1. Introduction
Among the top five human infections requiring medical treatment is dermatitis [
1]. Treatment of bacterial and fungal skin infections is usually based on antibiotic therapy, which is often ineffective due to the involvement of antibiotic-resistant microbial strains such as methicillin-resistant
Staphylococcus aureus (MRSA) [
2] and
Candida sp. [
3]. In recent decades, given the poor innovation in the discovery of new antimicrobials and the frequency of recalcitrant skin infections, the need for innovative anti-infective therapeutics is becoming more and more urgent. In this field, great interest in the last 20 years has been focused on the potential of natural products.
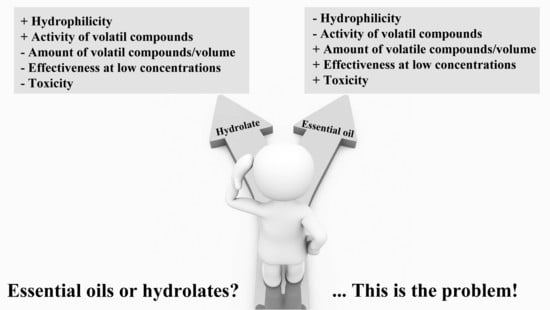
In recent years, there has been growing interest in natural products obtained from aromatic plant distillation: essential oils (EOs) and hydrolates (Hys). As such, there are many scientific articles about the effectiveness of EOs in various contexts: antimicrobials, immunomodulatory, antioxidants, anti-inflammatory, pain-relievers, etc., but there is little evidence on the activities of Hys.
Official Pharmacopoeias well define the two natural products. The EO is considered to be a complex odorous product obtained by steam distillation, hydro-distillation, or by the dry distillation of a plant, some of its parts, or, in the case of OEs obtained from
Citrus spp., through appropriate mechanical cold processes [
4]. Similarly, starting from 2012, the French Pharmacopoeia defines the Hy as a product obtained through the distillation of different parts of aromatic plants, which separates from the essential oil at the end of the distillation [
5].
While they originate from the same process, the two distillation products are quite different in terms of chemical composition and effectiveness.
EOs are hydrophobic mixtures mainly characterized by terpene molecules that, on the contrary, are extremely diluted in Hys. In fact, the Hys are hydrophilic solutions characterized, up to a maximum of 1 g/L, by the terpene components present in the corresponding EO [
6]. Furthermore, in the Hy, the relative ratio of each terpenic molecule will be conditioned by its hydrophilic characteristics. Owing to this, the major components of an EO may not be the same that is present in the corresponding Hy.
Due to the high oxicity of many terpene compounds [
7], essential oils require special warnings when used
per os or in topical applications [
8]. On the contrary, Hys resulting from dilution of terpenic solutions are less toxic and can be used more easily for the same applications.
However, only few studies have been carried out on EOs and Hys obtained from the same distillation process in order to compare their chemical composition [
9,
10,
11], or study some of their activities such as psychopharmacological and anti-cancer activities [
12,
13], or larvicidal and nematodicidal ones [
14,
15]. Our group participated in these early investigations, assessing the chemical composition and the antimicrobial activity of the EO and Hy obtained from
Monarda citriodora in a recent research. The study showed that, to achieve the same inhibitory effect of EO, a higher volume of Hy was necessary; however, in this volume, the concentration of active components was lower than that present in the corresponding EO, i.e., the EO from the same plant source [
16]. Therefore, data indicate a higher likelihood for the active compounds isolated from
M. citriodora Hy to be more active in the aqueous phase, because they can more easily reach their target, or because they are not contrasted with antagonistic compounds present only in the OE.
Given this background and in view of improving the knowledge on Hy potential uses, the first aim of this study was to evaluate the antimicrobial activity of six EOs and the companion Hys isolated from the same aromatic plant cultivated in Italy, towards fungal and bacterial strains potentially pathogenic for human skin. The following microorganisms isolated from patients with skin infections included the following. Six bacteria: methicillin-resistant Staphylococcus aureus (MRSA), methicillin- susceptible Staphylococcus aureus (MSSA), Streptococcus pyogenes, vancomycin-resistant enterococci (VRE) Enterococcus faecalis and Enterococcus faecium. Four drug-resistant yeasts: Candida albicans, Candida parapsilosis, Candida glabrata and Candida tropicalis. Five dermatophytes: Trichophyton soudanense, Trichophyton tonsurans, Trichophyton rubrum, Trichophyton violaceum and Microsporum canis. The second aim was to compare the relative concentration of active volatiles present in EOs and Hys obtained from the same plant by using the volatiles’ conversion factor (CF).
3. Discussion
For more than half a century, humans have relied primarily on antibiotics and vaccines to treat and prevent microbial infections. In recent decades, despite the great progress in the medical and pharmaceutical fields, the traditional treatment of infectious diseases is often ineffective due to the increased resistance of microbial strains to antibiotics. To date, one fifth of global deaths is due to infectious diseases [
17], as the uncontrolled use of antibiotics in the clinical, veterinary, and agricultural fields has led to the spread of multidrug-resistant microbial strains. While the pharmaceutical industry has addressed this problem by modifying existing antibiotics and developing new ones, microbial strains respond to the pharmaceutical industry by inactivating these new strategies with the development of antibiotic resistance. This scenario clearly highlights the need for new antimicrobial agents with different modes of action than those of traditional antibiotics.
Natural products are among the most promising candidates because they have low toxicity, low environmental impact, and a broad spectrum of action when compared to synthetic antimicrobial substances.
Many studies have shown the antimicrobial activity of various EOs [
18,
19] also regarding muti-drug resistant bacteria and fungi, due to a broad spectrum of cytocidal activity [
20,
21]. For example, the EO of
S. montana, in addition to anti-oxidant activity, proved effective against bacteria and dermatophytes; especially
T. violaceum,
T. rubrum,
T. tonsurans,
T. mentagrophytes and
P. oryzae [
22,
23], while the EO obtained from
O. hirtum showed antimicrobial activity against both Gram+ and Gram- strains [
24,
25]. The EOs belonging to the
Lavandula genus, in addition to having an antimicrobial activity against a broad spectrum of microorganisms [
26,
27,
28], show sedative properties on the central nervous system, as well as anti-inflammatory and re-epithelializing properties [
29,
30,
31]. Furthermore, EOs and Hys derived from non-native plants belonging to the
Monarda genus grown in Italy, have shown interesting antimicrobial activities towards Gram+, Gram- yeasts and environmental fungi [
32,
33,
34].
The effectiveness of active ingredients was also studied. β-Linalool is a non-toxic alcohol most common in nature. It is present in the phytocomplexes of lavender EOs but also of many other EOs. In the EO of
Cinnamomum camphora (Ho wood) it can reach concentrations higher than 90%. Literature data show its comprehensive range of bioactive properties including antimicrobial activity [
35]. The main component of both EO and Hy of
O. hirtum is the thymol, a phenol monoterpene isomer of carvacrol, particularly present in EOs obtained from species belonging to the
Thymus genus. This natural compound has an antimicrobial spectrum wider than that of β-linalool, including Gram-positive, Gram-negative bacteria (especially pathogens of the airways), and fungi. Finally, it shows the ability to interfere with the fungal transformation process from the cellular form to the hyphal form [
36]. The antimicrobial activity of carvacrol, main component of both
S. montana and
Monarda spp. natural products, is higher than that of the other volatile compounds due to the free hydroxyl group, hydrophobicity, and the phenol moiety. In particular, it shows a great activity against Gram- food-borne pathogens [
37].
Among the main active compounds analyzed, it is possible to identify an activity gradient (linalool < thymol < carvacrol). This gradient is consistent with the data of antimicrobial efficacy actually observed, as the least active natural compounds are those obtained from the Lavandula genus, while the others show stronger antimicrobial activities.
Moreover, several EOs have been shown to interfere with the ability of microorganisms to form biofilm, which is often linked to chronic, difficult-to-treat infections such as skin and wound infections [
38,
39].
S. montana EO was shown to be able to inhibit biofilm formation and interfere with preformed biofilms of Gram+ bacteria, including
S. aureus [
23].
Despite the high antimicrobial activity of EOs, use as such is not recommended due to their high concentration of hydrophobic active ingredients with a toxic potential. Therefore, to avoid toxic effects, EOs need to be used in low concentrations by diluting them in an appropriate vehicle before use.
On the contrary, Hys are hydrophilic solutions containing up to a maximum 1g/L of the EOs active compounds. Although more perishable than EOs, they are generally safe and do not need to be diluted in a vehicle before use. This feature of Hys makes them interesting both for oral intake and skin applications. The latter use becomes especially important in the presence of skin infections.
However, the antimicrobial activity of Hys would certainly appear to be milder than that of the corresponding EOs. In fact, the simple comparison of MIC values obtained from the antimicrobial analysis of the EOs and Hys used in this study evidence that the first are more effective at a lower concentration.
Table 1 and
Table 2 show that the EOs active on at least the 50% of the strains have inhibitory and cytocidal actions at concentrations ranging between 0.125%
v/
v and 2%
v/
v. Whereas, the Hys must be used at concentrations between 25%
v/
v and 50%
v/
v to reach the same antimicrobial activity, i.e., they need to be from 25 to 200 times more concentrated than EOs.
However, if we consider the relative concentration of active chemicals, can we say that Hys really have milder antimicrobial actions than the corresponding EOs?
Table 5 and
Table 6 show that this cannot be said. In fact, the calculated IR50
Hy/CF is lower than the IR50
EO, as well as the IC50
Hy/CF calculated for each microbial strain is lower than the IC50
EO. This means that, to obtain the inhibition of 50% of growth of both the initial inoculum of each strain and total microbial strains, a concentration of EOs’ volatiles greater than that of the corresponding Hys is required. It results, therefore, in the Hys’ volatiles being relatively more effective than those of EOs. This activity could be due to the hydrophilic environment of Hy, which provides a greater bioavailability of volatiles for the interaction with bacteria and fungi [
40], or to the antagonistic action present among chemical components of the EO phytocomplex.
These data are interesting because they show the antimicrobial activity of Hys from another point of view, especially as it concerns potential clinical applications for the treatment of skin infections. In fact, in these pathologies, local applications that are simultaneously effective for the patient and safe for intact or damaged skin are indispensable.
Potential applications encompass all small skin infections that need daily local treatments with antimicrobial creams and ointments, but also of more serious pathologies such as Tinea capitis generated by dermatophytes that essentially afflicts children, or antibiotic resistant/sensitive infections of sores or wounds whose treatment becomes important for skin re-epithelialization, or chronic vaginal infections induced by yeasts in which the topical use of concentrated EOs is absolutely contraindicated due to their toxicity.
In all cases, the use of Hys with antimicrobial activity compatible with a cutaneous or mucosal treatment would be of great interest. In fact, Hys are already on the market, and they can be used on the skin of non-allergic subjects without inducing adverse effects. Currently, Hys in Italy are used in formulations of cosmetic products for body care, or they are sold pure for cosmetic and food use. As is well known, the Italian market is a famous perfume and fragrance hub that is constantly looking for new products and is able to influence the Hys production of primary producers. Globally, the Hys market in Europe has been growing for several years, attaining, in 2018, a 40% share of the world market [
41]. From 2019 to 2024, this share is set to increase by an additional 5.2% [
42]. Owing to these reasons and in light of our preliminary data, it becomes more and more interesting to deepen the studies on Hys.
4. Materials and Methods
4.1. Clinical Strains
Fifteen clinical strains (six Gram-positive bacterial strains and nine fungal strains), which are potential skin pathogens provided by the UOC of Microbiology of Policlinico Universitario A. Gemelli of Rome, Italy, were used. Two of the six bacterial strains were resistant (R) to antibiotics. Bacterial strains were: Staphylococcus aureus MRSA (0.1R), Streptococcus pyogenes (0.2), Enterococcus faecalis VRE (0.3R), Enterococcus faecium (0.4), Staphylococcus aureus MSSA (0.5), Enterococcus faecalis (0.6). Whereas, four of the nine fungal strains were yeasts (Candida albicans (3.1), Candida parapsilosis (0.1R), Candida glabrata (0.2R), and Candida tropicalis (0.3R)), three of which were resistant to common antifungals, and five dermatophytes (Trichophyton rubrum, Trichophyton tonsurans, Trichophyton soudanense, Trichophyton violaceum, and Microsporum canis). Mueller Hinton medium (Becton Dickinson and Company, Cockeysville, MD, USA) was used to grow bacterial strains at 37 °C for 24 h, while fungal strains were grown on RPMI broth and Sabouraud agar medium (Oxoid, Wade Road, Basingstoke, Hants, UK). In particular, yeasts were grown at 37 °C for 24 h, and dermatophytes at 30 °C for 7 days.
4.2. Essential Oils and Related Hydrolates
EOs and Hy from six aromatic plants grown and processed in Italy were studied (S. montana, L. angustifolia, L. intermedia, O. hirtum, M. didyma, and M. fistulosa). All EOs and Hys were kindly granted by FX Laboratorio Benessere srl (Arzignano, Vicenza, Italy), except for those isolated from M. didyma and M. fistulosa species, which were provided by DISTAL, University of Bologna.
4.3. Gas Chromatography Mass Spectrometry Analysis
Analyses were performed on a Bruker ScionSQ gas chromatograph, coupled with a single quadrupole mass-spectrometer (GC-MS) (Bruker, Milan, Italy). Compounds were separated BD-5 a semi-standard non-polar column (30 m × 0.25 mm, i.d.0.25 μm) (Phenomenex, Bologna, Italy). EOs were diluted 1:1000 (
v/
v) in ethyl acetate, and 1 μL of this dilution was injected into GC-MS. Samples of hydrolate were diluted 1:5 (
v/
v) in ethanol (99.8%), and 1 μL of this dilution was injected into GC-MS. The percentage (
w/
w) of the amount of the compounds of EO present in Hy was carried out gravimetrically. Peaks were identified by comparing the retention times with those of authentic standard MS fragmentation patterns and final confirmation by matching with the components of the commercial library NIST mass spectral database (vers. 6.41). The percentage composition of the oils was computed by the normalization method from the GC peak areas. R.I. were generated by using a series of n-alkanes from C7 to C40 (Sigma-Aldrich, Milan, Italy) and compared with data reported in the literature [
43,
44,
45,
46]. All analyses were repeated in triplicate.
4.4. Gravimetric Analysis
Five mL of each Hy were subjected to liquid/liquid isolation with 5 mL of CH2Cl2 (n = 3). The organic phases were pooled, and the solvent evaporated by means of a rotary evaporator at reduced pressure. The residue obtained was weighed and the percentage (w/v) content of volatiles in the hydrolate evaluated.
4.5. Broth Microdilution Susceptibility Test
The broth microdilution (BMD) susceptibility test according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) international guidelines were performed. The BMD test was performed on a 96-well plate by adding 100 μL of a cell suspension equal to 5 × 105 CFU/mL to a final volume of 200 μL. Scalar dilutions, between 50% v/v (500 μL/mL) and 3.125% v/v (31.25 μL/mL) of Hy and between 2% (20 μL /mL) and 0.06% (0.6 μL/mL) of EO were tested. EOs and Hys were dissolved in a suitable nutrient agar (as specified in paragraph 4.1) and 0.5% v/v of Tween 80 was used to deliver the EOs into the hydrophilic medium. Plates were incubated overnight at 37 °C. After this period, MIC values were determined by spectrophotometric reading at 450 nm (EL808, Biotek, Winooski, VT, USA), except for MICs values of the dermatophytes, which were assessed by visual reading. To evaluate the MLC, 5 μL of the content of each well was seeded on Muller Hilton or Sabouraud agar plates, which were incubated for 24 h at 37 °C. The MIC is defined as the lowest concentration that completely inhibits the organism’s growth when compared to the growth of control. Whereas, the MLC is defined as the lowest concentration corresponding to the death of 99.9% or more of the initial inoculum. Each test was performed in triple, and both negative and positive controls were included. Values corresponding to the IR or LR of 50% and 90% of all strains were calculated. As discussed in the “Data management” paragraph, the value corresponding to a concentration of EOs or Hys necessary to obtain the inhibition of 50% of the initial inoculum was extrapolated for each strain analyzed.
4.6. Comparison Between EO and Hy
Hy and EO comparison was made, as described in Di Vito M et al. [
16]. Comparison was based on comparing the total volatiles content of EO with that of the corresponding Hy. Briefly, the Essential Oil Total volatiles Area (EOTA) and the Hydrolate Total volatiles Area (HYTA) were calculated by evaluating areas covered by the total volatiles in the chromatograms multiplied by EO and Hy respective dilutions prior to GC–MS (1000 and 5, respectively). The semi-quantitative volatiles’ Conversion Factor (CF) between the EO and the Hy was assumed to be the EOTA/HYTA ratio. Comparison between an EO and its corresponding Hy was made by dividing the IC50 or IR50 of each Hy by its CF. If the value of this ratio corresponds to the value of IC50 or IR50 of the EO, it means that the two natural products are equivalent in terms of relative antimicrobial activity, as the same amount of volatiles is needed in both EO and Hy to inhibit the growth of 50% of the initial inoculum. Whereas, values of this ratio lower or higher than the IC50 or IR50 of the OE show a relative antimicrobial activity of volatiles contained in the Hy higher or lower than that of the EO, respectively.
4.7. Data Management
The IC50 value of each natural substance (O. hirtum, S. montana, M. didyma and M. fistulosa) and distillation product (EO and Hy) vs. each microbial strain was obtained by interpolating the OD450 values corresponding to the tested dilutions with a regression line, and calculating the dilution value (% v/v) corresponding to half of the OD450 value of the positive control. All the values obtained from both the microbiological and chemical analyzes were processed obtaining mean and standard deviation values.