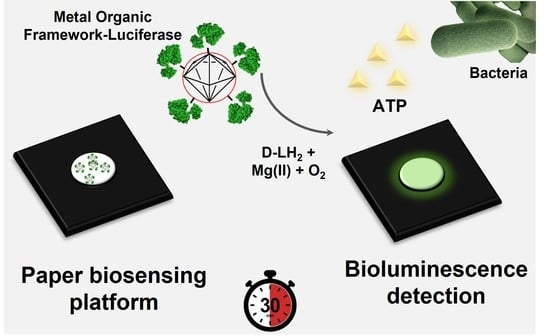
Super-Stable Metal–Organic Framework (MOF)/Luciferase Paper-Sensing Platform for Rapid ATP Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Portable Light Detectors
2.3. Synthesis of the ZIF-8@Luc Biocomposite
2.4. Fabrication of the ZIF-8@Luc Paper Sensor
2.5. BL Signal Acquisition and Data Treatment
2.6. Characterization of the ZIF-8@Luc Paper-Sensing Platform
2.7. Real Sample Analysis and Stability Studies
3. Results and Discussion
3.1. ZIF-8@Luc Paper Sensor Fabrication
3.2. Characterization of the ZIF-8@Luc Paper Sensor
3.3. ATP Quantification in Real Samples
3.4. Stability Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aycicek, H.; Oguz, U.; Karci, K. Comparison of Results of ATP Bioluminescence and Traditional Hygiene Swabbing Methods for the Determination of Surface Cleanliness at a Hospital Kitchen. Int. J. Hyg. Environ. Health 2006, 209, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Branchini, B.R.; Southworth, T.L.; Fontaine, D.M.; Kohrt, D.; Talukder, M.; Michelini, E.; Cevenini, L.; Roda, A.; Grossel, M.J. An Enhanced Chimeric Firefly Luciferase-Inspired Enzyme for ATP Detection and Bioluminescence Reporter and Imaging Applications. Anal. Biochem. 2015, 484, 148–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heathcote, R.; Stadelmann, B. Measuring of ATP Bioluminescence as a Means of Assessing Washer Disinfector Performance and Potentially as a Means of Validating the Decontamination Process. Healthc. Infect. 2009, 14, 147–151. [Google Scholar] [CrossRef]
- Bruno-Murtha, L.A.; Fridman, A.; Osgood, R. A Quantitative Assessment of Cleanliness in the Operating Room (OR). Am. J. Infect. Control 2014, 42, S36. [Google Scholar] [CrossRef]
- Calabretta, M.M.; Lopreside, A.; Montali, L.; Zangheri, M.; Evangelisti, L.; D’Elia, M.; Michelini, E. Portable Light Detectors for Bioluminescence Biosensing Applications: A Comprehensive Review from the Analytical Chemist’s Perspective. Anal. Chim. Acta 2022, 1200, 339583. [Google Scholar] [CrossRef] [PubMed]
- Gill, K.; Horsley, H.; Kupelian, A.S.; Baio, G.; De Iorio, M.; Sathiananamoorthy, S.; Khasriya, R.; Rohn, J.L.; Wildman, S.S.; Malone-Lee, J. Urinary ATP as an Indicator of Infection and Inflammation of the Urinary Tract in Patients with Lower Urinary Tract Symptoms. BMC Urol. 2015, 15, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q. Cellular ATP Dynamics. Cells 2022, 11, 1920. [Google Scholar] [CrossRef]
- Onyango, I.G.; Dennis, J.; Khan, S.M. Mitochondrial Dysfunction in Alzheimer’s Disease and the Rationale for Bioenergetics Based Therapies. Aging Dis. 2016, 7, 201–214. [Google Scholar] [CrossRef] [Green Version]
- Santangelo, M.F.; Libertino, S.; Turner, A.P.F.; Filippini, D.; Mak, W.C. Integrating Printed Microfluidics with Silicon Photomultipliers for Miniaturised and Highly Sensitive ATP Bioluminescence Detection. Biosens. Bioelectron. 2018, 99, 464–470. [Google Scholar] [CrossRef] [Green Version]
- Calabretta, M.M.; Álvarez-Diduk, R.; Michelini, E.; Roda, A.; Merkoçi, A. Nano-Lantern on Paper for Smartphone-Based ATP Detection. Biosens. Bioelectron. 2020, 150, 111902. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 Principles of Green Analytical Chemistry and the SIGNIFICANCE Mnemonic of Green Analytical Practices. TrAC-Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Nowak, P.M.; Wietecha-Posłuszny, R.; Pawliszyn, J. White Analytical Chemistry: An Approach to Reconcile the Principles of Green Analytical Chemistry and Functionality. TrAC-Trends Anal. Chem. 2021, 138, 116223. [Google Scholar] [CrossRef]
- Calabretta, M.M.; Martínez-Pérez-Cejuela, H.; Gregucci, D.; Michelini, E. A Luciferase Mutant with Improved Brightness and Stability for Whole-Cell Bioluminescent Biosensors and In Vitro Biosensing. Biosensors 2022, 12, 742. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Hosseinkhani, S.; Heydari, A.; Akbari, J. Simple and Rapid Immobilization of Firefly Luciferase on Functionalized Magnetic Nanoparticles; a Try to Improve Kinetic Properties and Stability. Biomacromolecular J. 2015, 1, 104–112. [Google Scholar]
- Palomba, S.; Berovic, N.; Palmer, R.E. Bioluminescence of Monolayers of Firefly Luciferase Immobilized on Graphite. Langmuir 2006, 22, 5451–5454. [Google Scholar] [CrossRef]
- Cruz-Aguado, J.A.; Chen, Y.; Zhang, Z.; Elowe, N.H.; Brook, M.A.; Brennan, J.D. Ultrasensitive ATP Detection Using Firefly Luciferase Entrapped in Sugar-Modified Sol-Gel-Derived Silica. J. Am. Chem. Soc. 2004, 126, 6878–6879. [Google Scholar] [CrossRef]
- Yousefi-Nejad, M.; Hosseinkhani, S.; Khajeh, K.; Ranjbar, B. Expression, Purification and Immobilization of Firefly Luciferase on Alkyl-Substituted Sepharose 4B. Enzym. Microb. Technol. 2007, 40, 740–746. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, Q.; Luo, M.; Li, M.; Wang, D.; Wang, Y.; Liu, Q. Immobilization of Firefly Luciferase on PVA-Co-PE Nanofibers Membrane as Biosensor for Bioluminescent Detection of ATP. ACS Appl. Mater. Interfaces 2015, 7, 20046–20052. [Google Scholar] [CrossRef]
- Nowroozi-Nejad, Z.; Bahramian, B.; Hosseinkhani, S. Efficient Immobilization of Firefly Luciferase in a Metal Organic Framework: Fe-MIL-88(NH2) as a Mighty Support for This Purpose. Enzym. Microb. Technol. 2019, 121, 59–67. [Google Scholar] [CrossRef]
- Nowroozi-Nejad, Z.; Bahramian, B.; Hosseinkhani, S. A Fast and Efficient Stabilization of Firefly Luciferase on MIL-53(Al) via Surface Adsorption Mechanism. Res. Chem. Intermed. 2019, 45, 2489–2501. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and Synthesis of an Exceptionally Stable and Highly. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef] [Green Version]
- Gkaniatsou, E.; Sicard, C.; Ricoux, R.; Mahy, J.P.; Steunou, N.; Serre, C. Metal-Organic Frameworks: A Novel Host Platform for Enzymatic Catalysis and Detection. Mater. Horiz. 2017, 4, 55–63. [Google Scholar] [CrossRef]
- Zhuang, J.; Young, A.P.; Tsung, C.K. Integration of Biomolecules with Metal–Organic Frameworks. Small 2017, 13, 1700880. [Google Scholar] [CrossRef]
- Pérez-Cejuela, H.M.; Herrero-Martínez, J.M.; Simó-Alfonso, E.F. Recent Advances in Affinity MOF-Based Sorbents with Sample Preparation Purposes. Molecules 2020, 25, 4216. [Google Scholar] [CrossRef]
- Vaidya, L.B.; Nadar, S.S.; Rathod, V.K. Metal-Organic Frameworks (MOFs) for Enzyme Immobilization. In Metal-Organic Frameworks for Biomedical Applications; Mozafari, M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 491–523. ISBN 9780128169841. [Google Scholar]
- Liao, X.; Fu, H.; Yan, T.; Lei, J. Electroactive Metal–Organic Framework Composites: Design and Biosensing Application. Biosens. Bioelectron. 2019, 146, 111743. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yin, S.; Lin, M.; Chen, X.; Pan, Y.; Peng, Y.; Sun, J.; Kumar, A.; Liu, J. Current status and prospects of metal–organic frameworks for bone therapy and bone repair. J. Mater. Chem. B 2022, 27, 5105–5128. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Nikkhoo, E.; Hussain, C.M. Application of MOF Materials as Drug Delivery Systems for Cancer Therapy and Dermal Treatment. Coord. Chem. Rev. 2022, 451, 214262. [Google Scholar] [CrossRef]
- Kou, X.; Tong, L.; Shen, Y.; Zhu, W.; Yin, L.; Huang, S.; Zhu, F.; Chen, G.; Ouyang, G. Smartphone-assisted robust enzymes@MOFs-based paper biosensor for point-of-care detection. Biosens Bioelectron 2020, 156, 112095. [Google Scholar] [CrossRef]
- Troyano, J.; Carné-Sánchez, A.; Avci, C.; Imaz, I.; Maspoch, D. Colloidal Metal-Organic Framework Particles: The Pioneering Case of ZIF-8. Chem. Soc. Rev. 2019, 48, 5534–5546. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. Biointerface between Zif-8 and Biomolecules and Their Applications. Biointerface Res. Appl. Chem. 2021, 11, 8283–8297. [Google Scholar] [CrossRef]
- Martínez-Pérez-Cejuela, H.; Gregucci, D.; Calabretta, M.M.; Simó-Alfonso, E.F.; Herrero-Martínez, J.M.; Michelini, E. Novel Nanozeolitic Imidazolate Framework (ZIF-8)-Luciferase Biocomposite for Nanosensing Applications. Anal. Chem. 2023, 95, 2540–2547. [Google Scholar] [CrossRef] [PubMed]
- Gundacker, S.; Heering, A. The Silicon Photomultiplier: Fundamentals and Applications of a Modern Solid-State Photon Detector. Phys. Med. Biol. 2020, 65, 17TR01. [Google Scholar] [CrossRef] [PubMed]
- Calabretta, M.M.; Montali, L.; Lopreside, A.; Fragapane, F.; Iacoangeli, F.; Roda, A.; Bocci, V.; D’Elia, M.; Michelini, E. Ultrasensitive On-Field Luminescence Detection Using a Low-Cost Silicon Photomultiplier Device. Anal. Chem. 2021, 93, 7388–7393. [Google Scholar] [CrossRef]
- Martínez-Pérez-Cejuela, H.; Mompó-Roselló, Ó.; Crespí-Sánchez, N.; Palomino Cabello, C.; Catalá-Icardo, M.; Simó-Alfonso, E.F.; Herrero-Martínez, J.M. Determination of Benzomercaptans in Environmental Complex Samples by Combining Zeolitic Imidazolate Framework-8-Based Solid-Phase Extraction and High-Performance Liquid Chromatography with UV Detection. J. Chromatogr. A 2020, 1631, 461580. [Google Scholar] [CrossRef]
- Lopreside, A.; Montali, L.; Wang, B.; Tassoni, A.; Ferri, M.; Calabretta, M.M.; Michelini, E. Orthogonal Paper Biosensor for Mercury(II) Combining Bioluminescence and Colorimetric Smartphone Detection. Biosens. Bioelectron. 2021, 194, 113569. [Google Scholar] [CrossRef]
- Bergua, J.F.; Álvarez-Diduk, R.; Hu, L.; Hassan, A.H.A.; Merkoçi, A. Improved Aliivibrio Fischeri Based-Toxicity Assay: Graphene-Oxide as a Sensitivity Booster with a Mobile-Phone Application. J. Hazard. Mater. 2021, 406, 124434. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, Z.; Yu, J.; Wang, H.; Li, Y.; Duan, Y. Highly Sensitive Microfluidic Detection of Carcinoembryonic Antigen via a Synergetic Fluorescence Enhancement Strategy Based on the Micro/Nanostructure Optimization of ZnO Nanorod Arrays and in Situ ZIF-8 Coating. Chem. Eng. J. 2020, 383, 123230. [Google Scholar] [CrossRef]
- Ye, J.; Zhu, L.; Yan, M.; Xiao, T.; Fan, L.; Xue, Y.; Huang, J.; Yang, X. An Intensive and Glow-Type Chemiluminescence of Luminol-Embedded, Guanosine-Derived Hydrogel. Talanta 2021, 230, 122351. [Google Scholar] [CrossRef]
- Wang, P.; Cheng, Z.; Chen, Q.; Qu, L.; Miao, X.; Feng, Q. Construction of a Paper-Based Electrochemical Biosensing Platform for Rapid and Accurate Detection of Adenosine Triphosphate (ATP). Sens. Actuators B Chem. 2018, 256, 931–937. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Ng, R.; Li, Y.; Wu, Z.; Leung, V.; Imbrogno, S.; Pelton, R.; Brennan, J.D.; Filipe, C.D.M. An Inkjet-Printed Bioactive Paper Sensor That Reports ATP through Odour Generation. Analyst 2014, 139, 4775–4778. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Kim, H.R.; Jung, J.H.; Lee, K.B.; Kim, B.C. The Development of Paper Discs Immobilized with Luciferase/D-Luciferin for the Detection of ATP from Airborne Bacteria. Sens. Actuators B Chem. 2018, 260, 274–281. [Google Scholar] [CrossRef]
- Reyes, S.; Rizzo, E.; Ting, A.; Dikici, A.E.; Daunert, S.; Deo, S.K. Metal organic framework encapsulated tamavidin-Gluc reporter: Application in COVID-19 spike antigen bioluminescent immunoassay. Sens. Diagn. 2022, 1, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Bakke, M. A Comprehensive Analysis of ATP Tests: Practical Use and Recent Progress in the Total Adenylate Test for the Effective Monitoring of Hygiene. J. Food Prot. 2022, 85, 1079–1095. [Google Scholar] [CrossRef] [PubMed]
- Montali, L.; Calabretta, M.M.; Lopreside, A.; D’Elia, M.; Guardigli, M.; Michelini, E. Multienzyme chemiluminescent foldable biosensor for on-site detection of acetylcholinesterase inhibitors. Biosens. Bioelectron. 2020, 162, 112232. [Google Scholar]





| ATP Conc. (mM) | Time (s) | Precision (RSD, %) | |
|---|---|---|---|
| Intra-Batch (n = 6) 1 | Inter-Batch (n = 6) 2 | ||
| 0.02 | 300 | 11.0 | 9.8 |
| 600 | 12.4 | 8.0 | |
| 900 | 16.7 | 15.2 | |
| 2.00 | 300 | 18.5 | 9.7 |
| 600 | 19.9 | 11.2 | |
| 900 | 19.5 | 8.2 | |
| Km (µM) | LOD (nM) | |||
|---|---|---|---|---|
| ATP | D-LH2 | LuminoSiPM | Smartphone | |
| ZIF-8@Luc | 834 ± 50 | 240 ± 50 | 400 ± 20 | 389 ± 40 |
| Free Luc | 2774 ± 300 | 301 ± 25 | 1995 ± 300 | 666 ± 45 |
| Urine | Added (mM) | Found (mM) | Recovery (%) |
|---|---|---|---|
| Male | 0.02 | 0.018 ± 0.005 | 93.5 |
| 2.00 | 1.83 ± 0.17 | 91.8 | |
| Female | 0.02 | 0.019 ± 0.002 | 99.5 |
| 2.00 | 1.72 ± 0.14 | 86.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Pérez-Cejuela, H.; Calabretta, M.M.; Bocci, V.; D’Elia, M.; Michelini, E. Super-Stable Metal–Organic Framework (MOF)/Luciferase Paper-Sensing Platform for Rapid ATP Detection. Biosensors 2023, 13, 451. https://doi.org/10.3390/bios13040451
Martínez-Pérez-Cejuela H, Calabretta MM, Bocci V, D’Elia M, Michelini E. Super-Stable Metal–Organic Framework (MOF)/Luciferase Paper-Sensing Platform for Rapid ATP Detection. Biosensors. 2023; 13(4):451. https://doi.org/10.3390/bios13040451
Chicago/Turabian StyleMartínez-Pérez-Cejuela, Héctor, Maria Maddalena Calabretta, Valerio Bocci, Marcello D’Elia, and Elisa Michelini. 2023. "Super-Stable Metal–Organic Framework (MOF)/Luciferase Paper-Sensing Platform for Rapid ATP Detection" Biosensors 13, no. 4: 451. https://doi.org/10.3390/bios13040451