Comparison of Different Approaches to Surface Functionalization of Biodegradable Polycaprolactone Scaffolds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrospinning of Poly-ε-Caprolactone (PCL) Nanofibers
2.2. Deposition of TiCaPCON Film
2.3. Deposition of COOH Plasma Polymers
2.4. Biomineralization of PCL Nanofibrous Scaffolds
2.5. Material Characterization
2.6. Morphometric Analysis
2.7. Actin Cytoskeleton and Focal Adhesions Staining
2.8. Proliferation of MC3T3-E1 Osteoblastic and IAR-2 Epithelial Cells
3. Results
3.1. Structural Characterization of PCL Nanofibers
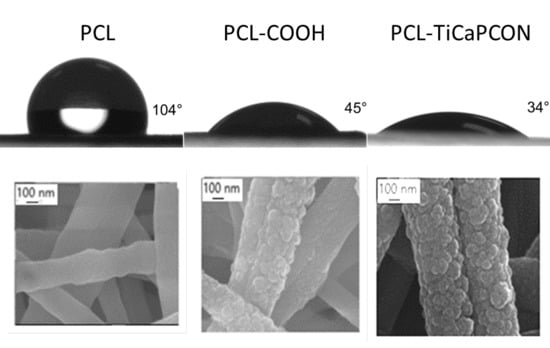
3.2. Water Contact Angle
3.3. Tests in Simulated Body Fluid (SBF)
3.4. Cytocompatibility
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Mao, A.S.; Mooney, D.J. Regenerative medicine: Current therapies and future directions. Proc. Natl. Acad. Sci. USA 2015, 112, 14452–14459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parvathi, K.; Krishnan, A.G.; Anitha, A.; Jayakumar, R.; Nair, M.B. Poly(L-lactic acid) nanofibers containing Cissus quadrangularis induced osteogenic differentiation in vitro. Int. J. Biol. Macromol. 2018, 110, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Al-Enizi, A.; Zagho, M.; Elzatahry, A. Polymer-Based Electrospun Nanofibers for Biomedical Applications. Nanomaterials 2018, 8, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.-H.; Chen, C.-H.; Chen, J.-P.; Li, M.-L.; Kuo, C.-Y. Response of Dermal Fibroblasts to Biochemical and Physical Cues in Aligned Polycaprolactone/Silk Fibroin Nanofiber Scaffolds for Application in Tendon Tissue Engineering. Nanomaterials 2017, 7, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeFrates, K.; Moore, R.; Lin, G.; Hu, X.; Beachley, V.; Borgesi, J.; Mulderig, T. Protein-Based Fiber Materials in Medicine: A Review. Nanomaterials 2018, 8, 457. [Google Scholar] [CrossRef] [Green Version]
- Stocco, T.D.; Bassous, N.J.; Zhao, S.; Granato, A.E.C.; Webster, T.J.; Lobo, A.O. Nanofibrous scaffolds for biomedical applications. Nanoscale 2018, 10, 12228–12255. [Google Scholar] [CrossRef]
- Du, Y.; Chen, X.; Hag Koh, Y.; Lei, B. Facilely fabricating PCL nanofibrous scaffolds with hierarchical pore structure for tissue engineering. Mater. Lett. 2014, 122, 62–65. [Google Scholar] [CrossRef]
- Lotfi, M.; Ghasemi, N.; Rahimi, S.; Vosoughhosseini, S.; Saghiri, M.A.; Shahidi, A. Resilon: A comprehensive literature review. J. Dent. Res. Dent. Clin. Dent. Prospect. 2013, 7, 119–130. [Google Scholar]
- Forsgren, J.; Svahn, F.; Jarmar, T.; Engqvist, H. Formation and adhesion of biomimetic hydroxyapatite deposited on titanium substrates. Acta Biomater. 2007, 3, 980–984. [Google Scholar] [CrossRef]
- Contreras-Cáceres, R.; Cabeza, L.; Perazzoli, G.; Díaz, A.; López-Romero, J.M.; Melguizo, C.; Prados, J. Electrospun nanofibers: Recent applications in drug delivery and cancer therapy. Nanomaterials 2019, 9, 656. [Google Scholar] [CrossRef] [Green Version]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Zhang, C.; Cao, M.; Lan, J.; Wei, P.; Cai, Q.; Yang, X. Regulating proliferation and differentiation of osteoblasts on poly(l-lactide)/gelatin composite nanofibers via timed biomineralization. J. Biomed. Mater. Res. Part A 2016, 104, 1968–1980. [Google Scholar] [CrossRef]
- Nagarajan, S.; Belaid, H.; Pochat-Bohatier, C.; Teyssier, C.; Iatsunskyi, I.; Coy, E.; Balme, S.; Cornu, D.; Miele, P.; Kalkura, N.S.; et al. Design of boron nitride/gelatin electrospun nanofibers for bone tissue engineering. ACS Appl. Mater. Interfaces 2017, 9, 33695–33706. [Google Scholar] [CrossRef]
- Gao, Y.; Shao, W.; Qian, W.; He, J.; Zhou, Y.; Qi, K.; Wang, L.; Cui, S.; Wang, R. Biomineralized poly (L-lactic-co-glycolic acid)-tussah silk fibroin nanofiber fabric with hierarchical architecture as a scaffold for bone tissue engineering. Mater. Sci. Eng. C 2018, 84, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, T.; Kishida, A.; Akashi, M. Hydroxyapatite formation on/in poly(vinyl alcohol) hydrogel matrices using a novel alternate soaking process. Chem. Lett. 1998, 27, 711–712. [Google Scholar] [CrossRef]
- Wei, K.; Li, Y.; Kim, K.O.; Nakagawa, Y.; Kim, B.S.; Abe, K.; Chen, G.Q.; Kim, I.S. Fabrication of nano-hydroxyapatite on electrospun silk fibroin nanofiber and their effects in osteoblastic behavior. J. Biomed. Mater. Res. Part A 2011, 97, 272–280. [Google Scholar] [CrossRef]
- Nie, W.; Gao, Y.; McCoul, D.J.; Gillispie, G.J.; Zhang, Y.; Liang, L.; He, C. Rapid mineralization of hierarchical poly(l-lactic acid)/poly(ε-caprolactone) nanofibrous scaffolds by electrodeposition for bone regeneration. Int. J. Nanomed. 2019, 14, 3929–3941. [Google Scholar] [CrossRef] [Green Version]
- Melnik, E.V.; Shkarina, S.N.; Ivlev, S.I.; Weinhardt, V.; Baumbach, T.; Chaikina, M.V.; Surmeneva, M.A.; Surmenev, R.A. In vitro degradation behaviour of hybrid electrospun scaffolds of polycaprolactone and strontium-containing hydroxyapatite microparticles. Polym. Degrad. Stab. 2019, 167, 21–32. [Google Scholar] [CrossRef]
- Shtansky, D.V.; Grigoryan, A.S.; Toporkova, A.K.; Arkhipov, A.V.; Sheveyko, A.N.; Kiryukhantsev-Korneev, P.V. Modification of polytetrafluoroethylene implants by depositing TiCaPCON films with and without stem cells. Surf. Coat. Technol. 2011, 206, 1188–1195. [Google Scholar] [CrossRef]
- Shtansky, D.V.; Glushankova, N.A.; Kiryukhantsev-Korneev, F.V.; Sheveiko, A.N.; Sigarev, A.A. A comparative study of the structure and cytotoxicity of polytetrafluoroethylene after ion etching and ion implantation. Phys. Solid State 2011, 53, 638–642. [Google Scholar] [CrossRef]
- Manakhov, A.; Kedroňová, E.; Medalová, J.; Černochová, P.; Obrusník, A.; Michlíček, M.; Shtansky, D.V.; Zajíčková, L. Carboxyl-anhydride and amine plasma coating of PCL nanofibers to improve their bioactivity. Mater. Des. 2017, 132, 257–265. [Google Scholar] [CrossRef]
- Shtansky, D.V.; Levashov, E.A.; Batenina, I.V.; Gloushankova, N.A.; Anisimova, N.Y.; Kiselevsky, M.V.; Reshetov, I.V. Recent progress in the field of multicomponent bioactive nanostructured films. RSC Adv. 2013, 3, 11107. [Google Scholar] [CrossRef]
- Manakhov, A.; Kiryukhantsev-Korneev, P.; Michlíček, M.; Permyakova, E.; Dvořáková, E.; Polčák, J.; Popov, Z.; Visotin, M.; Shtansky, D.V. Grafting of carboxyl groups using CO2/C2H4/Ar pulsed plasma: Theoretical modeling and XPS derivatization. Appl. Surf. Sci. 2018, 435, 1220–1227. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Manakhov, A.; Michlíček, M.; Felten, A.; Pireaux, J.-J.; Nečas, D.; Zajíčková, L. XPS depth profiling of derivatized amine and anhydride plasma polymers: Evidence of limitations of the derivatization approach. Appl. Surf. Sci. 2017, 394, 578–585. [Google Scholar] [CrossRef]
- Ponomarev, V.A.; Sukhorukova, I.V.; Sheveyko, A.N.; Permyakova, E.S.; Manakhov, A.M.; Ignatov, S.G.; Gloushankova, N.A.; Zhitnyak, I.Y.; Lebedev, O.I.; Polčak, J.; et al. Antibacterial Performance of TiCaPCON Films Incorporated with Ag, Pt, and Zn: Bactericidal Ions Versus Surface Microgalvanic Interactions. ACS Appl. Mater. Interfaces 2018, 10, 24406–24420. [Google Scholar] [CrossRef]
- Štrbková, L.; Manakhov, A.; Zajíčková, L.; Stoica, A.; Veselý, P.; Chmelík, R. The adhesion of normal human dermal fibroblasts to the cyclopropylamine plasma polymers studied by holographic microscopy. Surf. Coat. Technol. 2016, 295, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Montesano, R.; Vincent, L.S.; Drevon, C.; Tomatis, L. Production of epithelial and mesenchymal tumours with rat liver cells transformedin vitro. Int. J. Cancer 1975, 16, 550–558. [Google Scholar] [CrossRef]
- Roh, H.S.; Myung, S.W.; Jung, S.C.; Kim, B.H. Fabrication of 3D scaffolds with nano-hydroxyapatite for improving the preosteoblast cell-biological performance. J. Nanosci. Nanotechnol. 2015, 15, 5585–5588. [Google Scholar] [CrossRef]
- Erbetta, C.D.A.C.; Alves, R.J.; Resende, J.M.; de Souza Freitas, R.F.; de Sousa, R.G. Synthesis and Characterization of Poly(D,L-Lactide-co-Glycolide) Copolymer. J. Biomater. Nanobiotechnol. 2012, 3, 208–225. [Google Scholar] [CrossRef]
- Gardy, J.; Hassanpour, A.; Lai, X.; Ahmed, M.H. Synthesis of Ti(SO4)O solid acid nano-catalyst and its application for biodiesel production from used cooking oil. Appl. Catal. A Gen. 2016, 527, 81–95. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Peng, Z.-X. Nanofibers grafted on titanium alloy: The effects of fiber alignment and density on osteoblast mineralization. J. Mater. Sci. Mater. Med. 2017, 28, 140. [Google Scholar] [CrossRef] [PubMed]
- Zreiqat, H.; Standard, O.G.; Gengenbach, T.; Steele, J.G.; Howlett, C.R. The role of surface characteristics in the initial adhesion of human bone-derived cells on ceramics. Cells Mater. 1996, 6, 45–56. [Google Scholar]
- Onak, G.; Karaman, O. Accelerated mineralization on nanofibers via non-thermal atmospheric plasma assisted glutamic acid templated peptide conjugation. Regen. Biomater. 2019, 6, 231–240. [Google Scholar] [CrossRef]
- Brunette, D.M.; Chehroudi, B. The effects of the surface topography of micromachined titanium substrata on cell behavior in vitro and in vivo. J. Biomech. Eng. 1999, 121, 49–57. [Google Scholar] [CrossRef]
- Warita, K.; Aoki, R.; Kitamura, N.; Shibuya, I.; Hosaka, Y.Z. The precursor osteoblast-like cell, MC3T3-E1 cell line, enhances sodium–calcium exchanger 1 (Ncx1) gene expression by stretch stimuli prior to osteoblast differentiation. J. Vet. Med. Sci. 2019, 81, 508–512. [Google Scholar] [CrossRef]
- Yang, X.; Yang, F.; Walboomers, X.F.; Bian, Z.; Fan, M.; Jansen, J.A. The performance of dental pulp stem cells on nanofibrous PCL/gelatin/nHA scaffolds. J. Biomed. Mater. Res. Part. A 2010, 93, 247–257. [Google Scholar] [CrossRef] [Green Version]
- Park, H.; Park, H.; Lim, D.J.; Lee, S.H. Nanofibrous mineralized electrospun scaffold as a substrate for bone tissue regeneration. J. Biomed. Nanotechnol. 2016, 12, 2076–2082. [Google Scholar] [CrossRef]
- Fu, Q.W.; Zi, Y.P.; Xu, W.; Zhou, R.; Cai, Z.Y.; Zheng, W.J.; Chen, F.; Qian, Q.R. Electrospinning of calcium phosphate-poly(D,L-lactic acid) nanofibers for sustained release of water-soluble drug and fast mineralization. Int. J. Nanomed. 2016, 11, 5087–5097. [Google Scholar] [CrossRef] [Green Version]
- Miroshnichenko, S.; Timofeeva, V.; Permyakova, E.; Ershov, S.; Kiryukhantsev-Korneev, P.; Dvořaková, E.; Shtansky, D.; Zajíčková, L.; Solovieva, A.; Manakhov, A. Plasma-Coated Polycaprolactone Nanofibers with Covalently Bonded Platelet-Rich Plasma Enhance Adhesion and Growth of Human Fibroblasts. Nanomaterials 2019, 9, 637. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Wang, B.; Liu, X.; Pan, Z.; Liu, C.; Ma, H.; Liu, X.; Liu, L.; Jiang, C. The dosage effects of dexamethasone on osteogenic activity andbiocompatibility of poly(lactic-co-glycolic acid)/hydroxyapatite nanofibers. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1823–1832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouhi, M.; Jayarama Reddy, V.; Ramakrishna, S. GPTMS-Modified Bredigite/PHBV Nanofibrous Bone Scaffolds with Enhanced Mechanical and Biological Properties. Appl. Biochem. Biotechnol. 2019, 188, 357–368. [Google Scholar] [CrossRef] [PubMed]








| Sample Name | (C), at.% | (O), at.% | (N), at.% | (Ca), at.% | (Na), at.% | (Ti), at.% |
|---|---|---|---|---|---|---|
| PCL-ref | 76.0 | 24.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| PCL–TiCaPCON | 55.4 | 22.5 | 8.6 | 1.1 | 0.0 | 8.4 |
| PCL–COOH | 77.0 | 23.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| PCL–COOH-12h | 75.0 | 22.0 | 1.1 | 1.5 | 0.4 | 0.0 |
| PCL–COOH-24h | 77.3 | 20.1 | 1.0 | 1.2 | 0.3 | 0.0 |
| PCL–COOH-72h | 78.2 | 18.4 | 2.4 | 1.0 | 0.0 | 0.0 |
| Nanofiber Chemical Composition | Mineralization Method | Cell Type | Cell/Material Interaction | Reference |
|---|---|---|---|---|
| PCL/gelatin, PCL/gelatin/nano–hydroxyapatite (HA) | nano-HA-doped electrospun solution | DPSCs | Cell attachment and growth was not improved | [37] |
| PCL–COOH PCL–COOH-SBF | SBF solution | hASCs | Improved osteogenic differentiation of SBF-treated samples | [38] |
| PLA PLA/Ca3(PO4)2 PLA/Ca3(PO4)2/BSA | SBF-solution, deposition of amorphous Ca3(PO4)2 film | MG-63 | No significant differences for cell adhesion and spreading | [39] |
| PLA/TSF, PLA/TSF/HA | 1.5× SBF doped with 1 wt% of asparaginic acid | MSCs | Improved cell proliferation and differentiation on the PLA/TSF/HA | [14] |
| PLGA, PLGA–COOH, PLGA–COOH-Glu6 | 1.5× SBF | hMSCs | Improved proliferation | [34] |
| PCL/nano–HA PCL/nano–HA–COOH | nano-HAp-doped electrospun solution | MC3T3-E1 | Enhanced attachment, proliferation, and differentiation | [29] |
| PCL, PCL–COOH PCL–COOH/PRP | - | MRC-5 | The number of adhered cells did not differ significantly. More uniform cell distribution on the surface of PCL–COOH | [40] |
| PLGA (control), PLGA/HA PLGA/HA/ Dexamethasone | nano-HA-doped electrospun solution | MC3T3-E1 | Slow cell proliferation compared with control | [41] |
| PLLA/PCL | Electrochemical deposition | rBMSCs | enhanced the osteogenic differen- tiation and proliferation of rBMSCs for treated sample | [17] |
| BR or G-BR nanoparticles into PHBV nanofibers | Electrochemical deposition | hFOB | Enhanced cell proliferation | [42] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Permyakova, E.S.; Kiryukhantsev-Korneev, P.V.; Gudz, K.Y.; Konopatsky, A.S.; Polčak, J.; Zhitnyak, I.Y.; Gloushankova, N.A.; Shtansky, D.V.; Manakhov, A.M. Comparison of Different Approaches to Surface Functionalization of Biodegradable Polycaprolactone Scaffolds. Nanomaterials 2019, 9, 1769. https://doi.org/10.3390/nano9121769
Permyakova ES, Kiryukhantsev-Korneev PV, Gudz KY, Konopatsky AS, Polčak J, Zhitnyak IY, Gloushankova NA, Shtansky DV, Manakhov AM. Comparison of Different Approaches to Surface Functionalization of Biodegradable Polycaprolactone Scaffolds. Nanomaterials. 2019; 9(12):1769. https://doi.org/10.3390/nano9121769
Chicago/Turabian StylePermyakova, Elizaveta S., Philipp V. Kiryukhantsev-Korneev, Kristina Yu. Gudz, Anton S. Konopatsky, Josef Polčak, Irina Y. Zhitnyak, Natalia A. Gloushankova, D. V. Shtansky, and Anton M. Manakhov. 2019. "Comparison of Different Approaches to Surface Functionalization of Biodegradable Polycaprolactone Scaffolds" Nanomaterials 9, no. 12: 1769. https://doi.org/10.3390/nano9121769