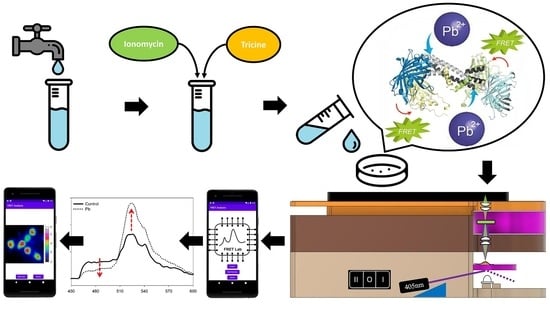
Portable FRET-Based Biosensor Device for On-Site Lead Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation of the Portable FRET-Based Pb-Biosensor Device (pMet-Lead)
2.2. Preparing of the Met-Lead-Containing Biochip
2.3. Sample Source and Preparation
2.4. Ratio Imaging under Epifluorescence FRET Microscope
2.5. Ratio Imaging under pMet-Lead
| Algorithm 1 Pre-processing of RAW (DNG) images taken with a smartphone camera |
|
2.6. Validation of the Y/C Ratio Data from pMet-Lead
2.7. Data Analysis
3. Results
3.1. Design and Outline of the Portable FRET-Based Pb-Biosensor Device (pMet-Lead)
3.2. Performance of pMet-Lead
3.3. Solution to the Selectivity Issue of Met-Leads and pMet-Lead
3.4. Practical Water Check by pMet-Lead
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rees, N.; Fuller, R. The Toxic Truth: Children’s Exposure to Lead Pollution Undermines a Generation of Future Potential; UNICEF: Hong Kong, China, 2020; ISBN 978-92-806-5140-9. [Google Scholar]
- Caldwell, K.L.; Cheng, P.-Y.; Jarrett, J.M.; Makhmudov, A.; Vance, K.; Ward, C.D.; Jones, R.L.; Mortensen, M.E. Measurement Challenges at Low Blood Lead Levels. Pediatrics 2017, 140, 20170272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldwell, K.L.; Cheng, P.-Y.; Vance, K.A.; Makhmudov, A.; Jarrett, J.M.; Caudill, S.P.; Ho, D.-P.; Jones, R.L. LAMP: A CDC Program to Ensure the Quality of Blood-Lead Laboratory Measurements. J. Public Health Manag. Pract. 2019, 25, S23–S30. [Google Scholar] [CrossRef]
- Paulson, J.A.; Brown, M.J. The CDC blood lead reference value for children: Time for a change. Environ. Health 2019, 18, 16. [Google Scholar] [CrossRef] [Green Version]
- Jarvis, P.; Fawell, J. Lead in drinking water—An ongoing public health concern? Curr. Opin. Environ. Sci. Health 2021, 20, 100239. [Google Scholar] [CrossRef]
- Chiu, T.-Y.; Yang, D.-M. Intracellular Pb2+ Content Monitoring Using a Protein-Based Pb2+ Indicator. Toxicol. Sci. 2012, 126, 436–445. [Google Scholar] [CrossRef]
- Yang, D.-M.; Manurung, R.V.; Lin, Y.-S.; Chiu, T.-Y.; Lai, W.-Q.; Chang, Y.-F.; Fu, T.-F. Monitoring the Heavy Metal Lead Inside Living Drosophila with a FRET-Based Biosensor. Sensors 2020, 20, 1712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.-M.; Fu, T.-F.; Lin, C.-S.; Chiu, T.-Y.; Huang, C.-C.; Huang, H.-Y.; Chung, M.-W.; Lin, Y.-S.; Manurung, R.V.; Nguyen, P.N.N.; et al. High-performance FRET biosensors for single-cell and in vivo lead detection. Biosens. Bioelectron. 2020, 168, 112571. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-J.; Lai, W.-Q.; Chang, Y.-F.; Wang, C.-L.; Yang, D.-M. Development and optimization of heavy metal lead biosensors in biomedical and environmental applications. J. Chin. Med. Assoc. 2021, 84, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.-M.; Chang, Y.-F. Versatile Cell and Animal Models for Advanced Investigation of Lead Poisoning. Biosensors 2021, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization, Ed.; World Health Organization: Geneva, Switzerland, 2017; ISBN 9789241549950. [Google Scholar]
- Breslauer, D.N.; Maamari, R.N.; Switz, N.; Lam, W.A.; Fletcher, D.A. Mobile Phone Based Clinical Microscopy for Global Health Applications. PLoS ONE 2009, 4, e6320. [Google Scholar] [CrossRef]
- Smith, Z.; Chu, K.; Espenson, A.R.; Rahimzadeh, M.; Gryshuk, A.; Molinaro, M.; Dwyre, D.M.; Lane, S.; Matthews, D.; Wachsmann-Hogiu, S. Cell-Phone-Based Platform for Biomedical Device Development and Education Applications. PLoS ONE 2011, 6, e17150. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yaglidere, O.; Su, T.-W.; Tseng, D.; Ozcan, A. Cost-effective and compact wide-field fluorescent imaging on a cell-phone. Lab Chip 2010, 11, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Switz, N.; D’Ambrosio, M.V.; Fletcher, D.A. Low-Cost Mobile Phone Microscopy with a Reversed Mobile Phone Camera Lens. PLoS ONE 2014, 9, e95330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knowlton, S.; Joshi, A.; Syrrist, P.; Coskun, A.F.; Tasoglu, S. 3D-printed smartphone-based point of care tool for fluorescence- and magnetophoresis-based cytometry. Lab Chip 2017, 17, 2839–2851. [Google Scholar] [CrossRef]
- Chen, P.-C.; Chen, K.-H.; Lin, C.-Y.; Yeh, Y.-C. Rapidly and simultaneously quantifying multiple biomarkers of L-tyrosine hydroxylase deficiency by using paper microfluidic devices and smartphone-based analysis system. Sens. Actuators B Chem. 2021, 349, 130722. [Google Scholar] [CrossRef]
- Sun, K.; Yang, Y.; Zhou, H.; Yin, S.; Qin, W.; Yu, J.; Chiu, D.T.; Yuan, Z.; Zhang, X.; Wu, C. Ultrabright Polymer-Dot Transducer Enabled Wireless Glucose Monitoring via a Smartphone. ACS Nano 2018, 12, 5176–5184. [Google Scholar] [CrossRef] [PubMed]
- Calabretta, M.M.; Álvarez-Diduk, R.; Michelini, E.; Roda, A.; Merkoçi, A. Nano-lantern on paper for smartphone-based ATP detection. Biosens. Bioelectron. 2020, 150, 111902. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, M.; Leichner, J.; Naja, G.M.; Li, C.-Z. Smart-phone, paper-based fluorescent sensor for ultra-low inorganic phosphate detection in environmental samples. Microsyst. Nanoeng. 2019, 5, 56. [Google Scholar] [CrossRef] [Green Version]
- Shan, Y.; Wang, B.; Huang, H.; Jian, D.; Wu, X.; Xue, L.; Wang, S.; Liu, F. On-site quantitative Hg2+ measurements based on selective and sensitive fluorescence biosensor and miniaturized smartphone fluorescence microscope. Biosens. Bioelectron. 2019, 132, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.W.; Wahid, K.A.; Islam, F.; Bernhard, W.; Geyer, C.R.; Vizeacoumar, F.J. A Low-Cost and Portable Smart Instrumentation for Detecting Colorectal Cancer Cells. Appl. Sci. 2019, 9, 3510. [Google Scholar] [CrossRef] [Green Version]
- Ng, E.; Yao, C.; Shultz, T.O.; Ross-Howe, S.; Wang, S.X. Magneto-nanosensor smartphone platform for the detection of HIV and leukocytosis at point-of-care. Nanomed. Nanotechnol. Biol. Med. 2019, 16, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Fozouni, P.; Son, S.; Derby, M.D.D.L.; Knott, G.J.; Gray, C.N.; D’Ambrosio, M.V.; Zhao, C.; Switz, N.A.; Kumar, G.R.; Stephens, S.I.; et al. Amplification-free detection of SARS-CoV-2 with CRISPR-Cas13a and mobile phone microscopy. Cell 2020, 184, 323–333.e9. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-F.; Teng, H.-C.; Cheng, S.-Y.; Wang, C.-T.; Chiou, S.-H.; Kao, L.-S.; Kao, F.-J.; Chiou, A.; Yang, D.-M. Orai1–STIM1 formed store-operated Ca2+ channels (SOCs) as the molecular components needed for Pb2+ entry in living cells. Toxicol. Appl. Pharmacol. 2008, 227, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Chiu, T.-Y.; Chen, P.-H.; Chang, C.-L.; Yang, D.-M. Live-Cell Dynamic Sensing of Cd2+ with a FRET-Based Indicator. PLoS ONE 2013, 8, e65853. [Google Scholar] [CrossRef] [Green Version]
- Suwa, H.; Saint-Amant, L.; Triller, A.; Drapeau, P.; Legendre, P. High-affinity zinc potentiation of inhibitory postsynaptic glycinergic currents in the zebrafish hindbrain. J. Neurophysiol. 2001, 85, 912–925. [Google Scholar] [CrossRef] [PubMed]
- Radford, R.J.; Lippard, S.J. Chelators for investigating zinc metalloneurochemistry. Curr. Opin. Chem. Biol. 2013, 17, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Alawsi, T.; Al-Bawi, Z. A review of smartphone point-of-care adapter design. Eng. Rep. 2019, 1, 12039. [Google Scholar] [CrossRef]
- Zhao, W.; Tian, S.; Huang, L.; Liu, K.; Dong, L.; Guo, J. A smartphone-based biomedical sensory system. Analyst 2020, 145, 2873–2891. [Google Scholar] [CrossRef]
- Chen, W.; Yao, Y.; Chen, T.; Shen, W.; Tang, S.; Lee, H.K. Application of smartphone-based spectroscopy to biosample analysis: A review. Biosens. Bioelectron. 2021, 172, 112788. [Google Scholar] [CrossRef]
- Hochreiter, B.; Pardo-Garcia, A.; Schmid, J.A. Fluorescent Proteins as Genetically Encoded FRET Biosensors in Life Sciences. Sensors 2015, 15, 26281–26314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marx, V. Probes: FRET sensor design and optimization. Nat. Methods 2017, 14, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Algar, W.R.; Hildebrandt, N.; Vogel, S.S.; Medintz, I.L. FRET as a biomolecular research tool—Understanding its potential while avoiding pitfalls. Nat. Methods 2019, 16, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, Y.; Yang, X.; Tang, Y.; Han, S.; Kang, A.; Deng, H.; Chi, Y.; Zhu, D.; Lu, Y. FÖrster resonance energy transfer (FRET)-based biosensors for biological applications. Biosens. Bioelectron. 2019, 138, 111314. [Google Scholar] [CrossRef] [PubMed]
- Watabe, T.; Terai, K.; Sumiyama, K.; Matsuda, M. Booster, a Red-Shifted Genetically Encoded Förster Resonance Energy Transfer (FRET) Biosensor Compatible with Cyan Fluorescent Protein/Yellow Fluorescent Protein-Based FRET Biosensors and Blue Light-Responsive Optogenetic Tools. ACS Sens. 2020, 5, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Mitra, P.; Sharma, S.; Purohit, P.; Sharma, P. Clinical and molecular aspects of lead toxicity: An update. Crit. Rev. Clin. Lab. Sci. 2017, 54, 506–528. [Google Scholar] [CrossRef]
- Reuben, A.; Caspi, A.; Belsky, D.W.; Broadbent, J.; Harrington, H.; Sugden, K.; Houts, R.M.; Ramrakha, S.; Poulton, R.; Moffitt, T.E. Association of Childhood Blood Lead Levels With Cognitive Function and Socioeconomic Status at Age 38 Years and With IQ Change and Socioeconomic Mobility Between Childhood and Adulthood. JAMA 2017, 317, 1244–1251. [Google Scholar] [CrossRef]
- Lanphear, B.P.; Rauch, S.; Auinger, P.; Allen, R.W.; Hornung, R.W. Low-level lead exposure and mortality in US adults: A population-based cohort study. Lancet Public Health 2018, 3, e177–e184. [Google Scholar] [CrossRef]
- Rocha, A.; Trujillo, K.A. Neurotoxicity of low-level lead exposure: History, mechanisms of action, and behavioral effects in humans and preclinical models. Neurotoxicology 2019, 73, 58–80. [Google Scholar] [CrossRef]
- O’Connor, D.; Hou, D.; Ok, Y.S.; Lanphear, B.P. The effects of iniquitous lead exposure on health. Nat. Sustain. 2020, 3, 77–79. [Google Scholar] [CrossRef]
- Frank, J.J.; Poulakos, A.G.; Tornero-Velez, R.; Xue, J. Systematic review and meta-analyses of lead (Pb) concentrations in environmental media (soil, dust, water, food, and air) reported in the United States from 1996 to 2016. Sci. Total Environ. 2019, 694, 133489. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pan, Y.-Q.; Wang, J.-F.; Zhang, Y.; Ding, Y.-J. A highly selective and sensitive half-salamo-based fluorescent chemosensor for sequential detection of Pb(II) ion and Cys. J. Photochem. Photobiol. A Chem. 2020, 400, 112719. [Google Scholar] [CrossRef]
- Salman, S.; Znad, H.; Hasan, N.; Hasan, M. Optimization of innovative composite sensor for Pb(II) detection and capturing from water samples. Microchem. J. 2021, 160, 105765. [Google Scholar] [CrossRef]
- Gumpu, M.B.; Veerapandian, M.; Krishnan, U.M.; Rayappan, J.B.B. Simultaneous electrochemical detection of Cd(II), Pb(II), As(III) and Hg(II) ions using ruthenium(II)-textured graphene oxide nanocomposite. Talanta 2017, 162, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, B.; Hou, H.; Huang, Z.; Zeinu, K.M.; Huang, L.; Yuan, X.; Guo, D.; Hu, J.; Yang, J. Alkaline intercalation of Ti3C2 MXene for simultaneous electrochemical detection of Cd(II), Pb(II), Cu(II) and Hg(II). Electrochim. Acta 2017, 248, 46–57. [Google Scholar] [CrossRef]
- Lu, Y.; Liang, X.; Xu, J.; Zhao, Z.; Tian, G. Synthesis of CuZrO3 nanocomposites/graphene and their application in modified electrodes for the co-detection of trace Pb(II) and Cd(II). Sens. Actuators B Chem. 2018, 273, 1146–1155. [Google Scholar] [CrossRef]
- Sarfo, D.K.; Izake, E.L.; O’Mullane, A.; Ayoko, G. Molecular recognition and detection of Pb(II) ions in water by aminobenzo-18-crown-6 immobilised onto a nanostructured SERS substrate. Sens. Actuators B Chem. 2018, 255, 1945–1952. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Wang, J.; Li, P.-H.; Yang, M.; Huang, X.-J. Highly sensitive electrochemical detection of Pb(II) based on excellent adsorption and surface Ni(II)/Ni(III) cycle of porous flower-like NiO/rGO nanocomposite. Sens. Actuators B Chem. 2019, 292, 136–147. [Google Scholar] [CrossRef]
- Borthwick, T.R.; Benson, G.D.; Schugar, H.J. Copper chelating agents. A comparison of cupruretic responses to various tetramines and D-penicillamine. J. Lab. Clin. Med. 1980, 95, 575–580. [Google Scholar] [PubMed]
- Guan, R.; Dai, H. Article Commentary: Chelation Therapy for Mercury Poisoning. Biochem. Insights 2009, 2, BCI.S3036. [Google Scholar] [CrossRef]
- Leal, J.; Guerreiro, B.; Amado, P.; Fernandes, A.; Barreira, L.; Paixão, J.; Cristiano, M. On the Development of Selective Chelators for Cadmium: Synthesis, Structure and Chelating Properties of 3-((5-(trifluoromethyl)-1,3,4-thiadiazol-2-yl)amino)benzo[d]isothiazole 1,1-dioxide, a Novel Thiadiazolyl Saccharinate. Molecules 2021, 26, 1501. [Google Scholar] [CrossRef] [PubMed]
- Akerboom, J.; Chen, T.-W.; Wardill, T.; Tian, L.; Marvin, J.; Mutlu, S.; Calderón, N.C.; Esposti, F.; Borghuis, B.G.; Sun, X.R.; et al. Optimization of a GCaMP Calcium Indicator for Neural Activity Imaging. J. Neurosci. 2012, 32, 13819–13840. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.L.; Tsai, C.Y.; Chen, T.H. Cell surface display of Chi92 onEscherichia coliusing ice nucleation protein for improved catalytic and antifungal activity. FEMS Microbiol. Lett. 2006, 256, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, H.; Yamamoto, Y.; Muraoka, M.; Akaishi, K.; Hori, Y.; Uemura, K.; Tsuji, N.; Harada, K.; Hirata, K.; Bamba, T.; et al. Development of surface-engineered yeast cells displaying phytochelatin synthase and their application to cadmium biosensors by the combined use of pyrene-excimer fluorescence. Biotechnol. Prog. 2013, 29, 1197–1202. [Google Scholar] [CrossRef] [PubMed]






| Water Samples | Mean of Y/C Ratio (with Standard Error) | ICP-MS (ppb) |
|---|---|---|
| Control | 2.7969 ± 0.1001 | NA 1 |
| 1 nM | 2.9136 ± 0.0800 | NA |
| 10 nM | 2.8310 ± 0.0112 | 2.85 |
| 20 nM | 2.8428 ± 0.0411 | 4.20 |
| 30 nM | 2.8413 ± 0.0611 | 6.14 |
| 40 nM | 3.5133 ± 0.0782 (***) 2 | 8.32 |
| 50 nM | 4.3811 ± 0.0994 (***) | 11.18 |
| 100 nM | 4.8107 ± 0.0470 (***) | 23.35 |
| 1 μM | 5.1732 ± 0.0298 (***) | 231.45 |
| 10 μM | 5.5572 ± 0.0138 (***) | 2327.71 |
| Water Samples | Mean of Y/C Ratio (with Standard Error) | ICP-MS (ppb) |
|---|---|---|
| Control | 2.7931 ± 0.025 | NA 1 |
| I | 2.3136 ± 0.0416 | NA |
| II | 2.2897 ± 0.0250 | 0.64 |
| III | 2.4554 ± 0.05369 | NA |
| IV | 3.4803 ± 0.0839 (***) 2 | 10.6 |
| V | 2.3581 ± 0.0258 | NA |
| VI | 3.7431 ± 0.1236 (***) 2 | 15.24 |
| Methods | LOD | Report Time | Performance/Cost Ratio | Note |
|---|---|---|---|---|
| ICP-MS | 0.003–0.01 ppb 1 | Long (lab/hospital) 1 | Low (high price) | Gold standard |
| GFAAS | 1–10 ppb 2 | Long (lab/hospital) 2 | Low (high price) | Gold standard |
| PB200 | ~0.1 ppb 3 | Real time (on-site) | High; no expandability | Not validated |
| PC2700 | 200 ppb | Fast (lab) | Low (poor LOD) | Not validated |
| Half-salamo-based chemosensor, HL [44] | ~11 ppb (56.5 nM), cal. | Fast (lab) | Medium (spectrophotometer); expandability | Not validated |
| Organic immobilized composite sensor [45] | 0.24 ppb (1.2 nM) | N. A. (lab) | Medium (color); no expandability; remove; resuses | Validated (ICP-AES) |
| Graphene oxide (GO) nanocomposite [46] | 0.28 ppb (1.41 nM) | N. A. (lab/on-site) | High (voltametry); expandability | Validated (AAS) |
| Alk-Ti3C2 MXenes [47] | ~8.2 ppb (41 nM) | Fast (lab) | Medium (SWASV); expandability | Not validated |
| CuZrO3 nanocopmposites [48] | 0.1 ppb | Fast (lab) | Medium (SWASV); expandability | Not validated |
| SERS sensor AB18C6-gold nanoparticles [49] | 0.69 pM | Fast (lab) | Medium (SERS); resuses | Validated (ICP-MS) |
| NiO/rGO Nanocomposite [50] | 2 ppb (10 nM) | Fast (lab/on-site) | High (SWASV); expandability | Validated (ICP-AES) |
| Met-lead 1.59 [6] | 100 ppb (500 nM) | Fast (lab) | Low (poor LOD) | Not validated |
| Met-lead 1.44 M1 [8] | 2 ppb (10 nM) | Fast (lab) | Medium (FRET fluorescence microscope); expandability | Validated (ICP-MS) |
| pMet-lead (this study) | 4.74 ppb (24 nM) | Real time (on-site) | High; expandability | Validated (ICP-MS) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, W.-Q.; Chang, Y.-F.; Chou, F.-N.; Yang, D.-M. Portable FRET-Based Biosensor Device for On-Site Lead Detection. Biosensors 2022, 12, 157. https://doi.org/10.3390/bios12030157
Lai W-Q, Chang Y-F, Chou F-N, Yang D-M. Portable FRET-Based Biosensor Device for On-Site Lead Detection. Biosensors. 2022; 12(3):157. https://doi.org/10.3390/bios12030157
Chicago/Turabian StyleLai, Wei-Qun, Yu-Fen Chang, Fang-Ning Chou, and De-Ming Yang. 2022. "Portable FRET-Based Biosensor Device for On-Site Lead Detection" Biosensors 12, no. 3: 157. https://doi.org/10.3390/bios12030157