Micelle-Assisted Strategy for the Direct Synthesis of Large-Sized Mesoporous Platinum Catalysts by Vapor Infiltration of a Reducing Agent
Abstract
:1. Introduction
2. Materials and Methods
3. Results
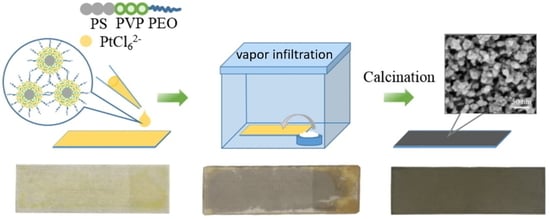
3.1. Polymeric Micelle Solution
3.2. Synthesis of Mesoporous Pt Particles
3.3. Methanol Electro-Oxidation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nie, Y.; Li, L.; Wei, Z. Recent advancements in Pt and Pt-free catalysts for oxygen reduction reaction. Chem. Soc. Rev. 2015, 44, 2168–2201. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Iocozzia, J.; Wang, Y.; Cui, X.; Chen, Y.; Zhao, S.; Li, Z.; Lin, Z. Noble metal–metal oxide nanohybrids with tailored nanostructures for efficient solar energy conversion, photocatalysis and environmental remediation. Energy Environ. Sci. 2017, 10, 402–434. [Google Scholar] [CrossRef]
- Cheng, N.; Stambula, S.; Wang, D.; Banis, M.N.; Liu, J.; Riese, A.; Xiao, B.; Li, R.; Sham, T.-K.; Liu, L.-M.; et al. Platinum single-atom and cluster catalysis of the hydrogen evolution reaction. Nat. Commun. 2016, 7, 13638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao-Horn, Y.; Sheng, W.C.; Chen, S.; Ferreira, P.J.; Holby, E.F.; Morgan, D. Instability of supported platinum nanoparticles in low-temperature fuel cells. Top. Catal. 2007, 46, 285–305. [Google Scholar] [CrossRef]
- Li, Y.; Bastakoti, B.P.; Li, C.; Malgras, V.; Ishihara, S.; Yamauchi, Y. Block copolymer-assisted solvothermal synthesis of bimetallic Pt-Pd nanoparticles. Electrochim. Acta 2015, 183, 119–124. [Google Scholar] [CrossRef]
- Takai, A.; Yamauchi, Y.; Kuroda, K. Fabrication of mesoporous Pt nanotubes utilizing dual templates under a reduced pressure condition. Chem. Commun. 2008, 4171–4173. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Jiang, Y.; Zhang, J.; Zhang, L.; Chen, Q.; Xie, Z.; Zheng, L. Unique excavated rhombic dodecahedral PtCu3 alloy nanocrystals constructed with ultrathin nanosheets of high-energy {110} facets. J. Am. Chem. Soc. 2014, 136, 3748–3751. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.; Jiang, M.; Camargo, P.H.C.; Cho, E.C.; Tao, J.; Lu, X.; Zhu, Y.; Xia, Y. Pd-Pt bimetallic nanodendrites with high activity for oxygen reduction. Science 2009, 324, 1302–1305. [Google Scholar] [CrossRef] [PubMed]
- Ataee-Esfahani, H.; Wang, L.; Nemoto, Y.; Yamauchi, Y. Synthesis of bimetallic Au@Pt nanoparticles with Au core and nanostructured Pt shell toward highly active electrocatalysts. Chem. Mater. 2010, 22, 6310–6318. [Google Scholar] [CrossRef]
- Wang, L.; Yamauchi, Y. Metallic Nanocages: Synthesis of Bimetallic Pt–Pd Hollow Nanoparticles with Dendritic Shells by Selective Chemical Etching. J. Am. Chem. Soc. 2013, 135, 16762–16765. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Kang, Y.; Huo, Z.; Zhu, Z.; Huang, W.; Xin, H.L.; Snyder, J.D.; Li, D.; Herron, J.A.; Mavrikakis, M.; et al. Highly crystalline multimetallic nanoframes with three-dimensional electrocatalytic surfaces. Science 2014, 343, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Figueroa-Cosme, L.; Yang, X.; Luo, M.; Liu, J.; Xie, Z.; Xia, Y. Pt-Based Icosahedral Nanocages: Using a Combination of {111} Facets, Twin Defects, and Ultrathin Walls to Greatly Enhance Their Activity toward Oxygen Reduction. Nano Lett. 2016, 16, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Li, C.; Malgras, V.; Imura, M.; Tominaka, S.; Yamauchi, Y. Mesoporous Pt nanospheres with designed pore surface as highly active electrocatalyst. Chem. Sci. 2016, 7, 1575–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Sato, T.; Yamauchi, Y. Electrochemical synthesis of one-dimensional mesoporous Pt nanorods using the assembly of surfactant micelles in confined space. Angew. Chem. 2013, 52, 8050–8053. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lin, J.; Iqbal, M.; Jiang, B.; Malgras, V.; Kim, J.; Yamauchi, Y. Electrochemical deposition: An advanced approach for templated synthesis of nanoporous metal architectures. Acc. Chem. Res. 2018, 51, 1764–1773. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.C.; Galeano, C.; Katsounaros, I.; Topalov, A.A.; Kostka, A.; Schüth, F.; Mayrhofer, K.J.J. Degradation mechanisms of Pt/C fuel cell catalysts under simulated start–stop conditions. ACS Catal. 2012, 2, 832–843. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Y.; Asakura, H.; Zhang, B.; Zhang, J.; Zhou, M.; Han, Y.; Tanaka, T.; Wang, A.; Zhang, T.; et al. Thermally stable single atom Pt/m-Al2O3 for selective hydrogenation and CO oxidation. Nat. Commun. 2017, 8, 16100. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bastakoti, B.P.; Malgras, V.; Li, C.; Tang, J.; Kim, J.H.; Yamauchi, Y. Polymeric micelle assembly for the smart synthesis of mesoporous platinum nanospheres with tunable pore sizes. Angew. Chem. Int. Ed. 2015, 54, 11073–11077. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tan, H.; Lin, J.; Luo, X.; Wang, S.; You, J.; Kang, Y.-M.; Bando, Y.; Yamauchi, Y.; Kim, J. Emerging Pt-based electrocatalysts with highly open nanoarchitectures for boosting oxygen reduction reaction. Nano Today 2018, 21, 91–105. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Sugiyama, A.; Morimoto, R.; Takai, A.; Kuroda, K. Mesoporous platinum with giant mesocages templated from lyotropic liquid crystals consisting of diblock copolymers. Angew. Chem. Int. Ed. 2008, 47, 5371–5373. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jeong, H.Y.; Imura, M.; Wang, L.; Radhakrishnan, L.; Fujita, N.; Castle, T.; Terasaki, O.; Yamauchi, Y. Shape- and size-controlled synthesis in hard templates: Sophisticated chemical reduction for mesoporous monocrystalline platinum nanoparticles. J. Am. Chem. Soc. 2011, 133, 14526–14529. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.C.-W.; Yamauchi, Y. Controlling physical features of mesoporous silica nanoparticles (MSNs) for emerging applications. J. Mater. Chem. 2012, 22, 1251–1256. [Google Scholar] [CrossRef]
- Wu, K.C.-W.; Jiang, X.; Yamauchi, Y. New trend on mesoporous films: precise controls of one-dimensional (1D) mesochannels toward innovative applications. J. Mater. Chem. 2011, 21, 8934–8939. [Google Scholar] [CrossRef]
- Tan, H.; Li, Y.; Jiang, X.; Tang, J.; Wang, Z.; Qian, H.; Mei, P.; Malgras, V.; Bando, Y.; Yamauchi, Y. Perfectly ordered mesoporous iron-nitrogen doped carbon as highly efficient catalyst for oxygen reduction reaction in both alkaline and acidic electrolytes. Nano Energy 2017, 36, 286–294. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Kuroda, K. Rational design of mesoporous metals and related nanomaterials by a soft-template approach. Chem. Asian J. 2008, 3, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, M.; Nakashima, K. Core–shell–corona polymeric micelles as a versatile template for synthesis of inorganic hollow nanospheres. Acc. Chem. Res. 2014, 47, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Cappillino, P.J.; Hattar, K.M.; Clark, B.G.; Hartnett, R.J.; Stavila, V.; Hekmaty, M.A.; Jacobs, B.W.; Robinson, D.B. Synthesis of mesoporous palladium with tunable porosity and demonstration of its thermal stability by in situ heating and environmental transmission electron microscopy. J. Mater. Chem. A 2013, 1, 602–610. [Google Scholar] [CrossRef]
- Chen, J.; Herricks, T.; Matthias Geissler, A.; Xia, Y. Single-crystal nanowires of platinum can be synthesized by controlling the reaction rate of a polyol process. J. Am. Chem. Soc. 2004, 126, 10854–10855. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xin, H.L.; Hoven, R.; Wang, H.; Yu, Y.; Muller, D.A.; DiSalvo, F.J.; Abruna, H.D. Structurally ordered intermetallic platinum–cobalt core–shell nanoparticles with enhanced activity and stability as oxygen reduction electrocatalysts. Nat. Mater. 2013, 12, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Qi, L.; Jiang, X.; Fu, G.; Xu, L.; Sun, D.; Gu, Z.; Tang, Y. FeOOH-Templated synthesis of hollow porous platinum nanotubes as superior electrocatalysts towards methanol electrooxidation. New J. Chem. 2017, 41, 8812–8817. [Google Scholar] [CrossRef]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liu, Y.; Yamauchi, Y.; Kaneti, Y.V.; Alsheri, S.M.; Ahamad, T.; Alhokbany, N.; Kim, J.; Ariga, K.; Wu, N.; et al. Micelle-Assisted Strategy for the Direct Synthesis of Large-Sized Mesoporous Platinum Catalysts by Vapor Infiltration of a Reducing Agent. Nanomaterials 2018, 8, 841. https://doi.org/10.3390/nano8100841
Li Y, Liu Y, Yamauchi Y, Kaneti YV, Alsheri SM, Ahamad T, Alhokbany N, Kim J, Ariga K, Wu N, et al. Micelle-Assisted Strategy for the Direct Synthesis of Large-Sized Mesoporous Platinum Catalysts by Vapor Infiltration of a Reducing Agent. Nanomaterials. 2018; 8(10):841. https://doi.org/10.3390/nano8100841
Chicago/Turabian StyleLi, Yunqi, Yuwei Liu, Yusuke Yamauchi, Yusuf Valentino Kaneti, Saad M. Alsheri, Tansir Ahamad, Norah Alhokbany, Jeonghun Kim, Katsuhiko Ariga, Ning Wu, and et al. 2018. "Micelle-Assisted Strategy for the Direct Synthesis of Large-Sized Mesoporous Platinum Catalysts by Vapor Infiltration of a Reducing Agent" Nanomaterials 8, no. 10: 841. https://doi.org/10.3390/nano8100841