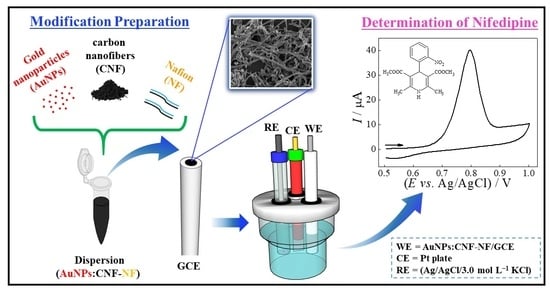
Voltammetric Sensing of Nifedipine Using a Glassy Carbon Electrode Modified with Carbon Nanofibers and Gold Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Apparatus
2.3. CNF Activation and CNF Modification with Gold Nanoparticles
2.4. Preparation of the Modified GCE
2.5. Preparation of the Samples
3. Results and Discussion
3.1. Morphological and Chemical Characterization
3.2. Electrochemical Characterization of Electrodes
3.3. Electrochemical Behavior of NIF
3.4. Mechanism Proposal of NIF Electrochemical Oxidation
3.5. Optimization of SWV Parameters
3.6. Voltammetric Determination of Nifedipine
3.7. Study of Repeatability, Reproducibility, and Selectivity
3.8. Applications in Biological and Environmental Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scremin, J.; Sartori, E.R. Simultaneous determination of nifedipine and atenolol in combined dosage forms using a boron-doped diamond electrode with differential pulse voltammetry. Can. J. Chem. 2018, 96, 1–7. [Google Scholar] [CrossRef]
- Mokhtari, B.; Nematollahi, D.; Salehzadeh, H. Electrochemical simultaneous determination of nifedipine and its main metabolite dehydronifedipine using MWCNT modified glassy carbon electrode. J. Mol. Liq. 2018, 264, 543–549. [Google Scholar] [CrossRef]
- Wang, X.-D.; Li, J.-L.; Lu, Y.; Chen, X.; Huang, M.; Chowbay, B.; Zhou, S.-F. Rapid and simultaneous determination of nifedipine and dehydronifedipine in human plasma by liquid chromatography–tandem mass spectrometry: Application to a clinical herb–drug interaction study. J. Chromatogr. B 2007, 852, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Awad Uday, Y.; Shravan Kumar, P.; Dinesh Kumar, A.; Bari, S. Estimation of nifedipine by reverse phase high performance liquid chromatography tablet dosage form. Int. J. Pharm. Life Sci. 2011, 2, 610–612. [Google Scholar]
- Asthana, S.; Kaur, V.; Chawla, P.; Saraf, S.A. Rapid and sensitive HPLC-UV method for simultaneous estimation of Nifedipine, Nateglinide and Lovastatin: Quantitative application to polypill based synthetic ternary mixture. Int. J. Pharmtech. Res. 2010, 2, 682–688. [Google Scholar]
- Lesko, L.J.; Miller, A.K.; Yeager, R.L.; Chatterji, D.C. Rapid GC method for quantitation of nifedipine in serum using electron capture detection. J. Chromatogr. Sci. 1983, 21, 415–419. [Google Scholar] [CrossRef]
- Shapovalov, V. Determination of nifedipine by differential pulse adsorptive stripping polarography. J. Anal. Chem. 2002, 57, 157–158. [Google Scholar] [CrossRef]
- Xue-Cai, T.; Zhi-Bin, M.; Dong-Ping, W.; Guang-Hui, D.; Zai-Yin, H.; Pei-Xiang, C. Determination of nifedipine using multiwalled carbon nanotubes-nafion composite film modified glassy carbon electrode. Chin. J. Anal. Chem. 2007, 35, 495. [Google Scholar]
- Agrawal, N.; Savalia, R.; Chatterjee, S. Nanostructured zinc oxide film amalgamated with functionalized carbon nanotubes for facile electrochemical determination of nifedipine. Colloids Surf. B Biointerfaces 2021, 201, 111635. [Google Scholar] [CrossRef]
- Gaichore, R.R.; Srivastava, A.K. Voltammetric determination of nifedipine using a β-cyclodextrin modified multi-walled carbon nanotube paste electrode. Sens. Actuators B Chem. 2013, 188, 1328–1337. [Google Scholar] [CrossRef]
- Baghayeri, M.; Namadchian, M.; Karimi-Maleh, H.; Beitollahi, H. Determination of nifedipine using nanostructured electrochemical sensor based on simple synthesis of Ag nanoparticles at the surface of glassy carbon electrode: Application to the analysis of some real samples. J. Electroanal. Chem. 2013, 697, 53–59. [Google Scholar] [CrossRef]
- Khairy, M.; Khorshed, A.A.; Rashwan, F.A.; Salah, G.A.; Abdel-Wadood, H.M.; Banks, C.E. Simultaneous voltammetric determination of antihypertensive drugs nifedipine and atenolol utilizing MgO nanoplatelet modified screen-printed electrodes in pharmaceuticals and human fluids. Sens. Actuators B Chem. 2017, 252, 1045–1054. [Google Scholar] [CrossRef]
- Wong, A.; Santos, A.M.; Cardenas-Riojas, A.A.; Baena-Moncada, A.M.; Sotomayor, M.D.P.T. Voltammetric sensor based on glassy carbon electrode modified with hierarchical porous carbon, silver sulfide nanoparticles and fullerene for electrochemical monitoring of nitrite in food samples. Food Chem. 2022, 383, 132384. [Google Scholar] [CrossRef]
- Santos, A.M.; Wong, A.; Feitosa, M.H.A.; Silva, L.P.; Fatibello-Filho, O.; Moraes, F.C. Using Carbon Paste Electrode Modified with Graphene and Nanodiamond for the Determination of Nimesulide in Biologic and Environmental Samples. Electroanalysis 2022, 34, 1441–1449. [Google Scholar] [CrossRef]
- Feitosa, M.H.A.; Santos, A.M.; Wong, A.; Rocha, R.S.; Moraes, F.C. Voltammetric Determination of Trimethoprim Using a Glassy Carbon Electrode Modified with Printex(6L) Carbon and Gold Nanoparticles. Analytica 2023, 4, 159–169. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Yang, B.; Liu, R. Structural and electronic properties of carbon nanowires made of linear carbon chains enclosed inside zigzag carbon nanotubes. Carbon 2006, 44, 456–462. [Google Scholar] [CrossRef]
- Kumar, A.; Avasthi, D.; Tripathi, A.; Filip, L.; Carey, J.; Pivin, J. Formation and characterization of carbon nanowires. J. Appl. Phys. 2007, 102, 044305. [Google Scholar] [CrossRef]
- Harsini, M.; Widyaningrum, B.A.; Fitriany, E.; Paramita, D.R.A.; Farida, A.N.; Baktir, A.; Kurniawan, F.; Sakti, S.C.W. Electrochemical synthesis of polymelamine/gold nanoparticle modified carbon paste electrode as voltammetric sensor of dopamine. Chin. J. Anal. Chem. 2022, 50, 100052. [Google Scholar] [CrossRef]
- Yılmaz, G.E.; Saylan, Y.; Göktürk, I.; Yılmaz, F.; Denizli, A. Selective Amplification of Plasmonic Sensor Signal for Cortisol Detection Using Gold Nanoparticles. Biosensors 2022, 12, 482. [Google Scholar] [CrossRef]
- Santos, A.M.; Wong, A.; Ferreira, L.M.C.; Soares, F.L.F.; Fatibello-Filho, O.; Moraes, F.C.; Vicentini, F.C. Multivariate optimization of a novel electrode film architecture containing gold nanoparticle-decorated activated charcoal for voltammetric determination of levodopa levels in pre-therapeutic phase of Parkinson`s disease. Electrochim. Acta 2021, 390, 138851. [Google Scholar] [CrossRef]
- Chang, F.; Ren, K.; Li, S.; Su, Q.; Peng, J.; Tan, J. A voltammetric sensor for bisphenol A using gold nanochains and carbon nanotubes. Ecotoxicol. Environ. Saf. 2023, 252, 114588. [Google Scholar] [CrossRef]
- Saki, A.; Pourghobadi, Z.; Derikvand, Z. Gold Nanoparticles Decorated with Multi-Walled Carbon Nanotubes/Graphene Oxide for Voltammetric Determination of Dopamine in the Presence of Acetaminophen. J. Electrochem. Soc. 2022, 169, 116507. [Google Scholar] [CrossRef]
- Cardenas-Riojas, A.A.; Calderon-Zavaleta, S.L.; Quiroz-Aguinaga, U.; López, E.O.; Ponce-Vargas, M.; Baena-Moncada, A.M. Evaluation of an electrochemical sensor based on gold nanoparticles supported on carbon nanofibers for detection of tartrazine dye. J. Solid State Electrochem. 2023, 27, 1969–1982. [Google Scholar] [CrossRef]
- Laube, N.; Mohr, B.; Hesse, A. Laser-probe-based investigation of the evolution of particle size distributions of calcium oxalate particles formed in artificial urines. J. Cryst. Growth 2001, 233, 367–374. [Google Scholar] [CrossRef]
- Duc Chinh, V.; Speranza, G. Synthesis of Gold Nanoparticles Decorated with Multiwalled Carbon Nanotubes (Au-MWCNTs) via Cysteaminium Chloride Functionalization. Sci. Rep. 2019, 9, 5667. [Google Scholar] [CrossRef] [PubMed]
- Nekounam, H.; Allahyari, Z.; Gholizadeh, S.; Mirzaei, E.; Shokrgozar, M.A.; Faridi-Majidi, R. Simple and robust fabrication and characterization of conductive carbonized nanofibers loaded with gold nanoparticles for bone tissue engineering applications. Mater. Sci. Eng. C 2020, 117, 111226. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J.; Faulkner, L.R.; White, H.S. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Nicholson, R.S. Theory and Application of Cyclic Voltammetry for Measurement of Electrode Reaction Kinetics. Anal. Chem. 1965, 37, 1351–1355. [Google Scholar] [CrossRef]
- Lavagnini, I.; Antiochia, R.; Magno, F. An Extended Method for the Practical Evaluation of the Standard Rate Constant from Cyclic Voltammetric Data. Electroanalysis 2004, 16, 505–506. [Google Scholar] [CrossRef]
- Şentürk, Z.; Özkan, S.A.; Özkan, Y. Electroanalytical study of nifedipine using activated glassy carbon electrode1Presented at the Fourth International Symposium on Pharmaceutical Science, June 1995, Ankara, Turkey.1. J. Pharm. Biomed. Anal. 1998, 16, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Zhao, F.; Zeng, B. Highly dispersive hollow PdAg alloy nanoparticles modified ionic liquid functionalized graphene nanoribbons for electrochemical sensing of nifedipine. Electrochim. Acta 2015, 168, 330–336. [Google Scholar] [CrossRef]
- Zeng, Q.; Wei, T.; Wang, M.; Huang, X.; Fang, Y.; Wang, L. Polyfurfural film modified glassy carbon electrode for highly sensitive nifedipine determination. Electrochim. Acta 2015, 186, 465–470. [Google Scholar] [CrossRef]








| Electrode | Technique | Linear Range (µmol L−1) | LOD (nmol L−1) | Matrice | Ref. |
|---|---|---|---|---|---|
| βCD-CNT-PE | DPAdSV | 0.0477–20.0 | 14.7 | Tablet, urine and serum | [10] |
| AgNPs/GCE | DPV | 0.80–60 | 720 | Tablet and urine | [11] |
| MgO-SPEs | DPV | 0.20–104 | 32 | Tablet and urine | [12] |
| GRs–IL–hPdAg/GCE | DPV | 0.010–4.0 | 4.0 | Tablet | [31] |
| BDD | DPV | 3.98–107 | 612 | Tablet | [1] |
| Polyfurfural film/GCE | DPV | 0.010–7.0 | 5.0 | Tablet and urine | [32] |
| AuNPs:CNF-NF/GCE | SWV | 0.020–2.5 | 2.8 | River water, urine and serum | This work |
| Samples | Added (µmol L−1) | Found (µmol L−1) * | Recovery (%) ** |
|---|---|---|---|
| Serum A | 0.100 | 0.101 ± 0.005 | 101 |
| Serum B | 1.00 | 1.05 ± 0.11 | 105 |
| Urine A | 0.100 | 0.103 ± 0.005 | 103 |
| Urine B | 1.00 | 0.960 ± 0.008 | 95.7 |
| River water A | 0.100 | 0.106 ± 0.009 | 106 |
| River water B | 1.00 | 1.08 ± 0.05 | 108 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, A.M.; Wong, A.; Feitosa, M.H.A.; Cardenas-Riojas, A.A.; Calderon-Zavaleta, S.L.; Baena-Moncada, A.M.; Sotomayor, M.D.P.T.; Moraes, F.C. Voltammetric Sensing of Nifedipine Using a Glassy Carbon Electrode Modified with Carbon Nanofibers and Gold Nanoparticles. Biosensors 2023, 13, 829. https://doi.org/10.3390/bios13080829
Santos AM, Wong A, Feitosa MHA, Cardenas-Riojas AA, Calderon-Zavaleta SL, Baena-Moncada AM, Sotomayor MDPT, Moraes FC. Voltammetric Sensing of Nifedipine Using a Glassy Carbon Electrode Modified with Carbon Nanofibers and Gold Nanoparticles. Biosensors. 2023; 13(8):829. https://doi.org/10.3390/bios13080829
Chicago/Turabian StyleSantos, Anderson M., Ademar Wong, Maria H. A. Feitosa, Andy A. Cardenas-Riojas, Sandy L. Calderon-Zavaleta, Angélica M. Baena-Moncada, Maria D. P. T. Sotomayor, and Fernando C. Moraes. 2023. "Voltammetric Sensing of Nifedipine Using a Glassy Carbon Electrode Modified with Carbon Nanofibers and Gold Nanoparticles" Biosensors 13, no. 8: 829. https://doi.org/10.3390/bios13080829