Suboptimal Concentrations of Ceftazidime/Avibactam (CAZ-AVI) May Select for CAZ-AVI Resistance in Extensively Drug-Resistant Pseudomonas aeruginosa: In Vivo and In Vitro Evidence
Abstract
:1. Introduction
2. Results
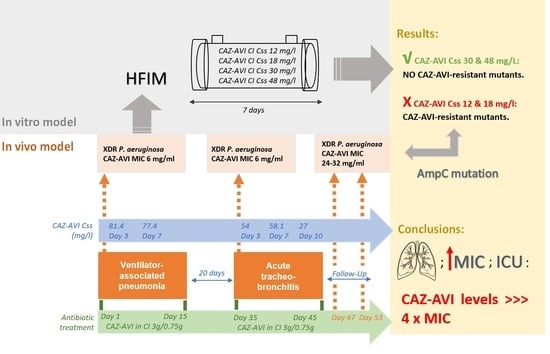
2.1. Clinical Study
2.2. HFIM
2.3. Resistance Studies
2.4. In Vitro Susceptibility and Resistance Mechanisms
2.5. Drug Concentrations
3. Discussion
4. Materials and Methods
4.1. The PseudoNOVA Project
4.2. Bacterial Isolates, Microbiological Studies, and Resistance Mechanisms
4.3. Antibiotics
4.4. HFIM
4.5. Antimicrobial Resistance Studies
4.6. Characterization of Resistance Mechanisms by WGS
4.7. Data Availability
4.8. Drug Concentrations in the HFIM
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HIFM | Hollow-Fiber Infection Model |
| CAZ-AVI | Ceftazidime-avibactam |
| CI | Continuous infusion |
| Css | steady-state concentrations |
| XDR PA | extensively drug-resistant Pseudomonas aeruginosa |
| MIC | minimum inhibitory concentration |
| ICU | Intensive Care Unit |
| WGS | Whole genome sequencing |
| GFR | Glomerular filtration rate |
| CFU | Colony-forming unit |
References
- Paterson, D.L.; Rice, L.B. Empirical Antibiotic Choice for the Seriously Ill Patient: Are Minimization of Selection of Resistant Organisms and Maximization of Individual Outcome Mutually Exclusive? Clin. Infect. Dis. 2003, 36, 1006–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, C.; Kim, S.; Kim, H.; Park, S.; Choe, Y.; Oh, M.; Kim, E.; Choe, K. Pseudomonas aeruginosa Bacteremia: Risk Factors for Mortality and Influence of Delayed Receipt of Effective Antimicrobial Therapy on Clinical Outcome. Clin. Infect. Dis. 2003, 37, 745–751. [Google Scholar] [CrossRef] [Green Version]
- Micek, S.T.; Lloyd, A.E.; Ritchie, D.J.; Reichley, R.M.; Fraser, V.J.; Kollef, M.H. Pseudomonas aeruginosa Bloodstream Infection: Importance of Appropriate Initial Antimicrobial Treatment. Antimicrob. Agents Chemother. 2005, 49, 1306–1311. [Google Scholar] [CrossRef] [Green Version]
- Sader, H.S.; Carvalhaes, C.G.; Streit, J.M.; Doyle, T.B.; Castanheira, M. Antimicrobial Activity of Ceftazidime-Avibactam, Ceftolozane-Tazobactam and Comparators Tested Against Pseudomonas aeruginosa and Klebsiella pneumoniae Isolates from United States Medical Centers in 2016–2018. Microb. Drug Resist. 2021, 27, 342–349. [Google Scholar] [CrossRef]
- Sanz-García, F.; Hernando-Amado, S.; Martínez, J.L. Mutation-Driven Evolution of Pseudomonas aeruginosa in the Presence of either Ceftazidime or Ceftazidime-Avibactam. Antimicrob. Agents Chemother. 2018, 62, e01379-18. [Google Scholar] [CrossRef] [Green Version]
- AVYCAZ (Ceftazidime and Avibactam) Safely and Effectively. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/206494s005,s006lbl.pdf (accessed on 7 August 2022).
- Sy, S.K.B.; Zhuang, L.; Beaudoin, M.-E.; Kircher, P.; Tabosa, M.A.M.; Cavalcanti, N.C.T.; Grunwitz, C.; Pieper, S.; Schuck, V.J.; Nichols, W.W.; et al. Potentiation of ceftazidime by avibactam against β-lactam-resistant Pseudomonas aeruginosa in an in vitro infection model. J. Antimicrob. Chemother. 2017, 72, 1109–1117. [Google Scholar]
- Kidd, J.M.; Stein, G.E.; Nicolau, D.P.; Kuti, J.L. Monte Carlo Simulation Methodologies for β-Lactam/β-Lactamase Inhibitor Combinations: Effect on Probability of Target Attainment Assessments. J. Clin. Pharmacol. 2020, 60, 172–180. [Google Scholar] [CrossRef]
- Haj-Darrah, R.; Leung, E.; Zvonar, R. Should Prolonged Infusion of ß-Lactams Become Standard of Practice? Can. J. Hosp. Pharm. 2017, 70, 156–160. [Google Scholar] [CrossRef] [Green Version]
- Goncette, V.; Layios, N.; Descy, J.; Frippiat, F. Continuous infusion, therapeutic drug monitoring and outpatient parenteral antimicrobial therapy with ceftazidime/avibactam: A retrospective cohort study. J. Glob. Antimicrob. Resist. 2021, 26, 15–19. [Google Scholar] [CrossRef]
- Huttner, A.; Harbarth, S.; Hope, W.W.; Lipman, J.; Roberts, J.A. Therapeutic drug monitoring of the β-lactam antibiotics: What is the evidence and which patients should we be using it for? J. Antimicrob. Chemother. 2015, 70, 3178–3183. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.R.; Miller, P.D.; Alzghari, S.K.; Blanco, D.D.; Hager, J.D.; Kuntz, K.S. Continuous Infusion Versus Intermittent Bolus of Beta-Lactams in Critically Ill Patients with Respiratory Infections: A Systematic Review and Meta-analysis. Eur. J. Drug Metab. Pharmacokinet. 2018, 43, 155–170. [Google Scholar] [CrossRef]
- Antibiotic Resistance and Pathogenicity of Bacterial Infections Group—IdISBa. PDC database. Available online: www.arpbigidisba.com (accessed on 17 August 2022).
- Blot, S.I.; Pea, F.; Lipman, J. The effect of pathophysiology on pharmacokinetics in the critically ill patient—Concepts appraised by the example of antimicrobial agents. Adv. Drug Deliv. Rev. 2014, 77, 3–11. [Google Scholar] [CrossRef]
- Nicolau, D.P.; Siew, L.; Armstrong, J.; Li, J.; Edeki, T.; Learoyd, M.; Das, S. Phase 1 study assessing the steady-state concentration of ceftazidime and avibactam in plasma and epithelial lining fluid following two dosing regimens. J. Antimicrob. Chemother. 2015, 70, 2862–2869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin-Loeches, I.; Coakley, J.; Nseir, S. Should We Treat Ventilator-Associated Tracheobronchitis with Antibiotics? Semin. Respir. Crit. Care Med. 2017, 38, 264–270. [Google Scholar] [PubMed]
- Rodríguez-Núñez, O.; Periañez-Parraga, L.; Oliver, A.; Munita, J.M.; Boté, A.; Gasch, O.; Nuvials, X.; Dinh, A.; Shaw, R.; Lomas, J.M.; et al. Higher MICs (>2 mg/L) Predict 30-Day Mortality in Patients with Lower Respiratory Tract Infections Caused by Multidrug- and Extensively Drug-Resistant Pseudomonas aeruginosa Treated with Ceftolozane/Tazobactam. Open Forum Infect. Dis. 2019, 6, ofz416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montero, M.M.; Domene Ochoa, S.; López-Causapé, C.; Luque, S.; Sorlí, L.; Campillo, N.; López Montesinos, I.; Padilla, E.; Prim, N.; Angulo-Brunet, A.; et al. Time-Kill Evaluation of Antibiotic Combinations Containing Ceftazidime-Avibactam against Extensively Drug-Resistant Pseudomonas aeruginosa and Their Potential Role against Ceftazidime-Avibactam-Resistant Isolates. Microbiol. Spectr. 2021, 9, e00585-21. [Google Scholar] [CrossRef]
- Tumbarello, M.; Raffaelli, F.; Giannella, M.; Mantengoli, E.; Mularoni, A.; Venditti, M.; De Rosa, F.G.; Sarmati, L.; Bassetti, M.; Brindicci, G.; et al. Ceftazidime-Avibactam Use for Klebsiella pneumoniae Carbapenemase–Producing K. pneumoniae Infections: A Retrospective Observational Multicenter Study. Clin. Infect. Dis. 2021, 73, 1664–1676. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, S.D.; Walkup, G.K.; Whiteaker, J.D.; Palmer, T.; McCormack, K.; Tanudra, M.A.; Nash, T.J.; Thresher, J.; Johnstone, M.R.; Hajec, L.; et al. Selection and molecular characterization of ceftazidime/avibactam-resistant mutants in Pseudomonas aeruginosa strains containing derepressed AmpC. J. Antimicrob. Chemother. 2015, 70, 1650–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraile-Ribot, P.A.; Cabot, G.; Mulet, X.; Periañez, L.; Martín-Pena, M.L.; Juan, C.; Pérez, J.L.; Oliver, A. Mechanisms leading to in vivo ceftolozane/tazobactam resistance development during the treatment of infections caused by MDR Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2018, 73, 658–663. [Google Scholar] [CrossRef] [Green Version]
- Cabot, G.; Bruchmann, S.; Mulet, X.; Zamorano, L.; Moyà, B.; Juan, C.; Haussler, S.; Oliver, A. Pseudomonas aeruginosa Ceftolozane-Tazobactam Resistance Development Requires Multiple Mutations Leading to Overexpression and Structural Modification of AmpC. Antimicrob. Agents Chemother. 2014, 58, 3091–3099. [Google Scholar] [CrossRef] [Green Version]
- Heffernan, A.J.; Sime, F.B.; Lipman, J.; Dhanani, J.; Andrews, K.; Ellwood, D.; Grimwood, K.; Roberts, J.A. Intrapulmonary pharmacokinetics of antibiotics used to treat nosocomial pneumonia caused by Gram-negative bacilli: A systematic review. Int. J. Antimicrob. Agents 2019, 53, 234–245. [Google Scholar] [CrossRef]
- Mouton, J.W.; Muller, A.E.; Canton, R.; Giske, C.G.; Kahlmeter, G.; Turnidge, J. MIC-based dose adjustment: Facts and fables. J. Antimicrob. Chemother. 2018, 73, 564–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [Green Version]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone. Available online: https://www.eucast.org/ast_of_bacteria/previous_versions_of_documents/ (accessed on 15 June 2022).
- Montero, M.; VanScoy, B.D.; López-Causapé, C.; Conde, H.; Adams, J.; Segura, C.; Zamorano, L.; Oliver, A.; Horcajada, J.P.; Ambrose, P.G. Evaluation of Ceftolozane-Tazobactam in Combination with Meropenem against Pseudomonas aeruginosa Sequence Type 175 in a Hollow-Fiber Infection Model. Antimicrob. Agents Chemother. 2018, 62, e00026-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montero, M.M.; Domene-Ochoa, S.; López-Causapé, C.; Luque, S.; Sorlí, L.; Campillo, N.; Padilla, E.; Prim, N.; Ferrer-Alapont, L.; Angulo-Brunet, A.; et al. Impact of ceftolozane/tazobactam concentrations in continuous infusion against extensively drug-resistant Pseudomonas aeruginosa isolates in a hollow-fiber infection model. Sci. Rep. 2021, 11, 22178. [Google Scholar] [CrossRef]
- Montero, M.; Domene Ochoa, S.; López-Causapé, C.; VanScoy, B.; Luque, S.; Sorlí, L.; Campillo, N.; Angulo-Brunet, A.; Padilla, E.; Prim, N.; et al. Efficacy of Ceftolozane-Tazobactam in Combination with Colistin against Extensively Drug-Resistant Pseudomonas aeruginosa, Including High-Risk Clones, in an In Vitro Pharmacodynamic Model. Antimicrob. Agents Chemother. 2020, 64, e02542-19. [Google Scholar] [CrossRef] [PubMed]
- Rico Caballero, V.; Almarzoky Abuhussain, S.; Kuti, J.L.; Nicolau, D.P. Efficacy of Human-Simulated Exposures of Ceftolozane-Tazobactam Alone and in Combination with Amikacin or Colistin against Multidrug-Resistant Pseudomonas aeruginosa in an In Vitro Pharmacodynamic Model. Antimicrob. Agents Chemother. 2018, 62, e02384-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montero, M.M.; Domene-Ochoa, S.; López-Causapé, C.; López-Montesinos, I.; Luque, S.; Sorlí, L.; Campillo, N.; Padilla, E.; Prim, N.; Ferrer Alapont, L.; et al. Comparison of Ceftolozane/Tazobactam Infusion Regimens in a Hollow-Fiber Infection Model against Extensively Drug-Resistant Pseudomonas aeruginosa Isolates. Microbiol. Spectr. 2022, 10, e00892-22. [Google Scholar] [CrossRef]
- Weinstein, M.P.; Patel, J.B.; Bobenchik, A.M.; Campeau, S.; Cullen, S.K.; Galas, M.F.; Gold, H.; Humphries, R.M.; Kirn, T.J., Jr.; Limbago, B.; et al. Performance Standards for Antimicrobial Susceptibility Testing: A CLSI Supplement for Global Application; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020; Available online: https://clsi.org/media/3481/m100ed30_sample.pdf (accessed on 15 June 2022).
- Cortes-Lara, S.; del Barrio-Tofiño, E.; López-Causapé, C.; Oliver, A.; Martínez-Martínez, L.; Bou, G.; Zamorano, L.; Sánchez-Diener, I.; Galán, F.; Gracia, I.; et al. Predicting Pseudomonas aeruginosa susceptibility phenotypes from whole genome sequence resistome analysis. Clin. Microbiol. Infect. 2021, 27, 1631–1637. [Google Scholar] [CrossRef] [PubMed]
- SPAdes v3.13. Available online: http://cab.spbu.ru/files/release3.13.1/ (accessed on 17 August 2022).
- Sequence Type. Available online: https://cge.cbs.dtu.dk/services (accessed on 17 August 2022).
- Sutherland, C.A.; Nicolau, D.P. Development of an HPLC Method for the Determination of Ceftolozane/Tazobactam in Biological and Aqueous Matrixes. J. Chromatogr. Sci. 2016, 54, 1037–1040. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, K.; Anjum, F.; Aslam, B.; Shahbaz, K.; Javed, I. Hplc-validation of moxifloxacin. Int. J. Res. Dev. Pharm. Life Sci. 2016, 5, 2092–2098. [Google Scholar]
- D’Argenio, D.Z.; Schumitzky, A.; Wang, X. ADAPT 5 User’s Guide: Pharmacokinetic/Pharmacodynamic Systems Analysis Software; Biomedical Simulations Resource: Los Angeles, CA, USA, 2009. [Google Scholar]




| PA Isolate | CAZ/AVI MIC | Resistome Summary |
|---|---|---|
| Episode 1 (Index isolate) | 6 mg/L | aadB, oprD (Q142X), mexZ (G195D), gyrA (T83I, D87N), ampR (G154R), parC (L168Q, S87W), armZ (V266M) |
| Episode 2 (day 35) | 6 mg/L | aadB, oprD (Q142X), mexZ (G195D), gyrA (T83I, D87N), ampR (G154R), parC (L168Q, S87W), armZ (V266M) |
| Follow up (day 46) | 24 mg/L | aadB, oprD (Q142X), mexZ (G195D), gyrA (T83I, D87N), ampR (G154R), parC (L168Q, S87W), armZ (V266M), ampC (Q146K) |
| Follow up (day 55) | 32 mg/L | aadB, oprD (Q142X), mexZ (G195D), gyrA (T83I, D87N), ampR (G154R), parC (L168Q, S87W), armZ (V266M), ampC (Q146K) |
| HFIM in vitro resistant mutants | >32 mg/L | aadB, oprD (Q142X), mexZ (G195D), gyrA (T83I, D87N), ampR (G154R), parC (L168Q, S87W), armZ (V266M), ampC (Δ236-242) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez-Montesinos, I.; Montero, M.M.; Domene-Ochoa, S.; López-Causapé, C.; Echeverria, D.; Sorlí, L.; Campillo, N.; Luque, S.; Padilla, E.; Prim, N.; et al. Suboptimal Concentrations of Ceftazidime/Avibactam (CAZ-AVI) May Select for CAZ-AVI Resistance in Extensively Drug-Resistant Pseudomonas aeruginosa: In Vivo and In Vitro Evidence. Antibiotics 2022, 11, 1456. https://doi.org/10.3390/antibiotics11111456
Lopez-Montesinos I, Montero MM, Domene-Ochoa S, López-Causapé C, Echeverria D, Sorlí L, Campillo N, Luque S, Padilla E, Prim N, et al. Suboptimal Concentrations of Ceftazidime/Avibactam (CAZ-AVI) May Select for CAZ-AVI Resistance in Extensively Drug-Resistant Pseudomonas aeruginosa: In Vivo and In Vitro Evidence. Antibiotics. 2022; 11(11):1456. https://doi.org/10.3390/antibiotics11111456
Chicago/Turabian StyleLopez-Montesinos, Inmaculada, María Milagro Montero, Sandra Domene-Ochoa, Carla López-Causapé, Daniel Echeverria, Luisa Sorlí, Nuria Campillo, Sonia Luque, Eduardo Padilla, Nuria Prim, and et al. 2022. "Suboptimal Concentrations of Ceftazidime/Avibactam (CAZ-AVI) May Select for CAZ-AVI Resistance in Extensively Drug-Resistant Pseudomonas aeruginosa: In Vivo and In Vitro Evidence" Antibiotics 11, no. 11: 1456. https://doi.org/10.3390/antibiotics11111456