranghino, A.; et al. Changes in Cytokines, Haemodynamics and Microcirculation in Patients with Sepsis/Septic Shock Undergoing Continuous Renal Replacement Therapy and Blood Purification with Cytosorb®. Blood Purif. 2020, 49, 107–113. [Google Scholar] [CrossRef]
- Schädler, D.; Pausch, C.; Heise, D.; Meier-Hellmann, A.; Brederlau, J.; Weiler, N.; Marx, G.; Putensen, C.; Spies, C.; Jörres, A.; et al. The Effect of a Novel Extracorporeal Cytokine Hemoadsorption Device on IL-6 Elimination in Septic Patients: A Randomized Controlled Trial. PLoS ONE 2017, 12, e0187015. [Google Scholar] [CrossRef] [Green Version]
- Scharf, C.; Schroeder, I.; Paal, M.; Winkels, M.; Irlbeck, M.; Zoller, M.; Liebchen, U. Can the Cytokine Adsorber Cytosorb® Help to Mitigate Cytokine Storm and Reduce Mortality in Critically Ill Patients? A Propensity Score Matching Analysis. Ann. Intensive Care 2021, 11, 115. [Google Scholar] [CrossRef]
- Goetz, G.; Hawlik, K.; Wild, C. Extracorporeal Cytokine Adsorption Therapy As a Preventive Measure in Cardiac Surgery and As a Therapeutic Add-On Treatment in Sepsis: An Updated Systematic Review of Comparative Efficacy and Safety. Crit. Care Med. 2021, 49, 1347–1357. [Google Scholar] [CrossRef]
- Putzu, A.; Schorer, R.; Lopez-Delgado, J.C.; Cassina, T.; Landoni, G. Blood Purification and Mortality in Sepsis and Septic Shock: A Systematic Review and Meta-Analysis of Randomized Trials. Anesthesiology 2019, 131, 580–593. [Google Scholar] [CrossRef]
- Harm, S.; Gruber, A.; Gabor, F.; Hartmann, J. Adsorption of Selected Antibiotics to Resins in Extracorporeal Blood Purification. Blood Purif. 2016, 41, 55–63. [Google Scholar] [CrossRef]
- König, C.; Röhr, A.C.; Frey, O.R.; Brinkmann, A.; Roberts, J.A.; Wichmann, D.; Braune, S.; Kluge, S.; Nierhaus, A. In Vitro Removal of Anti-Infective Agents by a Novel Cytokine Adsorbent System. Int. J. Artif. Organs 2019, 42, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.G.; André, P.; Scheier, J.; Schmidt, M.; Ziervogel, H.; Buclin, T.; Kindgen-Milles, D. Pharmacokinetics of Anti-Infective Agents during Cytosorb® Hemoadsorption. Sci. Rep. 2021, 11, 10493. [Google Scholar] [CrossRef] [PubMed]
- Biever, P.; Staudacher, D.L.; Sommer, M.J.; Triebel, H.; Neukamm, M.A.; Bode, C.; Supady, A.; Lother, A. Hemoadsorption Eliminates Remdesivir from the Circulation: Implications for the Treatment of COVID-19. Pharm. Res. Perspect 2021, 9, e00743. [Google Scholar] [CrossRef] [PubMed]
- Poli, E.C.; Simoni, C.; André, P.; Buclin, T.; Longchamp, D.; Perez, M.-H.; Ferry, T.; Schneider, A.G. Clindamycin Clearance during Cytosorb® Hemoadsorption: A Case Report and Pharmacokinetic Study. Int. J. Artif. Organs 2019, 42, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Dimski, T.; Brandenburger, T.; MacKenzie, C.; Kindgen-Milles, D. Elimination of Glycopeptide Antibiotics by Cytokine Hemoadsorption in Patients with Septic Shock: A Study of Three Cases. Int. J. Artif. Organs 2020, 43, 753–757. [Google Scholar] [CrossRef]
- Zurl, C.; Waller, M.; Schwameis, F.; Muhr, T.; Bauer, N.; Zollner-Schwetz, I.; Valentin, T.; Meinitzer, A.; Ullrich, E.; Wunsch, S.; et al. Isavuconazole Treatment in a Mixed Patient Cohort with Invasive Fungal Infections: Outcome, Tolerability and Clinical Implications of Isavuconazole Plasma Concentrations. J. Fungi 2020, 6, 90. [Google Scholar] [CrossRef]
- Köhler, T.; Schwier, E.; Kirchner, C.; Winde, G.; Henzler, D.; Eickmeyer, C. Hemoadsorption with Cytosorb® and the Early Course of Linezolid Plasma Concentration during Septic Shock. J. Artif. Organs 2021. [Google Scholar] [CrossRef]
- Liebchen, U.; Scharf, C.; Zoller, M.; Weinelt, F.; Kloft, C. CytoMero collaboration team No Clinically Relevant Removal of Meropenem by Cytokine Adsorber Cytosorb® in Critically Ill Patients with Sepsis or Septic Shock. Intensive Care Med. 2021, 47, 1332–1333. [Google Scholar] [CrossRef]
- Bottari, G.; Guzzo, I.; Marano, M.; Stoppa, F.; Ravà, L.; Di Nardo, M.; Cecchetti, C. Hemoperfusion with Cytosorb® in Pediatric Patients with Septic Shock: A Retrospective Observational Study. Int. J. Artif. Organs 2020, 43, 587–593. [Google Scholar] [CrossRef]
- Berlot, G.; Samola, V.; Barbaresco, I.; Tomasini, A.; di MAso, V.; Bianco, F. Gerini UEffects of the Timing and Intensity of Treatment on Septic Shock Patients Treated with CytoSorb®: Clinical Experience. Int. J. Artif. Organs 2022, in press. [Google Scholar]
| Agent | Variation (%) |
|---|---|
| Liposomal Amphotericin B | 74.9 |
| Anidulafungin | 22.7 |
| Cefepime | 1.2 |
| Ceftriaxone | 5.2 |
| Ciprofloxacin | 14.5 |
| Clarithromycin | 4.7 |
| Clindamycin | 6.4 |
| Flucloxacillin | 15.9 |
| Fluconazole | 282.2 |
| Linezolid | 114.6 |
| Meropenem | 6.3 |
| Metronidazole | 15.4 |
| Piperacillin | 19.4 |
| Posaconazole | 32.0 |
| Teicoplanin | 30.7 |
| Tobramycin | 5.5 |
| Agent | Mode | Administration | Removal | Reference |
|---|---|---|---|---|
| Clindamycin | Associated with ECMO and CRRT | Intermittent | Not significant | [26] |
| Isavuconazole | Associated with CRRT | Intermittent | Significant | [28] |
| Linezolid | Associated with CRRT | Intermittent | Significant after 4 h | [29] |
| Meropenem | Stand alone and associated with CRRT | Intermittent | Not significant | [30] |
| Teicoplanin + Vancomycin | Stand Alone | Intermittent | Significant | [27] |
| Vancomycin | Stand Alone | Continuous Infusion | Not significant | [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berlot, G.; Di Bella, S.; Tomasini, A.; Roman-Pognuz, E. The Effects of Hemoadsorption on the Kinetics of Antibacterial and Antifungal Agents. Antibiotics 2022, 11, 180. https://doi.org/10.3390/antibiotics11020180
Berlot G, Di Bella S, Tomasini A, Roman-Pognuz E. The Effects of Hemoadsorption on the Kinetics of Antibacterial and Antifungal Agents. Antibiotics. 2022; 11(2):180. https://doi.org/10.3390/antibiotics11020180
Chicago/Turabian StyleBerlot, Giorgio, Stefano Di Bella, Ariella Tomasini, and Erik Roman-Pognuz. 2022. "The Effects of Hemoadsorption on the Kinetics of Antibacterial and Antifungal Agents" Antibiotics 11, no. 2: 180. https://doi.org/10.3390/antibiotics11020180