Biosensing Dopamine and L-Epinephrine with Laccase (Trametes pubescens) Immobilized on a Gold Modified Electrode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Methods
2.3. Biosensor Preparation and Validation
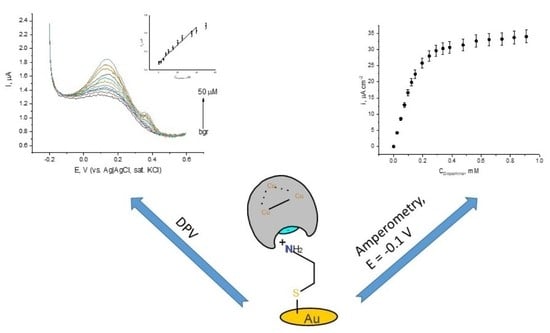
3. Results and Discussion
3.1. Characterization of Modified Electrode
3.2. Voltammetric Behavior of Immobilized Laccase in Aerated and Deaerated Buffer
3.3. Laccase Electrode in the Presence of Dopamine and L-Epinephrine
3.4. Interference Studies and Stability
4. Conclusions
- A conventional glassy carbon electrode has been modified with porous gold through electrodeposition. The resulting modified electrode has an electrochemically accessible surface area that is ca. 12 times larger than the surface area of a smooth gold electrode with the same diameter.
- The electrochemical behavior of Trametes pubescens laccase immobilized on porous gold-modified electrode has been investigated by cyclic and differential pulse voltammetry in both absence and presence of oxygen. Voltammetric studies revealed laccase susceptibility to direct electron transfer and bioelectrocatalytic oxygen reduction. In the presence of catecholamines dopamine and L-epinephrine studies showed the capability of the immobilized laccase to perform mediated oxygen reduction with efficiency dependent on substrate structure.
- Amperometric measurements performed at a constant potential of −0.1 V (vs. Ag|AgCl, KCl sat.) upon addition of either dopamine or L-epinephrine showed that the dependency of the current density on catecholamine concentrations follows the mechanism of Michaelis–Menten. The apparent kinetic constants have been found to depend on substrate structure with the values of for dopamine and for L-epinephrine. At the potentials of 0 and −0.1 V the electrochemical reduction of the catecholamines semi-quinones is superimposed over ORR, which results in increased electrode sensitivity. The calculated detection limits were found to be in the sub-micromolar concentration range.
- The concentrations of dopamine hydrochloride and L-epinephrine in ampules with solution for injections has been determined with the developed biosensor. The analytical recovery of the determination has been found to be within 99% and 106% for dopamine and between 89% and 105% for L-epinephrine.
- Interference studies have shown that at an operating potential of 0 V the presence of L-ascorbate will affect the laccase electrode response to L-epinephrine by 1.1% and to dopamine by 0.8%, whereas at an operating potential of −0.1 V the interference from L-ascorbate will contribute a 2.2% increase in the response to L-epinephrine, and 1.4% to dopamine.
- Stability tests have shown that after 21 h storage in the refrigerator, the biosensor response to dopamine decays to 1/3 of the initial one.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agrawal, K.; Chaturvedi, V.; Verma, P. Fungal laccase discovered but yet undiscovered. Bioresour. Bioprocess. 2018, 5, 4. [Google Scholar] [CrossRef]
- Arregui, L.; Ayala, M.; Gómez-Gil, X.; Gutiérrez-Soto, G.; Hernández-Luna, C.E.; Herrera de los Santos, M.; Levin, L.; Rojo-Domínguez, A.; Romero-Martínez, D.; Saparrat, M.C.N.; et al. Laccases: Structure, function, and potential application in water bioremediation. Microb. Cell Factories 2019, 18, 200. [Google Scholar] [CrossRef] [PubMed]
- Ivnitski, D.M.; Khripin, C.; Luckarift, H.R.; Johnson, G.R.; Atanassov, P. Surface characterization and direct bioelectrocatalysis of multicopper oxidases. Electrochim. Acta 2010, 55, 7385–7393. [Google Scholar] [CrossRef]
- Arrocha, A.A.; Cano-Castillo, U.; Aguila, S.A.; Vazquez-Duhalt, R. Enzyme orientation for direct electron transfer in an enzymatic fuel cell with alcohol oxidase and laccase electrodes. Biosens. Bioelectron. 2014, 61, 569–574. [Google Scholar] [CrossRef]
- Hoshikawa, Y.; Castro-Muñiz, A.; Tawata, H.; Nozaki, K.; Yamane, S.; Itoh, T.; Kyotani, T. Orientation Control of Trametes Laccases on a Carbon Electrode Surface to Understand the Orientation Effect on the Electrocatalytic Activity. Bioconjug. Chem. 2018, 29, 2927–2935. [Google Scholar] [CrossRef]
- Ulyanova, Y.; Babanova, S.; Pinchon, E.; Matanovic, I.; Singhal, S.; Atanassov, P. Effect of enzymatic orientation through the use of syringaldazine molecules on multiple multi-copper oxidase enzymes. Phys. Chem. Chem. Phys. 2014, 16, 13367–13375. [Google Scholar] [CrossRef]
- Hitaishi, V.P.; Clement, R.; Bourassin, N.; Baaden, M.; De Poulpiquet, A.; Sacquin-Mora, S.; Ciaccafava, A.; Lojou, E. Controlling Redox Enzyme Orientation at Planar Electrodes. Catalysts 2018, 8, 192. [Google Scholar] [CrossRef]
- Hitaishi, V.P.; Clément, R.; Quattrocchi, L.; Parent, P.; Duché, D.; Zuily, L.; Ilbert, M.; Lojou, E.; Mazurenko, I. Interplay between Orientation at Electrodes and Copper Activation of Thermus thermophilus Laccase for O2 Reduction. J. Am. Chem. Soc. 2020, 142, 1394–1405. [Google Scholar] [CrossRef]
- Lalaoui, N.; Rousselot-Pailley, P.; Robert, V.; Mekmouche, Y.; Villalonga, R.; Holzinger, M.; Cosnier, S.; Tron, T.; Le Goff, A. Direct Electron Transfer between a Site-Specific Pyrene-Modified Laccase and Carbon Nanotube/Gold Nanoparticle Supramolecular Assemblies for Bioelectrocatalytic Dioxygen Reduction. ACS Catal. 2016, 6, 1894–1900. [Google Scholar] [CrossRef]
- Albayati, S.A.R.; Kashanian, S.; Nazari, M.; Rezaei, S. Novel fabrication of a laccase biosensor to detect phenolic compounds using a carboxylated multiwalled carbon nanotube on the electropolymerized support. Bull. Mater. Sci. 2019, 42, 187. [Google Scholar] [CrossRef] [Green Version]
- Boubezari, I.; Bessueille, F.; Bonhomme, A.; Raimondi, G.; Zazoua, A.; Errachid, A.; Jaffrezic-Renault, N. Laccase-Based Biosensor Encapsulated in a Galactomannan-Chitosan Composite for the Evaluation of Phenolic Compounds. Biosensors 2020, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Casero, E.; Petit-Domínguez, M.D.; Vázquez, L.; Ramírez-Asperilla, I.; Parra-Alfambra, A.M.; Pariente, F.; Lorenzo, E. Laccase biosensors based on different enzyme immobilization strategies for phenolic compounds determination. Talanta 2013, 115, 401–408. [Google Scholar] [CrossRef]
- De Souza Gil, E.; Rezende, S.G.; Ribeiro Júnior, E.J.M.; Barcelos, H.T.; Scalize, P.S.; Santiago, M.F.; Quintino, M.P.; Somerset, V.S. Development of a Laccase biosensor for determination of phenolic micropollutants in surface waters. In Sensing in Electroanalysis; Kalcher, K.R.M., Švancara, I., Vytřas, K., Eds.; University Press Centre: Pardubice, Czech Republic, 2014; Volume 8, pp. 227–240. [Google Scholar]
- Gonzalez-Rivera, J.C.; Osma, J.F. Fabrication of an Amperometric Flow-Injection Microfluidic Biosensor Based on Laccase for In Situ Determination of Phenolic Compounds. BioMed Res. Int. 2015, 2015, 845261. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Luo, L.; Pang, Z.; Ding, L.; Wang, Q.; Ke, H.; Huang, F.; Wei, Q. Novel Phenolic Biosensor Based on a Magnetic Polydopamine-Laccase-Nickel Nanoparticle Loaded Carbon Nanofiber Composite. ACS Appl. Mater. Interfaces 2014, 6, 5144–5151. [Google Scholar] [CrossRef] [PubMed]
- Fuzi, M.F.; Jaafar, A.; Nor, A.Y.; Yusran, S.; Mohd, I.S.; Mohd, H.M.Z. Laccase Electrochemical Biosensor Based on Graphene-Gold/Chitosan Nanocomposite Film for Bisphenol A Detection. Curr. Anal. Chem. 2020, 16, 570–579. [Google Scholar]
- Caballero, S.J.; Guerrero, M.A.; Vargas, L.Y.; Ortiz, C.C.; Castillo, J.J.; Gutiérrez, J.A.; Blanco, S. Electroanalytical determination of catechol by a biosensor based on laccase from Aspergillus oryzae immobilized on gold screen-printed electrodes. J. Phys. Conf. Ser. 2018, 1119, 012009. [Google Scholar] [CrossRef]
- Palanisamy, S.; Ramaraj, S.K.; Chen, S.-M.; Yang, T.C.K.; Yi-Fan, P.; Chen, T.-W.; Velusamy, V.; Selvam, S. A novel Laccase Biosensor based on Laccase immobilized Graphene-Cellulose Microfiber Composite modified Screen-Printed Carbon Electrode for Sensitive Determination of Catechol. Sci. Rep. 2017, 7, 41214. [Google Scholar] [CrossRef]
- Mazlan, S.Z.; Lee, Y.H.; Hanifah, S.A. A New Laccase Based Biosensor for Tartrazine. Sensors 2017, 17, 2859. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Laccase and Tyrosinase Biosensors Used in the Determination of Hydroxycinnamic Acids. Int. J. Mol. Sci. 2021, 22, 4811. [Google Scholar] [CrossRef]
- Kurian, M.A.; Gissen, P.; Smith, M.; Heales, S.J.R.; Clayton, P.T. The monoamine neurotransmitter disorders: An expanding range of neurological syndromes. Lancet Neurol. 2011, 10, 721–733. [Google Scholar] [CrossRef]
- Hjemdahl, P. Plasma catecholamines—analytical challenges and physiological limitations. Baillière’s Clin. Endocrinol. Metab. 1993, 7, 307–353. [Google Scholar] [CrossRef]
- Ribeiro, J.A.; Fernandes, P.M.V.; Pereira, C.M.; Silva, F. Electrochemical sensors and biosensors for determination of catecholamine neurotransmitters: A review. Talanta 2016, 160, 653–679. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Tajiri, S.; Hyoguchi, M.; Koyanagi, R.; Shimura, A.; Takata, F.; Dohgu, S.; Matsui, T. Analysis of Catecholamine and Their Metabolites in Mice Brain by Liquid Chromatography–Mass Spectrometry Using Sulfonated Mixed-mode Copolymer Column. Anal. Sci. 2019, 35, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.D.P.; Silva, L.I.B.; Freitas, A.C.; Rocha-Santos, T.A.P.; Duarte, A.C. High performance liquid chromatography coupled to an optical fiber detector coated with laccase for screening catecholamines in plasma and urine. J. Chromatogr. A 2009, 1216, 7049–7054. [Google Scholar] [CrossRef]
- Ye, H.; Xu, H.; Xu, X.; Zheng, C.; Li, X.; Wang, L.; Liu, X.; Chen, G. An electrochemiluminescence sensor for adrenaline assay based on the tyrosinase/SiC/chitosan modified electrode. Chem. Commun. 2013, 49, 7070–7072. [Google Scholar] [CrossRef]
- Gunawardhana, S.M.; Bulgakova, G.A.; Barybin, A.M.; Thomas, S.R.; Lunte, S.M. Progress toward the development of a microchip electrophoresis separation-based sensor with electrochemical detection for on-line in vivo monitoring of catecholamines. Analyst 2020, 145, 1768–1776. [Google Scholar] [CrossRef]
- Lavanya, N.; Leonardi*, S.G.; Sekar, C.; Ficarra, S.; Galtieri, A.; Tellone, E.; Neri, G. Detection of Catecholamine Neurotransmitters by Nanostructured SnO2-Based Electrochemical Sensors: A Review of Recent Progress. Mini-Rev. Org. Chem. 2018, 15, 382–388. [Google Scholar] [CrossRef]
- Ges, I.A.; Currie, K.P.M.; Baudenbacher, F. Electrochemical detection of catecholamine release using planar iridium oxide electrodes in nanoliter microfluidic cell culture volumes. Biosens. Bioelectron. 2012, 34, 30–36. [Google Scholar] [CrossRef]
- Hardi, G.W.; Rahman, S.F. Amperometric Detection of Dopamine based on a Graphene Oxide/PEDOT:PSS Composite Electrode. Int. J. Technol. 2020, 11, 974–983. [Google Scholar] [CrossRef]
- Sanghavi, B.J.; Mobin, S.M.; Mathur, P.; Lahiri, G.K.; Srivastava, A.K. Biomimetic sensor for certain catecholamines employing copper(II) complex and silver nanoparticle modified glassy carbon paste electrode. Biosens. Bioelectron. 2013, 39, 124–132. [Google Scholar] [CrossRef]
- Álvarez-Martos, I.; Ferapontova, E.E. Electrochemical Label-Free Aptasensor for Specific Analysis of Dopamine in Serum in the Presence of Structurally Related Neurotransmitters. Anal. Chem. 2016, 88, 3608–3616. [Google Scholar] [CrossRef] [PubMed]
- Budantsev, A.Y. Biosensor for catecholamines with immobilized monoamine oxidase in tissue sections. Anal. Chim. Acta 1991, 249, 71–76. [Google Scholar] [CrossRef]
- Stoica, L.; Lindgren-Sjölander, A.; Ruzgas, T.; Gorton, L. Biosensor Based on Cellobiose Dehydrogenase for Detection of Catecholamines. Anal. Chem. 2004, 76, 4690–4696. [Google Scholar] [CrossRef] [PubMed]
- Molinnus, D.; Hardt, G.; Käver, L.; Willenberg, H.S.; Poghossian, A.; Keusgen, M.; Schöning, M.J. Detection of Adrenaline Based on Bioelectrocatalytical System to Support Tumor Diagnostic Technology. Proceedings 2017, 1, 506. [Google Scholar] [CrossRef]
- Josypčuk, O.; Barek, J.; Josypčuk, B. Amperometric Determination of Catecholamines by Enzymatic Biosensors in Flow Systems. Electroanalysis 2018, 30, 1163–1171. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Nhat Nguyen, U.T.; Hsieh, H.-Y.; Tahara, H.; Chang, Y.-S.; Wang, B.-Y.; Gu, B.-C.; Dai, Y.-H.; Wu, C.-C.; Tsai, I.J.; et al. Peptide-based electrochemical sensor with nanogold enhancement for detecting rheumatoid arthritis. Talanta 2022, 236, 122886. [Google Scholar] [CrossRef]
- Santos, A.M.; Wong, A.; Fatibello-Filho, O.; Moraes, F.C. Amperometric Biosensor Based on Laccase Enzyme, Gold Nanoparticles, and Glutaraldehyde for the Determination of Dopamine in Biological and Environmental Samples. C J. Carbon Res. 2022, 8, 40. Available online: https://www.mdpi.com/2311-5629/8/3/40 (accessed on 16 August 2022). [CrossRef]
- Silva, T.R.; Vieira, I.C. A biosensor based on gold nanoparticles stabilized in poly(allylamine hydrochloride) and decorated with laccase for determination of dopamine. Analyst 2016, 141, 216–224. [Google Scholar] [CrossRef]
- Dimcheva, N.; Horozova, E. Improved operational stability of a laccase-based electrode applicable in biofuel cells. Bulg. Chem. Commun. 2018, 50, 130–135. [Google Scholar]
- Song, J.; Li, Y.; Yin, F.; Zhang, Z.; Ke, D.; Wang, D.; Yuan, Q.; Zhang, X.-E. Enhanced Electrochemical Impedance Spectroscopy Analysis of Microbial Biofilms on an Electrochemically In Situ Generated Graphene Interface. ACS Sens. 2020, 5, 1795–1803. [Google Scholar] [CrossRef]
- Gupta, G.; Rajendran, V.; Atanassov, P. Bioelectrocatalysis of oxygen reduction reaction by laccase on gold electrodes. Electroanalysis 2004, 16, 1182–1185. [Google Scholar] [CrossRef]
- Williams, M. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 15th ed.; O′Neil, M.J., Ed.; Royal Society of Chemistry: Cambridge, UK, 2013; p. 2708. ISBN 9781849736701. [Google Scholar] [CrossRef]
- Deng, M.; Zhao, H.; Zhang, S.; Tian, C.; Zhang, D.; Du, P.; Liu, C.; Cao, H.; Li, H. High catalytic activity of immobilized laccase on core–shell magnetic nanoparticles by dopamine self-polymerization. J. Mol. Catal. B Enzym. 2015, 112, 15–24. [Google Scholar] [CrossRef]
- Decarli, N.O.; Zapp, E.; de Souza, B.S.; Santana, E.R.; Winiarski, J.P.; Vieira, I.C. Biosensor based on laccase-halloysite nanotube and imidazolium zwitterionic surfactant for dopamine determination. Biochem. Eng. J. 2022, 186, 108565. [Google Scholar] [CrossRef]











| Kinetic Constants | Dopamine | L-Epinephrine |
|---|---|---|
| Vmaxapp, (A cm−2) | (4.08 ± 0.16) × 10−5 | (3.36 ± 0.17) × 10−5 |
| KM app (mM) | 0.116 ± 0.015 | 0.245 ± 0.031 |
| Coefficient of determination, R2 | 0.999 | 0.998 |
| Operational Parameters | Dopamine | L-Epinephrine |
|---|---|---|
| Maximum current density imax (Acm−2) | (33.76 ± 1.2) × 10−6 | (25.80 ± 1.3) × 10−6 |
| Sensitivity A L mol−1 cm−2 | 0.178 ± 0.005 | 0.123 ± 0.002 |
| Linear dynamic range, mM | 0.12 | 0.19 |
| Limit of detection LOD, M | 3.74 × 10−8 | 5.41 × 10−8 |
| Limit of quantification, LOQ, M | 1.25 × 10−7 | 1.80 × 10−7 |
| Biosensor Type | Method of Analysis | Analyte | LOD, μM | Reference |
|---|---|---|---|---|
| Lac-mesoporous silica biosensor | Amperometry (FIA) | Dopamine L-Epinephrine | 5.46 15.5 | [36] |
| Lac-Glu-AuNPs/CPE | Amperometry (E = 0.3 V) | Dopamine | 0.06 | [38] |
| AuNP-PAH-LAC/CPE | SWV | Dopamine | 0.26 | [39] |
| Lac-HNT-ImS3–14/CPE | SWV | Dopamine | 0.252 | [45] |
| Lac-GA-NH2C2H4S-AuNS/GC | Amperometry (E = −0.1 V) | Dopamine L-Epinephrine | 0.037 0.054 | This work |
| Analyte | Spiked Volume, μL | Concentration Determined, mg mL−1 | Recovery, % |
|---|---|---|---|
| Dopamine | 10 20 30 | 39.6 42.3 41.5 | 99 106 104 |
| L-Epinephrine | 20 40 60 | 0.89 1.046 0.909 | 89 105 91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pimpilova, M.; Kamarska, K.; Dimcheva, N. Biosensing Dopamine and L-Epinephrine with Laccase (Trametes pubescens) Immobilized on a Gold Modified Electrode. Biosensors 2022, 12, 719. https://doi.org/10.3390/bios12090719
Pimpilova M, Kamarska K, Dimcheva N. Biosensing Dopamine and L-Epinephrine with Laccase (Trametes pubescens) Immobilized on a Gold Modified Electrode. Biosensors. 2022; 12(9):719. https://doi.org/10.3390/bios12090719
Chicago/Turabian StylePimpilova, Mariya, Kalina Kamarska, and Nina Dimcheva. 2022. "Biosensing Dopamine and L-Epinephrine with Laccase (Trametes pubescens) Immobilized on a Gold Modified Electrode" Biosensors 12, no. 9: 719. https://doi.org/10.3390/bios12090719