Highly Sensitive and Selective Nanogap-Enhanced SERS Sensing Platform
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Plasmonic Nanostructures and PDMS Encapsulation
2.2. Measurement and Chracterizations
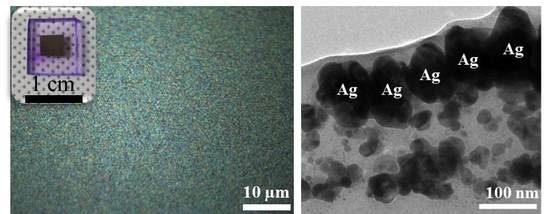
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jeon, T.Y.; Kim, D.J.; Park, S.G.; Kim, S.H.; Kim, D.H. Nanostructured Plasmonic Substrates for Use as SERS Sensors. Nano Converg. 2016, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-G.; Kang, M.; Kim, S.; Jung, H.S.; Kim, D.-H. 3D-Assembled Ag Nanowires for Use in Plasmon-Enhanced Spectroscopic Sensors. Appl. Spectrosc. Rev. 2018, 1467440, 1–23. [Google Scholar] [CrossRef]
- Park, S.-G.; Mun, C.; Lee, M.; Jeon, T.Y.; Shim, H.-S.; Lee, Y.-J.; Kwon, J.-D.; Kim, C.S.; Kim, D.-H. 3D Hybrid Plasmonic Nanomaterials for highly Efficient Optical Absorbers and Sensors. Adv. Mater. 2015, 27, 4290–4295. [Google Scholar] [CrossRef] [PubMed]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with Plasmonic Nanosensors. Nat. Mater. 2008, 7, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, R.; Sonnefraud, Y.; Fernández-Domínguez, A.I.; Giannini, V.; Maier, S.A. Design Considerations for Near-Field Enhancement in Optical Antennas. Contemp. Phys. 2014, 55, 1–11. [Google Scholar] [CrossRef]
- Zuloaga, J.; Prodan, E.; Nordlander, P. Quantum Description of the Plasmon Resonances of a Nanoparticle Dimer. Nano Lett. 2009, 9, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Christopher, P.; Xin, H.; Marimuthu, A.; Linic, S. Singular Characteristics and Unique Chemical Bond Activation Mechanisms of Photocatalytic Reactions on Plasmonic Nanostructures. Nat. Mater. 2012, 11, 1044–1050. [Google Scholar] [CrossRef]
- Linh, V.T.N.; Xiao, X.; Jung, H.S.; Giannini, V.; Maier, S.A.; Kim, D.-H.; Lee, Y.-L.; Park, S.-G. Compact Integration of TiO2 Nanoparticles into the Cross-Points of 3D Vertically Stacked Ag Nanowires for Plasmon-Enhanced Photocatalysis. Nanomaterials 2019, 9, 468. [Google Scholar] [CrossRef] [PubMed]
- Moreau, A.; Ciracì, C.; Mock, J.J.; Hill, R.T.; Wang, Q.; Wiley, B.J.; Chilkoti, A.; Smith, D.R. Controlled-Reflectance Surfaces with Film-Coupled Colloidal Nanoantennas. Nature 2012, 492, 86–89. [Google Scholar] [CrossRef]
- Ciracì, C.; Chen, X.; Mock, J.J.; McGuire, F.; Liu, X.; Oh, S.-H.; Smith, D.R. Film-coupled Nanoparticles by Atomic Layer Deposition: Comparison with organic spacing layers. Appl. Phys. Lett. 2014, 104, 023109. [Google Scholar] [CrossRef]
- Li, J.; Cushing, S.K.; Meng, F.; Senty, T.R.; Bristow, A.D.; Wu, N. Plasmon-induced Resonance Energy Transfer for Solar Energy Conversion. Nat. Photonics 2015, 9, 601–607. [Google Scholar] [CrossRef]
- Atwater, H.A.; Polman, A. Plasmonics for Improved Photovoltaic Devices. Nat. Mater. 2010, 9, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Green, M.A.; Pillai, S. Harnessing Plasmonics for Solar Cells. Nat. Photonics 2012, 6, 130–132. [Google Scholar] [CrossRef]
- Kim, A.; Ou, F.S.; Ohlberg, D.A.A.; Hu, M.; Williams, R.S.; Li, Z. Study of Molecular Trapping Inside Gold Nanofinger Arrays on Surface-Enhanced Raman Substrates. J. Am. Chem. Soc. 2011, 133, 8234–8239. [Google Scholar] [CrossRef] [PubMed]
- Ou, F.S.; Hu, M.; Naumov, I.; Kim, A.; Wu, W.; Bratkovsky, A.M.; Li, X.; Williams, R.S.; Li, Z. Hot-Spot Engineering in Polygonal Nanofinger Assemblies for Surface Enhanced Raman Spectroscopy. Nano Lett. 2011, 11, 2538–2542. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.S.; Hübner, J.; Boisen, A. Large Area Fabrication of Leaning Silicon Nanopillars for Surface Enhanced Raman Spectroscopy. Adv. Mater. 2012, 24, OP11–OP18. [Google Scholar] [CrossRef]
- Park, S.-G.; Mun, C.; Xiao, X.; Braun, A.; Kim, S.; Giannini, V.; Maier, S.A.; Kim, D.-H. Surface Energy-Controlled SERS Substrates for Molecular Concentration at Plasmonic Nanogaps. Adv. Funct. Mater. 2017, 27, 1703376. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Sun, Y.; Jin, Z.; Yang, L.; Liu, J. Capillarity-Constructed Reversible Hot Spots for Molecular Trapping Inside Silver Nanorod Arrays Light Up Ultrahigh SERS Enhancement. Chem. Sci. 2013, 4, 3490–3496. [Google Scholar] [CrossRef]
- Hu, M.; Ou, F.S.; Wu, W.; Naumov, I.; Li, X.; Bratkovsky, A.M.; Williams, R.S.; Li, Z. Gold Nanofingers for Molecule Trapping and Detection. J. Am. Chem. Soc. 2010, 132, 12820–12822. [Google Scholar] [CrossRef]
- Brown, B.S. Biological Membranes; The Biochemical Society: London, UK, 1996. [Google Scholar]
- Singleton, P. Bacteria in Biology, Biotechnology and Medicine; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Petersen, R.J. Composite Reverse Osmosis and Nanofiltration Membranes. J. Membr. Sci. 1993, 83, 81–150. [Google Scholar] [CrossRef]
- Park, S.-G.; Jeon, T.Y.; Jeon, H.C.; Yang, S.-M.; Kwon, J.-D.; Mun, C.-W.; Cho, B.; Kim, C.S.; Kim, D.-H. Fabrication of 3D ZnO Hollow Shell Structures by Prism Holographic Lithography and Atomic Layer Deposition. J. Mater. Chem. C 2014, 2, 1957–1961. [Google Scholar] [CrossRef]
- Jung, T.-H.; Park, J.-S.; Kim, D.-H.; Jeong, Y.; Park, S.-G.; Kwon, J.-D. Effect of in Situ Hydrogen Plasma Treatment on Zinc Oxide Grown Using Low Temperature Atomic Layer Deposition. J. Vac. Sci. Technol. A 2013, 31, 01A124. [Google Scholar] [CrossRef]
- Di Mundo, R.; Troia, M.; Palumbo, F.; Trotta, M.; d′Agostino, R. Nano-Texturing of Transparent Polymers with Plasma Etching: Tailoring Topography for A Low Reflectivity. Plasma Process. Polym. 2012, 9, 947–954. [Google Scholar] [CrossRef]
- Ko, T.-J.; Oh, K.H.; Moon, M.-W. Plasma-Induced Hetero-Nanostructures on A Polymer with Selective Metal Co-Deposition. Adv. Mater. Interfaces 2015, 2, 1400431. [Google Scholar] [CrossRef]
- Yun, J.; Wang, W.; Kim, S.M.; Bae, T.-S.; Lee, S.; Kim, D.; Lee, G.-H.; Lee, H.-S.; Song, M. Light Trapping in Bendable Organic Solar Cells Using Silica Nanoparticle Arrays. Energy Environ. Sci. 2015, 8, 932–940. [Google Scholar] [CrossRef]
- Yun, J.; Bae, T.-S.; Kwon, J.-D.; Lee, S.; Lee, G.-H. Antireflective Silica Nanoparticle Array Directly Deposited on Flexible Polymer Substrates by Chemical Vapor Deposition. Nanoscale 2012, 4, 7221–7230. [Google Scholar] [CrossRef]
- Materials Data Book; Cambridge University Engineering Department: Cambridge, UK, 2003.
- Fan, X.; Zheng, W.; Singh, D.J. Light Scattering and Surface Plasmons on Small Spherical Particles. Light Sci. Appl. 2014, 3, e179. [Google Scholar] [CrossRef]
- Mühlschlegel, P.; Eisler, H.-J.; Martin, O.J.F.; Hecht, B.; Pohl, D.W. Resonant Optical Antennas. Science 2005, 308, 1607–1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, M.; Novo, C.; Funston, A.; Wang, H.; Staleva, H.; Zou, S.; Mulvaney, P.; Xia, Y.; Hartland, G.V. Dark-Field Microscopy Studies of Single Metal Nanoparticles: Understanding the Factors that Influence the Linewidth of the Localized Surface Plasmon Resonance. J. Mater. Chem. 2008, 18, 1949–1960. [Google Scholar] [CrossRef]
- Raman, N.U.L.; Schmidt, M.S.; Ju, J.; Lin, Q. Surface-Enhanced Raman Bioassay on Aptamer-Functionalized Mapping. ACS Nano 2013, 7, 5350–5359. [Google Scholar]
- Campbell, C.T. Ultrathin Metal Films and Particles on Oxide Surfaces: Structural, Electronic and Chemisorptive Properties. Surf. Sci. Rep. 1997, 27, 1–111. [Google Scholar] [CrossRef]
- Tapily, K.; Gu, D.; Baumgart, H.; Namkoong, G.; Stegall, D.; Elmustafa, A.A. Mechanical and Structural Characterization of Atomic Layer Deposition-Based Zno Films. Semicond. Sci. Technol. 2011, 26, 115005. [Google Scholar] [CrossRef]
- Ru, P.G.L.; Eric, C.; Etchegoin, P. Principles of Surface-Enhanced Raman Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Shahzad, M.I.; Giorcelli, M.; Shahzad, N.; Guastella, S.; Castellino, M.; Jagdale, P.; Tagliaferro, A. Study of Carbon Nanotubes Based Polydimethylsiloxane Composite Films. J. Phys. Conf. Ser. 2013, 439, 012010. [Google Scholar] [CrossRef]
- Zabow, G.; Dodd, S.J.; Koretsky, A.P. Shape-Changing Magnetic Assemblies as High-Sensitivity Nmr-Readable Nanoprobes. Nature 2015, 520, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.-F.; Akdemir, Ö.; Hoth, A. Point by Point Comparison of two Thermosensitive Polymers Exhibiting A Similar Lcst: Is the Age of Poly(Nipam) Over? J. Am. Chem. Soc. 2006, 128, 13046–13047. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-H.; Moon, J.H.; Lee, S.-K.; Park, S.-G.; Jang, S.G.; Yang, S.; Yang, S.-M. Thermoresponsive Hydrogel Photonic Crystals by Three-Dimensional Holographic Lithography. Adv. Mater. 2008, 20, 3061–3065. [Google Scholar] [CrossRef]
- Kim, D.J.; Park, S.-G.; Kim, D.-H.; Kim, S.-H. SERS-Active-Charged Microgels for Size- and Charge- Selective Molecular Analysis of Complex Biological Samples. Small 2018, 14, 1802520. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Jeon, T.Y.; Baek, Y.-K.; Park, S.-G.; Kim, D.-H.; Kim, S.-H. Metal Nanoparticle-Loaded Microgels with Selective Permeability for Direct Detection of Small Molecules in Biological Fluids. Chem. Mater. 2016, 28, 1559–1565. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mun, C.; Linh, V.T.N.; Kwon, J.-D.; Jung, H.S.; Kim, D.-H.; Park, S.-G. Highly Sensitive and Selective Nanogap-Enhanced SERS Sensing Platform. Nanomaterials 2019, 9, 619. https://doi.org/10.3390/nano9040619
Mun C, Linh VTN, Kwon J-D, Jung HS, Kim D-H, Park S-G. Highly Sensitive and Selective Nanogap-Enhanced SERS Sensing Platform. Nanomaterials. 2019; 9(4):619. https://doi.org/10.3390/nano9040619
Chicago/Turabian StyleMun, ChaeWon, Vo Thi Nhat Linh, Jung-Dae Kwon, Ho Sang Jung, Dong-Ho Kim, and Sung-Gyu Park. 2019. "Highly Sensitive and Selective Nanogap-Enhanced SERS Sensing Platform" Nanomaterials 9, no. 4: 619. https://doi.org/10.3390/nano9040619