Characterization of the Antibacterial Activity of an SiO2 Nanoparticular Coating to Prevent Bacterial Contamination in Blood Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Labile Blood Products
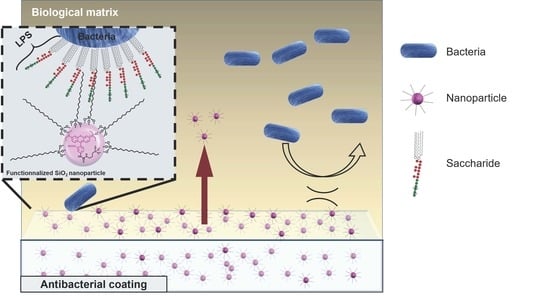
2.2. Medical Antibacterial Antiadhesive Coating
2.3. Characterization Techniques
2.4. Antibacterial Activity
2.4.1. Decontamination Sample
2.4.2. Microorganisms and Growth Conditions
2.4.3. Antibacterial Activity on Polymeric Material Surfaces
2.5. In Vitro Cytotoxicity
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization
3.2. Antibacterial Activity
3.3. MAAC In Vitro Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blajchman, M. Reducing the risk of bacterial contamination of cellular blood components. Dev. Biol. 2000, 102, 183–193. [Google Scholar]
- Kracalik, I.; Mowla, S.; Basavaraju, S.V.; Sapiano, M.R. Transfusion-related adverse reactions: Data from the National Healthcare Safety Network Hemovigilance Module—United States, 2013–2018. Transfusion 2021, 61, 1424–1434. [Google Scholar] [CrossRef]
- Hodgson, S.D.; Greco-Stewart, V.; Jimenez, C.S.; Sifri, C.D.; Brassinga, A.K.C.; Ramirez-Arcos, S. Enhanced pathogenicity of biofilm-negative Staphylococcus epidermidis isolated from platelet preparations. Transfusion 2014, 54, 461–470. [Google Scholar]
- Goldman, M.; Delage, G.; Beauregard, P.; Pruneau, F.D.; Ismail, J.; Robillard, P. A fatal case of transfusion-transmitted Staphylococcus epidermidis sepsis. Transfusion 2001, 41, 1075–1076. [Google Scholar] [CrossRef] [PubMed]
- Kou, Y.; Pagotto, F.; Hannach, B.; Ramirez-Arcos, S. Fatal false-negative transfusion infection involving a buffy coat platelet pool contaminated with biofilm-positive Staphylococcus epidermidis: A case report. Transfusion 2015, 55, 2384–2389. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Greco-Stewart, V.S.; Jacobs, M.R.; Yomtovian, R.A.; Rood, I.G.H.; de Korte, D.; Ramírez-Arcos, S.M. Characterization of the growth dynamics and biofilm formation of Staphylococcus epidermidis strains isolated from contaminated platelet units. J. Med. Microbiol. 2014, 63, 884–891. [Google Scholar] [CrossRef]
- Greco, C.; Martincic, I.; Gusinjac, A.; Kalab, M.; Yang, A.-F.; Ramírez-Arcos, S. Staphylococcus epidermidis forms biofilms under simulated platelet storage conditions. Transfusion 2007, 47, 1143–1153. [Google Scholar] [CrossRef]
- Ramirez-Arcos, S.; Chin-Yee, I.; Hume, H.; Fearon, M.; Goldman, M.; Eckert, K.; Martincic, I.; Peters, G.; Kovach, D.; Richardson, S.E. Fatal septic shock associated with transfusion-transmitted Serratia marcescens. Transfusion 2006, 46, 679–681. [Google Scholar] [CrossRef] [PubMed]
- Loza-Correa, M.; Kou, Y.; Taha, M.; Kalab, M.; Ronholm, J.; Schlievert, P.; Cahill, M.P.; Skeate, R.; Cserti-Gazdewich, C.; Ramirez-Arcos, S. Septic transfusion case caused by a platelet pool with visible clotting due to contamination with Staphylococcus aureus. Transfusion 2017, 57, 1299–1303. [Google Scholar] [CrossRef] [PubMed]
- Martincic, I.; Mastronardi, C.; Chung, A.; Ramirez-Arcos, S. Unexplained agglutination of stored red blood cells in Alsever’s solution caused by the gram-negative bacterium Serratia liquefaciens. Immunohematology 2020, 24, 39–44. [Google Scholar] [CrossRef]
- Campos-Cortés, C.L.; González, G.M.; Andrade, A.; Treviño-Rangel, R. Epidemiological Panorama of Serratia marcescens: Antimicrobial Resistance and Virulence Factors. Med. Univ. 2018, 20, 91–98. [Google Scholar]
- Ramírez-Arcos, S.; Goldman, M. Bacterial contamination. In Practical Transfusion Medicine, 5th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 168–175. [Google Scholar] [CrossRef]
- Benjamin, R.J.; Dy, B.; Perez, J.; Eder, A.F.; Wagner, S.J. Bacterial culture of apheresis platelets: A mathematical model of the residual rate of contamination based on unconfirmed positive results. Vox Sang. 2013, 106, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Daurat, A.; Roger, C.; Gris, J.; Daurat, G.; Feissel, M.; Le Manach, Y.; Lefrant, J.; Muller, L. Apheresis platelets are more frequently associated with adverse reactions than pooled platelets both in recipients and in donors: A study from F rench hemovigilance data. Transfusion 2016, 56, 1295–1303. [Google Scholar] [CrossRef]
- Bolton-Maggs, P. Conference report: The 2015 SHOT symposium and report–what’s new? Transfus. Med. 2015, 25, 295–298. [Google Scholar] [CrossRef]
- Hong, H.; Xiao, W.; Lazarus, H.M.; Good, C.E.; Maitta, R.W.; Jacobs, M.R. Detection of septic transfusion reactions to platelet transfusions by active and passive surveillance. Blood 2016, 127, 496–502. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Arcos, S.; Jenkins, C.; Dion, J.; Bernier, F.; Delage, G.; Goldman, M. Canadian experience with detection of bacterial contamination in apheresis platelets. Transfusion 2007, 47, 421–429. [Google Scholar] [CrossRef]
- Murphy, W.G.; Foley, M.; Doherty, C.; Tierney, G.; Kinsella, A.; Salami, A.; Cadden, E.; Coakley, P. Screening platelet concentrates for bacterial contamination: Low numbers of bacteria and slow growth in contaminated units mandate an alternative approach to product safety. Vox Sang. 2008, 95, 13–19. [Google Scholar] [CrossRef]
- Corash, L. Bacterial contamination of platelet components: Potential solutions to prevent transfusion-related sepsis. Expert Rev. Hematol. 2011, 4, 509–525. [Google Scholar] [CrossRef]
- Salunkhe, V.; De Cuyper, I.M.; Papadopoulos, P.; van der Meer, P.F.; Daal, B.B.; Villa, F.M.; de Korte, D.; van den Berg, T.K.; Gutiérrez, L. A comprehensive proteomics study on platelet concentrates: Platelet proteome, storage time and Mirasol pathogen reduction technology. Platelets 2019, 30, 368–379. [Google Scholar] [CrossRef]
- Magron, A.; Laugier, J.; Provost, P.; Boilard, E. Pathogen reduction technologies: The pros and cons for platelet transfusion. Platelets 2017, 29, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Marschner, S.; Goodrich, R. Pathogen Reduction Technology Treatment of Platelets, Plasma and Whole Blood Using Riboflavin and UV Light. Transfus. Med. Hemother. 2011, 38, 8–18. [Google Scholar] [CrossRef]
- Levy, J.H.; Neal, M.D.; Herman, J.H. Bacterial contamination of platelets for transfusion: Strategies for prevention. Crit. Care 2018, 22, 271. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, A.; Lindgren, B.; Marschner, S.; Aznar, M.; Zalba, S.; Sánchez, P.; Ayape, M.; Luisa, O.E.; Antelo, M.L. Evaluation of the post-transfusion platelet increment and safety of riboflavin-based pathogen reduction technology (PRT) treated platelet products stored in platelet additive solution for 5 days or less versus 6–7 days. Transfus. Apher. Sci. 2016, 54, 248–252. [Google Scholar] [CrossRef]
- Li, J.; De Korte, D.; Woolum, M.D.; Ruane, P.H.; Keil, S.D.; Lockerbie, O.; McLean, R.; Goodrich, R.P. Pathogen reduction of buffy coat platelet concentrates using riboflavin and light: Comparisons with pathogen-reduction technology-treated apheresis platelet products. Vox Sang. 2004, 87, 82–90. [Google Scholar] [CrossRef] [PubMed]
- De Souza e Silva, J.M.; Hanchuk, T.D.M.; Santos, M.I.; Kobarg, J.; Bajgelman, M.C.; Cardoso, M.B. Viral Inhibition Mechanism Mediated by Surface-Modified Silica Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 16564–16572. [Google Scholar] [CrossRef] [PubMed]
- Botequim, D.; Maia, J.; Lino, M.M.F.; Lopes, L.M.F.; Simões, P.N.; Ilharco, L.M.; Ferreira, L. Nanoparticles and Surfaces Presenting Antifungal, Antibacterial and Antiviral Properties. Langmuir 2012, 28, 7646–7656. [Google Scholar] [CrossRef]
- Carvalho, D.; Sousa, T.; Morais, P.; Piedade, A. Polymer/metal nanocomposite coating with antimicrobial activity against hospital isolated pathogen. Appl. Surf. Sci. 2016, 379, 489–496. [Google Scholar] [CrossRef]
- Saeed, A.M.; El-Fattah, M.A.; Azzam, A.M.; Dardir, M.; Bader, M.M. Synthesis of cuprous oxide epoxy nanocomposite as an environmentally antimicrobial coating. Int. J. Biol. Macromol. 2016, 89, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Garric, X.; Nottelet, B.; Pinese, C.; Leroy, A.; Coudane, J. Polymères synthétiques dégradables pour la conception de dispositifs médicaux implantables-Le cas de la ligamentoplastie. Med. Sci. 2017, 33, 39–45. [Google Scholar]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling Coatings: Recent Developments in the Design of Surfaces That Prevent Fouling by Proteins, Bacteria, and Marine Organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef] [PubMed]
- Green, J.-B.D.; Fulghum, T.; Nordhaus, M.A. A review of immobilized antimicrobial agents and methods for testing. Biointerphases 2011, 6, MR13–MR28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singha, P.; Locklin, J.; Handa, H. A review of the recent advances in antimicrobial coatings for urinary catheters. Acta Biomater. 2016, 50, 20–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayorga, J.L.C.; Randazzo, W.; Fabra, M.J.; Lagaron, J.; Aznar, R.; Sánchez, G. Antiviral properties of silver nanoparticles against norovirus surrogates and their efficacy in coated polyhydroxyalkanoates systems. LWT 2017, 79, 503–510. [Google Scholar] [CrossRef] [Green Version]
- Moongraksathum, B.; Chien, M.-Y.; Chen, Y.-W. Antiviral and Antibacterial Effects of Silver-Doped TiO2 Prepared by the Peroxo Sol-Gel Method. J. Nanosci. Nanotechnol. 2019, 19, 7356–7362. [Google Scholar] [CrossRef] [PubMed]
- Gabbay, J. Antimicrobial and Antiviral Polymeric Materials. Google Patents US 7,169.402 B2, 2007. Available online: https://patents.google.com/patent/US7169402B2/en (accessed on 11 January 2022).
- Sugiura, K. Antiviral Agent, Coating Composition, Resin Composition and Antiviral Product. Google Patents US 2019/0045793 A1, 2019. Available online: https://patents.google.com/patent/US20190045793A1/en (accessed on 11 January 2022).
- Reijnders, L. The release of TiO2 and SiO2 nanoparticles from nanocomposites. Polym. Degrad. Stab. 2009, 94, 873–876. [Google Scholar] [CrossRef]
- Ammar, S.; Ramesh, K.; Vengadaesvaran, B.; Ramesh, S.; Arof, A. A novel coating material that uses nano-sized SiO2 particles to intensify hydrophobicity and corrosion protection properties. Electrochim. Acta 2016, 220, 417–426. [Google Scholar] [CrossRef]
- Busscher, H.J.; Van Der Mei, H.C.; Subbiahdoss, G.; Jutte, P.C.; van den Dungen, J.J.A.M.; Zaat, S.A.J.; Schultz, M.J.; Grainger, D.W. Biomaterial-Associated Infection: Locating the Finish Line in the Race for the Surface. Sci. Transl. Med. 2012, 4, 153rv10. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Carmona, M.; Gun’ko, Y.K.; Vallet-Regí, M. Mesoporous silica materials as drug delivery: “The Nightmare” of bacterial infection. Pharmaceutics 2018, 10, 279. [Google Scholar] [CrossRef] [Green Version]
- Wawrzyniak, R.; Kosnowska, A.; Macioszek, S.; Bartoszewski, R.; Markuszewski, M.J. New plasma preparation approach to enrich metabolome coverage in untargeted metabolomics: Plasma protein bound hydrophobic metabolite release with proteinase K. Sci. Rep. 2018, 8, 9541. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Orski, S.; Woys, A.M.; Yuan, G.; Zarraga, I.E.; Wagner, N.J.; Liu, Y. Adsorption of polysorbate 20 and proteins on hydrophobic polystyrene surfaces studied by neutron reflectometry. Colloids Surf. B Biointerfaces 2018, 168, 94–102. [Google Scholar] [CrossRef]
- Misono, T. Measurement Techniques and Practices of Colloid and Interface Phenomena 65–69; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Ben-Jacob, E.; Cohen, I.; Golding, I.; Gutnick, D.L.; Tcherpakov, M.; Helbing, D.; Ron, I.G. Bacterial cooperative organization under antibiotic stress. Phys. A Stat. Mech. Its Appl. 2000, 282, 247–282. [Google Scholar] [CrossRef]
- Chen, D.; Ganesh, S.; Wang, W.; Amiji, M. Plasma protein adsorption and biological identity of systemically administered nanoparticles. Nanomedicine 2017, 12, 2113–2135. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Winter, K.M.; Kwok, M.; Reid, S.; Marks, D.C. Evaluation of the quality of blood components prepared using the Reveos automated blood processing system. Vox Sang. 2013, 105, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Arkles, B. Silane Coupling Agents: Connecting Across Boundaries, 3rd ed.; Gelest Inc.: Morrisville, PA, USA, 2014; Available online: https://www.gelest.com/wp-content/uploads/Silane_Coupling_Agents.pdf (accessed on 13 January 2022).
- Falke, S.; Betzel, C. Radiation in Bioanalysis; Springer: Berlin/Heidelberg, Germany, 2019; pp. 173–193. [Google Scholar]
- ISO 22196. Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces; International Organization for Standardization: Geneva, Switzerland, 2011; Available online: https://www.iso.org/standard/54431.html (accessed on 11 January 2022).
- ISO 10993-5. Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; International Organization for Standardization: Geneva, Switzerland, 2009; Available online: https://www.iso.org/standard/36406.html (accessed on 11 January 2022).
- Ferreira, T.; Rasband, W. ImageJ User Guide; ImageJ/Fiji 1: 2012; pp. 155–161. Available online: https://imagej.nih.gov/ij/docs/guide/user-guide.pdf (accessed on 13 January 2022).
- Ouellet, S. Lumière sur le Transfert D’énergie Résonant Entre Métal et Fluorophores: À la Recherche des Photons Perdus; Université Laval: Québec, QC, Canada, 2020. [Google Scholar]
- Formosa, D.; Li, X.; Sammons, R.; Dong, H. Development and characterisation of novel anti-bacterial S-phase based coatings. Thin Solid Film. 2017, 644, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Szatanek, R.; Baj-Krzyworzeka, M.; Zimoch, J.; Lekka, M.; Siedlar, M.; Baran, J. The Methods of Choice for Extracellular Vesicles (EVs) Characterization. Int. J. Mol. Sci. 2017, 18, 1153. [Google Scholar] [CrossRef]
- Mu, S.; Rafaelsen, J. Quantification and Precision in Particle Analysis Using SEM and EDS. Microsc. Microanal. 2019, 25, 708–709. [Google Scholar] [CrossRef] [Green Version]
- Dirisu, J.O.; Oyedepo, S.O.; Fayomi, O.S.I.; Okokpujie, I.P.; Asere, A.A.; Oyekunle, J.A.; Afolalu, S.A.; Abioye, A.A. Effects of Emission Characteristics on Elemental Composition of Selected PVC Ceiling Materials. Mater. Focus 2018, 7, 566–572. [Google Scholar] [CrossRef]
- Yu, Z.; Shi, Z.; Zheng, Z.; Han, J.; Yang, W.; Lu, R.; Lin, W.; Zheng, Y.; Nie, D.; Chen, G. DEHP induce cholesterol imbalance via disturbing bile acid metabolism by altering the composition of gut microbiota in rats. Chemosphere 2020, 263, 127959. [Google Scholar] [CrossRef]
- Xiu, F.-R.; Wang, Y.; Yu, X.; Li, Y.; Lu, Y.; Zhou, K.; He, J.; Song, Z.; Gao, X. A novel safety treatment strategy of DEHP-rich flexible polyvinyl chloride waste through low-temperature critical aqueous ammonia treatment. Sci. Total Environ. 2019, 708, 134532. [Google Scholar] [CrossRef]
- Hadjesfandiari, N.; Schubert, P.; Toosi, S.F.; Chen, Z.; Culibrk, B.; Ramirez-Arcos, S.; Devine, D.V.; Brooks, D.E. Effect of texture of platelet bags on bacterial and platelet adhesion. Transfusion 2016, 56, 2808–2818. [Google Scholar] [CrossRef]
- Elmehbad, N.Y.; Mohamed, N.A. Preparation and characterization of some new antimicrobial thermally stable PVC formulations. Polym. Bull. 2020, 78, 6183–6204. [Google Scholar] [CrossRef]
- Yuan, Y.; Hays, M.P.; Hardwidge, P.R.; Kim, J. Surface characteristics influencing bacterial adhesion to polymeric substrates. RSC Adv. 2017, 7, 14254–14261. [Google Scholar] [CrossRef] [Green Version]
- Tavakoli, S.; Nemati, S.; Kharaziha, M.; Akbari-Alavijeh, S. Embedding CuO nanoparticles in PDMS-SiO2 coating to improve antibacterial characteristic and corrosion resistance. Colloid Interface Sci. Commun. 2019, 28, 20–28. [Google Scholar] [CrossRef]
- Nicolai, L.; Gaertner, F.; Massberg, S. Platelets in Host Defense: Experimental and Clinical Insights. Trends Immunol. 2019, 40, 922–938. [Google Scholar] [CrossRef] [PubMed]
- Capeletti, L.B.; De Oliveira, L.F.; Gonçalves, K.D.A.; De Oliveira, J.F.A.; Saito, A.; Kobarg, J.; Dos Santos, J.H.Z.; Cardoso, M.B. Tailored Silica–Antibiotic Nanoparticles: Overcoming Bacterial Resistance with Low Cytotoxicity. Langmuir 2014, 30, 7456–7464. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.; Migonney, V.; Falentin-Daudre, C. Review of silicone surface modification techniques and coatings for antibacterial/antimicrobial applications to improve breast implant surfaces. Acta Biomater. 2020, 121, 68–88. [Google Scholar] [CrossRef]




| Material | PVC-DEHP | PVC-BTHC | Polyurethane/Silicone | |||
|---|---|---|---|---|---|---|
| Incubation temperatures (°C) a | 35 | 4 | 35 | 22 | 35 | |
| Gram | Positive | S. aureus | S. aureus | S. aureus | ||
| N/A | S. epidermidis | E. faecalis | ||||
| Negative | E. coli | E. coli | E. coli | |||
| S. marcescens | N/A | K. pneumoniae | ||||
| Material | Bacteria | Medium | Log Reduction a | Reduction (%) * |
|---|---|---|---|---|
| PVC-DEHP | S. aureus | NB | 3.8 ± 0.6 | 99.99 |
| RCC | 0.3 ± 0.6 | <90 | ||
| E. coli | NB | 3.1 ± 0.1 | 99.9 | |
| RCC | 1.6 ± 0.3 | 99 | ||
| S. marcescens | NB | 3.9 ± 1.7 | 99.99 | |
| RCC | 1.6 ± 0.1 | 99 | ||
| PVC-BTHC | S. aureus | NB | 2.2 ± 2.1 | 99 |
| PC | 0.0 ± 0.0 | <90 | ||
| E. coli | NB | 1.4 ± 2.1 | 90 | |
| PC | 0.2 ± 0.2 | <90 | ||
| S. epidermidis | NB | 1.4 ± 1.3 | 90 | |
| PC | 2.2 ± 0.2 | 99 | ||
| PVC | S. aureus | NB | 2.3 ± 1.3 | 99 |
| E. coli | NB | 4.6 ± 1.5 | 99.999 | |
| Polyurethane | S. aureus | NB | 4.9 ± 0.4 | 99.999 |
| E. coli | NB | 4.0 ± 0.5 | 99.99 | |
| K. pneumoniae | NB | 4.6 ± 0.6 | 99.999 | |
| E. faecalis | NB | 2.9 ± 0.8 | 99.9 | |
| Silicone | S. aureus | NB | 5.2 ± 0.8 | 99.999 |
| E. coli | NB | 5.2 ± 1.9 | 99.999 | |
| K. pneumoniae | NB | 5.5 ± 1.1 | 99.9999 | |
| E. faecalis | NB | 3.6 ± 0.9 | 99.99 |
| Medium | Dilution | Log reduction | Reduction (%) * |
|---|---|---|---|
| PC ¥ | ND | 0.2 ± 0.2 | <90 |
| Platelet ¥ | ND | 0.8 ± 0.8 | 90 |
| 1 × 10−3 | 0.2 ± 0.2 | <90 | |
| 1 × 10−6 | 0.2 ± 0.2 | <90 | |
| Plasma ¥ | ND | 0.4 ± 0.4 | <90 |
| 1 × 10−3 | 0.2 ± 0.2 | <90 | |
| 1 × 10−6 | 0.3 ± 0.2 | <90 | |
| RCC € | ND | 0.7 ± 0.2 | 90 |
| RBC € | ND | 0.7 ± 0.1 | 90 |
| 1 × 10−3 | 1.2 ± 0.3 | 90 | |
| 1 × 10−6 | 1.0 ± 0.6 | 90 | |
| 1 × 10−9 | 1.3 ± 0.7 | 90 | |
| SN € | ND | 1.1 ± 0.4 | 90 |
| Viability (%) | |||
|---|---|---|---|
| Colorimetry (MTT) | Microscopy (AO/DAPI) | Cytometry (7-AAD) | |
| L929 control | N/A | 94.1 ± 1.8 | 92.1 ± 2.8 |
| L929 with MAAC | 96.2 ± 6.6 | 91.5 ± 6.2 | 90.9 ± 2.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca, S.; Cayer, M.-P.; Ahmmed, K.M.T.; Khadem-Mohtaram, N.; Charette, S.J.; Brouard, D. Characterization of the Antibacterial Activity of an SiO2 Nanoparticular Coating to Prevent Bacterial Contamination in Blood Products. Antibiotics 2022, 11, 107. https://doi.org/10.3390/antibiotics11010107
Fonseca S, Cayer M-P, Ahmmed KMT, Khadem-Mohtaram N, Charette SJ, Brouard D. Characterization of the Antibacterial Activity of an SiO2 Nanoparticular Coating to Prevent Bacterial Contamination in Blood Products. Antibiotics. 2022; 11(1):107. https://doi.org/10.3390/antibiotics11010107
Chicago/Turabian StyleFonseca, Sahra, Marie-Pierre Cayer, K. M. Tanvir Ahmmed, Nima Khadem-Mohtaram, Steve J. Charette, and Danny Brouard. 2022. "Characterization of the Antibacterial Activity of an SiO2 Nanoparticular Coating to Prevent Bacterial Contamination in Blood Products" Antibiotics 11, no. 1: 107. https://doi.org/10.3390/antibiotics11010107