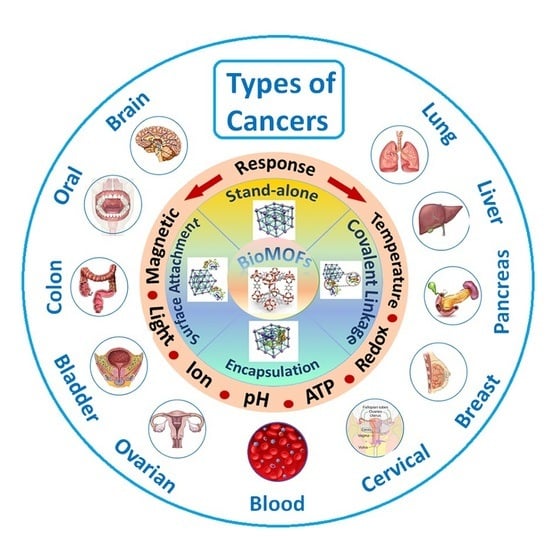
BioMOF-Based Anti-Cancer Drug Delivery Systems
Abstract
:1. Introduction
2. MOFs and Their Biomedical Applications
2.1. Biocompatibility of Metal Ions
2.2. Biocompatibility of Linkers

3. Synthesis of BioMOFs
3.1. Solvothermal/Hydrothermal
3.2. Sonochemical
3.3. Electrochemical
3.4. Microwave-Assisted
3.5. Vapor Diffusion
3.6. Reverse-Phase Microemulsions
4. Surface Modification of BioMOFs
5. BioMOFs for Drug Delivery Imaging Applications
- ○
- absorption into the pores of MOFs;
- ○
- attachment on the external surface of MOF crystals;
- ○
- in situ encapsulation into MOF crystals as ‘crystal defect’; and
- ○
- directly used as ligands to synthesize MOFs.

5.1. Stimuli-Responsive BioMOFs
5.1.1. pH-Responsive BioMOFs
5.1.2. Ion-Responsive BioMOFs
5.1.3. Magnetically Responsive BioMOFs
5.1.4. Temperature-Responsive BioMOFs
5.1.5. Redox-Responsive BioMOFs
5.1.6. ATP-Responsive BioMOFs
5.1.7. Light-Responsive BioMOFs
6. BioMOFs for Cancer Treatment
6.1. Breast Cancer
6.2. Lung Cancer
6.3. Liver Cancer
6.4. Colon Cancer
6.5. Pancreatic Cancer
6.6. Bladder Cancer
6.7. Ovarian and Cervical Cancer
6.8. Oral Cancer
6.9. Brain Cancer
6.10. Blood Cancer
7. Conclusions and Outlooks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Hammadi, M.M.; Arias, J.L. Chapter 11—Nanomedicine for vaginal drug delivery. In Theory and Applications of Nonparenteral Nanomedicines; Kesharwani, P., Taurin, S., Greish, K., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 235–257. [Google Scholar]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.; Shin, D.M. Therapeutic Nanoparticles for Drug Delivery in Cancer. Clin. Cancer Res. 2008, 14, 1310. [Google Scholar] [CrossRef] [Green Version]
- Patel, D.K.; Rana, D.; Aswal, V.K.; Srivastava, S.; Roy, P.; Maiti, P. Influence of graphene on self-assembly of polyurethane and evaluation of its biomedical properties. Polymer 2015, 65, 183–192. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Sign. Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Xia, L.; Jo, A.; Davis, R.M.; Bissel, P.; Ehrich, M.F.; Kingston, D.G.I. Synthesis and Evaluation of Doxorubicin-Loaded Gold Nanoparticles for Tumor-Targeted Drug Delivery. Bioconj. Chem. 2018, 29, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-K.; Ghosh, P.; Rotello, V.M. Multimodal drug delivery using gold nanoparticles. Nanoscale 2009, 1, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Kooti, M.; Sedeh, A.N.; Motamedi, H.; Rezatofighi, S.E. Magnetic graphene oxide inlaid with silver nanoparticles as antibacterial and drug delivery composite. Appl. Microbiol. Biotechnol. 2018, 102, 3607–3621. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yang, W.; Chen, P.; Huang, Y.; Li, F. Disulfiram Copper Nanoparticles Prepared with a Stabilized Metal Ion Ligand Complex Method for Treating Drug-Resistant Prostate Cancers. ACS Appl. Mater. Interfaces 2018, 10, 41118–41128. [Google Scholar] [CrossRef]
- Lu, Y.; Yue, Z.; Xie, J.; Wang, W.; Zhu, H.; Zhang, E.; Cao, Z. Micelles with ultralow critical micelle concentration as carriers for drug delivery. Nat. Biomed. Eng. 2018, 2, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, L.; Yang, T.; Wu, H. Stimuli-responsive polymeric micelles for drug delivery and cancer therapy. Int. J. Nanomed. 2018, 13, 2921–2942. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, M.; Yuba, E.; Hayashi, H.; Harada, A.; Kono, K. Hyaluronic Acid-Based pH-Sensitive Polymer-Modified Liposomes for Cell-Specific Intracellular Drug Delivery Systems. Bioconj. Chem. 2018, 29, 44–55. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhou, Y.; Zhou, Z.; Piao, Y.; Kalva, N.; Liu, X.; Tang, J.; Shen, Y. Synthesis of enzyme-responsive phosphoramidate dendrimers for cancer drug delivery. Polym. Chem. 2018, 9, 438–449. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Chou, H.-Y.; Yuh, C.-H.; Mekuria, S.L.; Kao, Y.-C.; Tsai, H.-C. Radiation-Sensitive Dendrimer-Based Drug Delivery System. Adv. Sci. 2018, 5, 1700339. [Google Scholar] [CrossRef] [PubMed]
- Ghawanmeh, A.A.; Ali, G.A.M.; Algarni, H.; Sarkar, S.M.; Chong, K.F. Graphene oxide-based hydrogels as a nanocarrier for anticancer drug delivery. Nano Res. 2019, 12, 973–990. [Google Scholar] [CrossRef]
- Hyun, H.; Park, M.H.; Jo, G.; Kim, S.Y.; Chun, H.J.; Yang, D.H. Photo-Cured Glycol Chitosan Hydrogel for Ovarian Cancer Drug Delivery. Mar. Drugs 2019, 17, 41. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, M.; Kawano, K.; Yokoyama, M.; Opanasopit, P.; Okano, T.; Maitani, Y. Preparation of camptothecin-loaded polymeric micelles and evaluation of their incorporation and circulation stability. Int. J. Pharm. 2006, 308, 183–189. [Google Scholar] [CrossRef]
- Mignani, S.; El Kazzouli, S.; Bousmina, M.; Majoral, J.-P. Expand classical drug administration ways by emerging routes using dendrimer drug delivery systems: A concise overview. Adv. Drug Deliv. Rev. 2013, 65, 1316–1330. [Google Scholar] [CrossRef]
- Mignani, S.; Shi, X.; Rodrigues, J.; Roy, R.; Muñoz-Fernández, Á.; Ceña, V.; Majoral, J.-P. Dendrimers toward Translational Nanotherapeutics: Concise Key Step Analysis. Bioconj. Chem. 2020, 31, 2060–2071. [Google Scholar] [CrossRef] [PubMed]
- Ogawara, K.-I.; Un, K.; Tanaka, K.-I.; Higaki, K.; Kimura, T. In vivo anti-tumor effect of PEG liposomal doxorubicin (DOX) in DOX-resistant tumor-bearing mice: Involvement of cytotoxic effect on vascular endothelial cells. J. Control. Release 2009, 133, 4–10. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef] [Green Version]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Jeremias, F. Synthesis and Characterization of Metal-Organic Frameworks for Heat Transformation Applications. Ph.D. Thesis, Institute for Inorganic and Structural Chemistry, Düsseldorf, Germany, 2015. [Google Scholar]
- Czaja, A.U.; Trukhan, N.; Müller, U. Industrial applications of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1284–1293. [Google Scholar] [CrossRef]
- Gu, J.-Z.; Lu, W.-G.; Jiang, L.; Zhou, H.-C.; Lu, T.-B. 3D Porous Metal−Organic Framework Exhibiting Selective Adsorption of Water over Organic Solvents. Inorg. Chem. 2007, 46, 5835–5837. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-N.; Meng, X.; Yang, G.-S.; Wang, X.-L.; Shao, K.-Z.; Su, Z.-M.; Wang, C.-G. Stepwise assembly of metal–organic framework based on a metal–organic polyhedron precursor for drug delivery. Chem. Commun. 2011, 47, 7128–7130. [Google Scholar] [CrossRef]
- Wang, Z.; Cohen, S.M. Postsynthetic modification of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1315–1329. [Google Scholar] [CrossRef]
- Rojas, S.; Devic, T.; Horcajada, P. Metal organic frameworks based on bioactive components. J. Mater. Chem. B 2017, 5, 2560–2573. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, Y.-W. Metal–Organic Frameworks for Biomedical Applications. Small 2020, 16, 1906846. [Google Scholar] [CrossRef]
- Giménez-Marqués, M.; Hidalgo, T.; Serre, C.; Horcajada, P. Nanostructured metal–organic frameworks and their bio-related applications. Coord. Chem. Rev. 2016, 307, 342–360. [Google Scholar] [CrossRef]
- Falsafi, M.; Saljooghi, A.S.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Smart metal organic frameworks: Focus on cancer treatment. Biomater. Sci. 2021, 9, 1503–1529. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Gao, Y.; Li, Z.; Xing, J.; Ren, W.; Zhang, L.; Li, A.; Lu, G.; Wu, A.; et al. ZD2-Engineered Gold Nanostar@Metal-Organic Framework Nanoprobes for T1-Weighted Magnetic Resonance Imaging and Photothermal Therapy Specifically Toward Triple-Negative Breast Cancer. Adv. Healthc. Mater. 2018, 7, 1801144. [Google Scholar] [CrossRef]
- Laha, D.; Pal, K.; Chowdhuri, A.R.; Parida, P.K.; Sahu, S.K.; Jana, K.; Karmakar, P. Fabrication of curcumin-loaded folic acid-tagged metal organic framework for triple negative breast cancer therapy in in vitro and in vivo systems. New J. Chem. 2019, 43, 217–229. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, M.; Zheng, W.; Xu, T.; Deng, H.; Liu, J. Se/Ru-Decorated Porous Metal–Organic Framework Nanoparticles for The Delivery of Pooled siRNAs to Reversing Multidrug Resistance in Taxol-Resistant Breast Cancer Cells. ACS Appl. Mater. Interfaces 2017, 9, 6712–6724. [Google Scholar] [CrossRef]
- Haddad, S.; Abánades Lázaro, I.; Fantham, M.; Mishra, A.; Silvestre-Albero, J.; Osterrieth, J.W.M.; Kaminski Schierle, G.S.; Kaminski, C.F.; Forgan, R.S.; Fairen-Jimenez, D. Design of a Functionalized Metal–Organic Framework System for Enhanced Targeted Delivery to Mitochondria. J. Am. Chem. Soc. 2020, 142, 6661–6674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alijani, H.; Noori, A.; Faridi, N.; Bathaie, S.Z.; Mousavi, M.F. Aptamer-functionalized Fe3O4@MOF nanocarrier for targeted drug delivery and fluorescence imaging of the triple-negative MDA-MB-231 breast cancer cells. J. Solid State Chem. 2020, 292, 121680. [Google Scholar] [CrossRef]
- Liang, S.; Xiao, X.; Bai, L.; Liu, B.; Yuan, M.; Ma, P.; Pang, M.; Cheng, Z.; Lin, J. Conferring Ti-Based MOFs with Defects for Enhanced Sonodynamic Cancer Therapy. Adv. Mater. 2021, 33, 2100333. [Google Scholar] [CrossRef] [PubMed]
- Nejadshafiee, V.; Naeimi, H.; Goliaei, B.; Bigdeli, B.; Sadighi, A.; Dehghani, S.; Lotfabadi, A.; Hosseini, M.; Nezamtaheri, M.S.; Amanlou, M.; et al. Magnetic bio-metal–organic framework nanocomposites decorated with folic acid conjugated chitosan as a promising biocompatible targeted theranostic system for cancer treatment. Mater. Sci. Eng. C 2019, 99, 805–815. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, Y.; Sun, S.; Li, Z.; Wu, A.; Zeng, L. pH-Responsive metal–organic framework encapsulated gold nanoclusters with modulated release to enhance photodynamic therapy/chemotherapy in breast cancer. J. Mater. Chem. B 2020, 8, 1739–1747. [Google Scholar] [CrossRef]
- Tian, Z.; Yao, X.; Ma, K.; Niu, X.; Grothe, J.; Xu, Q.; Liu, L.; Kaskel, S.; Zhu, Y. Metal–Organic Framework/Graphene Quantum Dot Nanoparticles Used for Synergistic Chemo- and Photothermal Therapy. ACS Omega 2017, 2, 1249–1258. [Google Scholar] [CrossRef] [Green Version]
- Pooresmaeil, M.; Asl, E.A.; Namazi, H. A new pH-sensitive CS/Zn-MOF@GO ternary hybrid compound as a biofriendly and implantable platform for prolonged 5-Fluorouracil delivery to human breast cancer cells. J. Alloy. Compd. 2021, 885, 160992. [Google Scholar] [CrossRef]
- Deng, Z.; Fang, C.; Ma, X.; Li, X.; Zeng, Y.-J.; Peng, X. One Stone Two Birds: Zr-Fc Metal–Organic Framework Nanosheet for Synergistic Photothermal and Chemodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 20321–20330. [Google Scholar] [CrossRef]
- Sun, X.; He, G.; Xiong, C.; Wang, C.; Lian, X.; Hu, L.; Li, Z.; Dalgarno, S.J.; Yang, Y.-W.; Tian, J. One-Pot Fabrication of Hollow Porphyrinic MOF Nanoparticles with Ultrahigh Drug Loading toward Controlled Delivery and Synergistic Cancer Therapy. ACS Appl. Mater. Interfaces 2021, 13, 3679–3693. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jin, H.; Jiang, X.; Gui, R. Assembly of Black Phosphorus Nanosheets and MOF to Form Functional Hybrid Thin-Film for Precise Protein Capture, Dual-Signal and Intrinsic Self-Calibration Sensing of Specific Cancer-Derived Exosomes. Anal. Chem. 2020, 92, 2866–2875. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, Y.S.; Jin, Z.; Zhao, P.; Zhang, H.; Wen, Y.; He, Q. Porphyrin–palladium hydride MOF nanoparticles for tumor-targeting photoacoustic imaging-guided hydrogenothermal cancer therapy. Nanoscale Horiz. 2019, 4, 1185–1193. [Google Scholar] [CrossRef]
- Wang, L.; Qu, X.; Zhao, Y.; Weng, Y.; Waterhouse, G.I.N.; Yan, H.; Guan, S.; Zhou, S. Exploiting Single Atom Iron Centers in a Porphyrin-like MOF for Efficient Cancer Phototherapy. ACS Appl. Mater. Interfaces 2019, 11, 35228–35237. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, M.; Jin, G.; Jiang, Y.; Luan, Y. Cu-MOF chemodynamic nanoplatform via modulating glutathione and H2O2 in tumor microenvironment for amplified cancer therapy. J. Colloid Interface Sci. 2021, 587, 358–366. [Google Scholar] [CrossRef]
- Alves, R.C.; Schulte, Z.M.; Luiz, M.T.; Bento da Silva, P.; Frem, R.C.G.; Rosi, N.L.; Chorilli, M. Breast Cancer Targeting of a Drug Delivery System through Postsynthetic Modification of Curcumin@N3-bio-MOF-100 via Click Chemistry. Inorg. Chem. 2021, 60, 11739–11744. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hu, M.; Li, Z.; He, L.; Song, Y.; Jia, Q.; Zhang, Z.; Du, M. Construction of Tb-MOF-on-Fe-MOF conjugate as a novel platform for ultrasensitive detection of carbohydrate antigen 125 and living cancer cells. Biosens. Bioelectron. 2019, 142, 111536. [Google Scholar] [CrossRef]
- Kim, K.; Lee, S.; Jin, E.; Palanikumar, L.; Lee, J.H.; Kim, J.C.; Nam, J.S.; Jana, B.; Kwon, T.-H.; Kwak, S.K.; et al. MOF × Biopolymer: Collaborative Combination of Metal–Organic Framework and Biopolymer for Advanced Anticancer Therapy. ACS Appl. Mater. Interfaces 2019, 11, 27512–27520. [Google Scholar] [CrossRef]
- Li, Z.; Wu, X.; Wang, W.; Gai, C.; Zhang, W.; Li, W.; Ding, D. Fe(II) and Tannic Acid-Cloaked MOF as Carrier of Artemisinin for Supply of Ferrous Ions to Enhance Treatment of Triple-Negative Breast Cancer. Nanoscale Res. Lett. 2021, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Afzalinia, A.; Mirzaee, M. Ultrasensitive Fluorescent miRNA Biosensor Based on a “Sandwich” Oligonucleotide Hybridization and Fluorescence Resonance Energy Transfer Process Using an Ln(III)-MOF and Ag Nanoparticles for Early Cancer Diagnosis: Application of Central Composite Design. ACS Appl. Mater. Interfaces 2020, 12, 16076–16087. [Google Scholar] [CrossRef]
- Wang, S.; Wu, H.; Sun, K.; Hu, J.; Chen, F.; Liu, W.; Chen, J.; Sun, B.; Hossain, A.M.S. A novel pH-responsive Fe-MOF system for enhanced cancer treatment mediated by the Fenton reaction. New J. Chem. 2021, 45, 3271–3279. [Google Scholar] [CrossRef]
- Sun, L.; Xu, Y.; Gao, Y.; Huang, X.; Feng, S.; Chen, J.; Wang, X.; Guo, L.; Li, M.; Meng, X.; et al. Synergistic Amplification of Oxidative Stress–Mediated Antitumor Activity via Liposomal Dichloroacetic Acid and MOF-Fe2+. Small 2019, 15, 1901156. [Google Scholar] [CrossRef]
- Zhang, K.; Meng, X.; Yang, Z.; Dong, H.; Zhang, X. Enhanced cancer therapy by hypoxia-responsive copper metal-organic frameworks nanosystem. Biomaterials 2020, 258, 120278. [Google Scholar] [CrossRef]
- Wei, D.; Xin, Y.; Rong, Y.; Li, Y.; Zhang, C.; Chen, Q.; Qin, S.; Wang, W.; Hao, Y. A Mesoporous Gd-MOF with Lewis Basic Sites for 5-Fu Delivery and Inhibition of Human Lung Cancer Cells In Vivo and In Vitro. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1121–1131. [Google Scholar] [CrossRef]
- Tan, G.; Zhong, Y.; Yang, L.; Jiang, Y.; Liu, J.; Ren, F. A multifunctional MOF-based nanohybrid as injectable implant platform for drug synergistic oral cancer therapy. Chem. Eng. J. 2020, 390, 124446. [Google Scholar] [CrossRef]
- Xiang, Z.; Qi, Y.; Lu, Y.; Hu, Z.; Wang, X.; Jia, W.; Hu, J.; Ji, J.; Lu, W. MOF-derived novel porous Fe3O4@C nanocomposites as smart nanomedical platforms for combined cancer therapy: Magnetic-triggered synergistic hyperthermia and chemotherapy. J. Mater. Chem. B 2020, 8, 8671–8683. [Google Scholar] [CrossRef]
- Leng, X.; Dong, X.; Wang, W.; Sai, N.; Yang, C.; You, L.; Huang, H.; Yin, X.; Ni, J. Biocompatible Fe-Based Micropore Metal-Organic Frameworks as Sustained-Release Anticancer Drug Carriers. Molecules 2018, 23, 2490. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Song, Y.; Zhang, W.; Xu, J.; Hou, J.; Feng, X.; Zhu, W. MOF nanoparticles with encapsulated dihydroartemisinin as a controlled drug delivery system for enhanced cancer therapy and mechanism analysis. J. Mater. Chem. B 2020, 8, 7382–7389. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, W.; Zhu, D.; Wang, Q.; Chen, B.; Liu, Z.; Wang, Y.; Liu, Q. Cancer cell membrane-camouflaged MOF nanoparticles for a potent dihydroartemisinin-based hepatocellular carcinoma therapy. RSC Adv. 2020, 10, 7194–7205. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Liu, W.; Shang, Y.; Cao, P.; Cui, J.; Li, Z.; Yin, X.; Li, Y. Folic acid-nanoscale gadolinium-porphyrin metal-organic frameworks: Fluorescence and magnetic resonance dual-modality imaging and photodynamic therapy in hepatocellular carcinoma. Int. J. Nanomed. 2018, 14, 57–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Leng, X.; Luo, J.; You, L.; Qu, C.; Dong, X.; Huang, H.; Yin, X.; Ni, J. In Vitro Toxicity Study of a Porous Iron(III) Metal-Organic Framework. Molecules 2019, 24, 1211. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.-L.; Li, Y.-H.; Shi, H. A Ketone Functionalized Gd(III)-MOF with Low Cytotoxicity for Anti-Cancer Drug Delivery and Inhibiting Human Liver Cancer Cells. J. Clust. Sci. 2019, 30, 251–258. [Google Scholar] [CrossRef]
- Hu, J.; Wu, W.; Qin, Y.; Liu, C.; Wei, P.; Hu, J.; Seeberger, P.H.; Yin, J. Fabrication of Glyco-Metal-Organic Frameworks for Targeted Interventional Photodynamic/Chemotherapy for Hepatocellular Carcinoma through Percutaneous Transperitoneal Puncture. Adv. Funct. Mater. 2020, 30, 1910084. [Google Scholar] [CrossRef]
- Ke, F.; Yuan, Y.-P.; Qiu, L.-G.; Shen, Y.-H.; Xie, A.-J.; Zhu, J.-F.; Tian, X.-Y.; Zhang, L.-D. Facile fabrication of magnetic metal–organic framework nanocomposites for potential targeted drug delivery. J. Mater. Chem. 2011, 21, 3843–3848. [Google Scholar] [CrossRef]
- Sharma, S.; Mittal, D.; Verma, A.K.; Roy, I. Copper-Gallic Acid Nanoscale Metal–Organic Framework for Combined Drug Delivery and Photodynamic Therapy. ACS Appl. Bio Mater. 2019, 2, 2092–2101. [Google Scholar] [CrossRef]
- Lu, X.-C.; Wang, H.-L.; Wang, X.; Li, Q.-Z.; Liao, L. A New Zn(II)-Diisophthalate MOF for Solvent-Free Cyanosilylation of Aldehydes and Anti-colon Cancer Activity Study. J. Clust. Sci. 2019, 30, 1673–1681. [Google Scholar] [CrossRef]
- Li, H.-T.; Song, S.-J.; Pei, X.-R.; Lu, D.-B. A InIII-MOF with Imidazole Decorated Pores as 5-Fu Delivery System to Inhibit Colon Cancer Cells Proliferation and Induce Cell Apoptosis in vitro and in vivo. Z. Anorg. Allg. Chem. 2019, 645, 801–809. [Google Scholar] [CrossRef]
- Lv, C.; Liu, Y.; Meng, G.; Li, J.; Ti, Z. A new Er(III)-based MOF showing anti-colon cancer activity by inhibiting IL-6-STAT3 signaling pathway and reducing TNF-α and IL-1β production. J. Coord. Chem. 2020, 73, 1478–1489. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Wang, L.; Xie, Z. Endogenous Hydrogen Sulfide-Triggered MOF-Based Nanoenzyme for Synergic Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 30213–30220. [Google Scholar] [CrossRef]
- Duan, F.; Hu, M.; Guo, C.; Song, Y.; Wang, M.; He, L.; Zhang, Z.; Pettinari, R.; Zhou, L. Chromium-based metal-organic framework embedded with cobalt phthalocyanine for the sensitively impedimetric cytosensing of colorectal cancer (CT26) cells and cell imaging. Chem. Eng. J. 2020, 398, 125452. [Google Scholar] [CrossRef]
- Wu, Y.-Y.; Fan, H.-Q.; Chen, H.-J.; Jiang, L.-X.; Zhang, W.-P. A Zn(II)-MOF with Suitable Pore Surroundings for Cyanosilylation Reaction and Protective Effect on Bladder Cancer Cells by Regulating miR-130 and CYLD. J. Inorg. Organomet. Polym. Mater. 2021, 31, 520–527. [Google Scholar] [CrossRef]
- He, C.; Lu, K.; Liu, D.; Lin, W. Nanoscale Metal–Organic Frameworks for the Co-Delivery of Cisplatin and Pooled siRNAs to Enhance Therapeutic Efficacy in Drug-Resistant Ovarian Cancer Cells. J. Am. Chem. Soc. 2014, 136, 5181–5184. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Shi, W.; Cheng, P.; Zaworotko, M.J. A Mixed-Crystal Lanthanide Zeolite-like Metal–Organic Framework as a Fluorescent Indicator for Lysophosphatidic Acid, a Cancer Biomarker. J. Am. Chem. Soc. 2015, 137, 12203–12206. [Google Scholar] [CrossRef]
- Sun, R.W.-Y.; Zhang, M.; Li, D.; Zhang, Z.-F.; Cai, H.; Li, M.; Xian, Y.-J.; Ng, S.W.; Wong, A.S.-T. Dinuclear Gold(I) Pyrrolidinedithiocarbamato Complex: Cytotoxic and Antimigratory Activities on Cancer Cells and the Use of Metal–Organic Framework. Chem. A Eur. J. 2015, 21, 18534–18538. [Google Scholar] [CrossRef]
- Chen, D.; Li, B.; Jiang, L.; Li, Y.; Yang, Y.; Luo, Z.; Wang, J. Pristine Cu-MOF Induces Mitotic Catastrophe and Alterations of Gene Expression and Cytoskeleton in Ovarian Cancer Cells. ACS Appl. Bio Mater. 2020, 3, 4081–4094. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lu, D.; Moya, S.; Yan, H.; Qiu, M.; Chen, J.; Wang, X.; Li, Y.; Pan, H.; Chen, G.; et al. Bispecific T-cell engager (BiTE) immunotherapy of ovarian cancer based on MIL-88A MOF/MC gene delivery system. Appl. Mater. Today 2020, 20, 100701. [Google Scholar] [CrossRef]
- Xiang, X.; Pang, H.; Ma, T.; Du, F.; Li, L.; Huang, J.; Ma, L.; Qiu, L. Ultrasound targeted microbubble destruction combined with Fe-MOF based bio-/enzyme-mimics nanoparticles for treating of cancer. J. Nanobiotechnol. 2021, 19, 92. [Google Scholar] [CrossRef]
- Chen, W.-H.; Luo, G.-F.; Sohn, Y.S.; Nechushtai, R.; Willner, I. miRNA-Specific Unlocking of Drug-Loaded Metal–Organic Framework Nanoparticles: Targeted Cytotoxicity toward Cancer Cells. Small 2019, 15, 1900935. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-F.; Guo, H.; Huang, Y.; Li, C.-M.; Liu, Y.; Han, J. A porous Cu(II)-based metal-organic framework with one-dimensional hexagonal channels for solvent-free cyanosilylation and anti-ovarian cancer activity. J. Polym. Res. 2020, 27, 63. [Google Scholar] [CrossRef]
- Yan, Z.; Li, X.; Fan, Q.; Bai, H.; Wu, S.; Zhang, Z.-F.; Pan, L. A water-stable and biofriendly Zn-MOF with pyrazine decorated pores as 5-Fu delivery system to induce human ovarian cancer cells apoptosis and abrogate their growth. J. Mol. Struct. 2020, 1204, 127477. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, S.; Guan, X.; Xie, Z. One-Step Synthesis of Nanoscale Zeolitic Imidazolate Frameworks with High Curcumin Loading for Treatment of Cervical Cancer. ACS Appl. Mater. Interfaces 2015, 7, 22181–22187. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Ding, L.; Yao, H.; Chen, Y.; Shi, J. A Metal-Organic Framework (MOF) Fenton Nanoagent-Enabled Nanocatalytic Cancer Therapy in Synergy with Autophagy Inhibition. Adv. Mater. 2020, 32, 1907152. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; He, L.; Fan, W.; Tang, W.; Wang, Z.; Jiang, C.; Zhang, D.; Liu, Y.; Deng, H.; Zou, J.; et al. Cascade Reactions Catalyzed by Planar Metal–Organic Framework Hybrid Architecture for Combined Cancer Therapy. Small 2020, 16, 2004016. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.-M.; Ma, Z.-Z.; Wang, L.-N.; Li, M.; Li, Z.-J. Three-Dimensional Ni(II)-MOF Containing an Asymmetric Pyridyl-Carboxylate Ligand: Catalytic Cyanosilylation of Aldehydes and Inhibits Human Promyelocytic Leukemia Cancer Cells. J. Clust. Sci. 2019, 30, 1455–1464. [Google Scholar] [CrossRef]
- Cai, H.; Huang, Y.-L.; Li, D. Biological metal–organic frameworks: Structures, host–guest chemistry and bio-applications. Coord. Chem. Rev. 2019, 378, 207–221. [Google Scholar] [CrossRef]
- Vaidya, L.B.; Nadar, S.S.; Rathod, V.K. Biological metal organic framework (bio-MOF) of glucoamylase with enhanced stability. Colloids Surf. B Biointerfaces 2020, 193, 111052. [Google Scholar] [CrossRef] [PubMed]
- Shoueir, K.; Wassel, A.R.; Ahmed, M.K.; El-Naggar, M.E. Encapsulation of extremely stable polyaniline onto Bio-MOF: Photo-activated antimicrobial and depletion of ciprofloxacin from aqueous solutions. J. Photochem. Photobiol. A Chem. 2020, 400, 112703. [Google Scholar] [CrossRef]
- Giles-Mazón, E.A.; Germán-Ramos, I.; Romero-Romero, F.; Reinheimer, E.; Toscano, R.A.; Lopez, N.; Barrera-Díaz, C.E.; Varela-Guerrero, V.; Ballesteros-Rivas, M.F. Synthesis and characterization of a Bio-MOF based on mixed adeninate/tricarboxylate ligands and zinc ions. Inorg. Chim. Acta 2018, 469, 306–311. [Google Scholar] [CrossRef]
- Sattar, T. Cu@nano-bio-MOF-7 composite: Having more potential for in vitro drug adsorption/release and photocatalytic water splitting as compared to its parent nano-bio-MOF-7. Appl. Nanosci. 2018, 8, 1831–1841. [Google Scholar] [CrossRef]
- Azhar, M.R.; Vijay, P.; Tadé, M.O.; Sun, H.; Wang, S. Submicron sized water-stable metal organic framework (bio-MOF-11) for catalytic degradation of pharmaceuticals and personal care products. Chemosphere 2018, 196, 105–114. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Farha, O.K.; Hupp, J.T.; Pohl, E.; Yeh, J.I.; Rosi, N.L. Metal-adeninate vertices for the construction of an exceptionally porous metal-organic framework. Nat. Commun. 2012, 3, 604. [Google Scholar] [CrossRef] [Green Version]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal–Organic Frameworks in Biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef] [PubMed]
- Alnaqbi, M.A.; Alzamly, A.; Ahmed, S.H.; Bakiro, M.; Kegere, J.; Nguyen, H.L. Chemistry and applications of s-block metal–organic frameworks. J. Mater. Chem. A 2021, 9, 3828–3854. [Google Scholar] [CrossRef]
- Grall, R.; Hidalgo, T.; Delic, J.; Garcia-Marquez, A.; Chevillard, S.; Horcajada, P. In vitro biocompatibility of mesoporous metal (III.; Fe, Al, Cr) trimesate MOF nanocarriers. J. Mater. Chem. B 2015, 3, 8279–8292. [Google Scholar] [CrossRef]
- Abánades Lázaro, I.; Wells, C.J.R.; Forgan, R.S. Multivariate Modulation of the Zr MOF UiO-66 for Defect-Controlled Combination Anticancer Drug Delivery. Angew. Chem. Int. Ed. Engl. 2020, 59, 5211–5217. [Google Scholar] [CrossRef]
- Devic, T.; Horcajada, P.; Serre, C.; Salles, F.; Maurin, G.; Moulin, B.; Heurtaux, D.; Clet, G.; Vimont, A.; Grenèche, J.-M.; et al. Functionalization in Flexible Porous Solids: Effects on the Pore Opening and the Host−Guest Interactions. J. Am. Chem. Soc. 2010, 132, 1127–1136. [Google Scholar] [CrossRef]
- Horcajada, P.; Salles, F.; Wuttke, S.; Devic, T.; Heurtaux, D.; Maurin, G.; Vimont, A.; Daturi, M.; David, O.; Magnier, E.; et al. How Linker’s Modification Controls Swelling Properties of Highly Flexible Iron(III) Dicarboxylates MIL-88. J. Am. Chem. Soc. 2011, 133, 17839–17847. [Google Scholar] [CrossRef]
- Taddei, M.; McPherson, M.J.; Gougsa, A.; Lam, J.; Sewell, J.; Andreoli, E. An Optimised Compaction Process for Zr-Fumarate (MOF-801). Inorganics 2019, 7, 110. [Google Scholar] [CrossRef] [Green Version]
- Al Neyadi, S.S.; Al Blooshi, A.G.; Nguyen, H.L.; Alnaqbi, M.A. UiO-66-NH2 as an effective solid support for quinazoline derivatives for antibacterial agents against Gram-negative bacteria. New J. Chem. 2021, 45, 20386–20395. [Google Scholar] [CrossRef]
- Miller, S.R.; Heurtaux, D.; Baati, T.; Horcajada, P.; Grenèche, J.-M.; Serre, C. Biodegradable therapeutic MOFs for the delivery of bioactive molecules. Chem. Commun. 2010, 46, 4526–4528. [Google Scholar] [CrossRef] [PubMed]
- Burrows, A.D.; Jurcic, M.; Keenan, L.L.; Lane, R.A.; Mahon, M.F.; Warren, M.R.; Nowell, H.; Paradowski, M.; Spencer, J. Incorporation by coordination and release of the iron chelator drug deferiprone from zinc-based metal–organic frameworks. Chem. Commun. 2013, 49, 11260–11262. [Google Scholar] [CrossRef] [Green Version]
- Burrows, A.D.; Jurcic, M.; Mahon, M.F.; Pierrat, S.; Roffe, G.W.; Windle, H.J.; Spencer, J. Bismuth coordination networks containing deferiprone: Synthesis, characterisation, stability and antibacterial activity. Dalton Trans. 2015, 44, 13814–13817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, D.-R.; Sun, D.-Z.; Liu, J.-L.; Zhang, G.-J.; Chen, H.-Y.; He, J.-H.; Yan, S.-W.; Yuan, R.; Wang, E.-B. Two Unprecedented Entangled Metal–Olsalazine Complexes with Coexistence of 2D→3D Polycatenation and meso-Helix. Eur. J. Inorg. Chem. 2011, 2011, 3656–3663. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Xiao, D.-R.; Yan, S.-W.; He, J.-H.; Yang, J.; Wang, X.; Yuan, R.; Wang, E.-B. Two three-dimensional pillared metal–olsalazine complexes based on infinite rod-shaped secondary building units. Inorg. Chim. Acta 2012, 387, 283–288. [Google Scholar] [CrossRef]
- André, V.; da Silva, A.R.F.; Fernandes, A.; Frade, R.; Garcia, C.; Rijo, P.; Antunes, A.M.M.; Rocha, J.; Duarte, M.T. Mg- and Mn-MOFs Boost the Antibiotic Activity of Nalidixic Acid. ACS Appl. Bio Mater. 2019, 2, 2347–2354. [Google Scholar] [CrossRef]
- Su, H.; Sun, F.; Jia, J.; He, H.; Wang, A.; Zhu, G. A highly porous medical metal–organic framework constructed from bioactive curcumin. Chem. Commun. 2015, 51, 5774–5777. [Google Scholar] [CrossRef]
- Čelič, T.B.; Jagličič, Z.; Lazar, K.; Logar, N.Z. Structure and magnetic properties of a new iron(II) citrate coordination polymer. Acta Crystallogr. Sect. B 2013, 69, 490–495. [Google Scholar] [CrossRef]
- Jiang, J.; Huang, L.; Liu, X.; Ai, L. Bioinspired Cobalt–Citrate Metal–Organic Framework as an Efficient Electrocatalyst for Water Oxidation. ACS Appl. Mater. Interfaces 2017, 9, 7193–7201. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Trickett, C.A.; Alahmadi, S.B.; Alshammari, A.S.; Yaghi, O.M. Calcium l-Lactate Frameworks as Naturally Degradable Carriers for Pesticides. J. Am. Chem. Soc. 2017, 139, 8118–8121. [Google Scholar] [CrossRef]
- Livage, C.; Egger, C.; Férey, G. Hydrothermal versus Nonhydrothermal Synthesis for the Preparation of Organic−Inorganic Solids: The Example of Cobalt(II) Succinate. Chem. Mater. 2001, 13, 410–414. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, L.; Yang, X.; Xu, T.; Sun, T.; Wang, L.; Zhang, Q. A luminescent Terbium-Succinate MOF fabricated by co-precipitation for sensing of Fe3+ in aqueous environment. J. Mater. Sci. Mater. Electron. 2017, 28, 7326–7332. [Google Scholar] [CrossRef]
- Nagaraja, C.M.; Haldar, R.; Maji, T.K.; Rao, C.N.R. Chiral Porous Metal–Organic Frameworks of Co(II) and Ni(II): Synthesis, Structure, Magnetic Properties, and CO2 Uptake. Cryst. Growth Des. 2012, 12, 975–981. [Google Scholar] [CrossRef]
- Yutkin, M.P.; Zavakhina, M.S.; Samsonenko, D.G.; Dybtsev, D.N.; Fedin, V.P. Synthesis and characterization of expected and unexpected topologies of homochiral porous metal(II) malate frameworks. Inorg. Chim. Acta 2013, 394, 367–372. [Google Scholar] [CrossRef]
- Zavakhina, M.S.; Samsonenko, D.G.; Virovets, A.V.; Dybtsev, D.N.; Fedin, V.P. Homochiral Cu(II) and Ni(II) malates with tunable structural features. J. Solid State Chem. 2014, 210, 125–129. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, S.; Zingiryan, A.; Bu, X. Integrated Molecular Chirality, Absolute Helicity, and Intrinsic Chiral Topology in Three-Dimensional Open-Framework Materials. J. Am. Chem. Soc. 2008, 130, 17246–17247. [Google Scholar] [CrossRef] [Green Version]
- Senthil Raja, D.; Luo, J.-H.; Chang, T.-G.; Lo, S.-H.; Wu, C.-Y.; Lin, C.-H. Synthesis, Crystal Structure, and Luminescence Properties of a New Calcium(II) Coordination Polymer Based on L-Malic Acid. J. Chem. 2013, 2013, 980243. [Google Scholar] [CrossRef] [Green Version]
- Katsoulidis, A.P.; Antypov, D.; Whitehead, G.F.S.; Carrington, E.J.; Adams, D.J.; Berry, N.G.; Darling, G.R.; Dyer, M.S.; Rosseinsky, M.J. Chemical control of structure and guest uptake by a conformationally mobile porous material. Nature 2019, 565, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Kirchon, A.; Day, G.S.; Fang, Y.; Banerjee, S.; Ozdemir, O.K.; Zhou, H.-C. Suspension Processing of Microporous Metal-Organic Frameworks: A Scalable Route to High-Quality Adsorbents. iScience 2018, 5, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Della Rocca, J.; Liu, D.; Lin, W. Nanoscale Metal–Organic Frameworks for Biomedical Imaging and Drug Delivery. Acc. Chem. Res. 2011, 44, 957–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, Y.; Xiong, N.; Zhu, G. Technology for the Remediation of Water Pollution: A Review on the Fabrication of Metal Organic Frameworks. Processes 2018, 6, 122. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-R.; Kim, J.; Ahn, W.-S. Synthesis of metal-organic frameworks: A mini review. Korean J. Chem. Eng. 2013, 30, 1667–1680. [Google Scholar] [CrossRef]
- Lestari, W.W.; Arvinawati, M.; Martien, R.; Kusumaningsih, T. Green and facile synthesis of MOF and nano MOF containing zinc(II) and benzen 1,3,5-tri carboxylate and its study in ibuprofen slow-release. Mater. Chem. Phys. 2018, 204, 141–146. [Google Scholar] [CrossRef]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Smaldone, R.A.; Forgan, R.S.; Furukawa, H.; Gassensmith, J.J.; Slawin, A.M.Z.; Yaghi, O.M.; Stoddart, J.F. Metal–Organic Frameworks from Edible Natural Products. Angew. Chem. Int. Ed. 2010, 49, 8630–8634. [Google Scholar] [CrossRef]
- Taylor, K.M.L.; Rieter, W.J.; Lin, W. Manganese-Based Nanoscale Metal−Organic Frameworks for Magnetic Resonance Imaging. J. Am. Chem. Soc. 2008, 130, 14358–14359. [Google Scholar] [CrossRef]
- Sun, S.; Murray, C.B.; Weller, D.; Folks, L.; Moser, A. Monodisperse FePt Nanoparticles and Ferromagnetic FePt Nanocrystal Superlattices. Science 2000, 287, 1989. [Google Scholar] [CrossRef] [PubMed]
- Mokari, T.; Zhang, M.; Yang, P. Shape, Size, and Assembly Control of PbTe Nanocrystals. J. Am. Chem. Soc. 2007, 129, 9864–9865. [Google Scholar] [CrossRef]
- Peng, X.; Manna, L.; Yang, W.; Wickham, J.; Scher, E.; Kadavanich, A.; Alivisatos, A.P. Shape control of CdSe nanocrystals. Nature 2000, 404, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Manna, L.; Milliron, D.J.; Meisel, A.; Scher, E.C.; Alivisatos, A.P. Controlled growth of tetrapod-branched inorganic nanocrystals. Nat. Mater. 2003, 2, 382–385. [Google Scholar] [CrossRef]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef]
- He, S.; Wu, L.; Li, X.; Sun, H.; Xiong, T.; Liu, J.; Huang, C.; Xu, H.; Sun, H.; Chen, W.; et al. Metal-organic frameworks for advanced drug delivery. Acta Pharm. Sin. B 2021, 11, 2362–2395. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Liu, Y.; Zeng, G.; Zhao, L.; Lai, Z. Rapid synthesis of zeolitic imidazolate framework-8 (ZIF-8) nanocrystals in an aqueous system. Chem. Commun. 2011, 47, 2071–2073. [Google Scholar] [CrossRef]
- Li, Z.-Q.; Qiu, L.-G.; Xu, T.; Wu, Y.; Wang, W.; Wu, Z.-Y.; Jiang, X. Ultrasonic synthesis of the microporous metal–organic framework Cu3(BTC)2 at ambient temperature and pressure: An efficient and environmentally friendly method. Mater. Lett. 2009, 63, 78–80. [Google Scholar] [CrossRef]
- Rajkumar, T.; Kukkar, D.; Kim, K.-H.; Sohn, J.R.; Deep, A. Cyclodextrin-metal–organic framework (CD-MOF): From synthesis to applications. J. Ind. Eng. Chem. 2019, 72, 50–66. [Google Scholar] [CrossRef]
- Li, H.; Lv, N.; Li, X.; Liu, B.; Feng, J.; Ren, X.; Guo, T.; Chen, D.; Fraser Stoddart, J.; Gref, R.; et al. Composite CD-MOF nanocrystals-containing microspheres for sustained drug delivery. Nanoscale 2017, 9, 7454–7463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Q.; Li, Z.; Guo, C.; Huang, X.; Song, Y.; Zhou, N.; Wang, M.; Zhang, Z.; He, L.; Du, M. A γ-cyclodextrin-based metal–organic framework embedded with graphene quantum dots and modified with PEGMA via SI-ATRP for anticancer drug delivery and therapy. Nanoscale 2019, 11, 20956–20967. [Google Scholar] [CrossRef]
- Li, S.-D.; Huang, L. Pharmacokinetics and Biodistribution of Nanoparticles. Mol. Pharm. 2008, 5, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Abánades Lázaro, I.; Haddad, S.; Rodrigo-Muñoz, J.M.; Marshall, R.J.; Sastre, B.; del Pozo, V.; Fairen-Jimenez, D.; Forgan, R.S. Surface-Functionalization of Zr-Fumarate MOF for Selective Cytotoxicity and Immune System Compatibility in Nanoscale Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 31146–31157. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Marqués, M.; Bellido, E.; Berthelot, T.; Simón-Yarza, T.; Hidalgo, T.; Simón-Vázquez, R.; González-Fernández, Á.; Avila, J.; Asensio, M.C.; Gref, R.; et al. GraftFast Surface Engineering to Improve MOF Nanoparticles Furtiveness. Small 2018, 14, 1801900. [Google Scholar] [CrossRef] [PubMed]
- Chowdhuri, A.R.; Singh, T.; Ghosh, S.K.; Sahu, S.K. Carbon Dots Embedded Magnetic Nanoparticles @Chitosan @Metal Organic Framework as a Nanoprobe for pH Sensitive Targeted Anticancer Drug Delivery. ACS Appl. Mater. Interfaces 2016, 8, 16573–16583. [Google Scholar] [CrossRef]
- Yang, Q.; Zhu, Y.; Luo, B.; Lan, F.; Wu, Y.; Gu, Z. pH-Responsive magnetic metal–organic framework nanocomposites for selective capture and release of glycoproteins. Nanoscale 2017, 9, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Chen, Z.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. In Nanoscience and Technology; Macmillan Publishers Ltd.: London, UK, 2009; pp. 239–250. [Google Scholar]
- Batool, M.; Tawakkul, N.; Batool, S.; Zafar, M.N.; Nazar, M.F. Chapter 8—Porous metal–organic framework nanoscale carriers as a potential platform for drug delivery. In Novel Platforms for Drug Delivery Applications; Das, S., Thomas, S., Das, P.P., Eds.; Woodhead Publishing: Sawston, UK, 2023; pp. 153–176. [Google Scholar]
- Ding, M.; Liu, W.; Gref, R. Nanoscale MOFs: From synthesis to drug delivery and theranostics applications. Adv. Drug Deliv. Rev. 2022, 190, 114496. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, N.; Atarod, M.; Tavakolizadeh, M.; Asgari, S.; Rezaei, M.; Akhavan, O.; Pourjavadi, A.; Jouyandeh, M.; Lima, E.C.; Hamed Mashhadzadeh, A.; et al. Green metal-organic frameworks (MOFs) for biomedical applications. Microporous Mesoporous Mater. 2022, 335, 111670. [Google Scholar] [CrossRef]
- An, H.; Li, M.; Gao, J.; Zhang, Z.; Ma, S.; Chen, Y. Incorporation of biomolecules in Metal-Organic Frameworks for advanced applications. Coord. Chem. Rev. 2019, 384, 90–106. [Google Scholar] [CrossRef]
- Férey, G.; Serre, C.; Mellot-Draznieks, C.; Millange, F.; Surblé, S.; Dutour, J.; Margiolaki, I. A Hybrid Solid with Giant Pores Prepared by a Combination of Targeted Chemistry, Simulation, and Powder Diffraction. Angew. Chem. Int. Ed. 2004, 43, 6296–6301. [Google Scholar] [CrossRef]
- Férey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surblé, S.; Margiolaki, I. A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area. Science 2005, 309, 2040. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Shi, J. Mesoporous silica nanoparticle based nano drug delivery systems: Synthesis, controlled drug release and delivery, pharmacokinetics and biocompatibility. J. Mater. Chem. 2011, 21, 5845–5855. [Google Scholar] [CrossRef]
- Horcajada, P.; Serre, C.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Metal–Organic Frameworks as Efficient Materials for Drug Delivery. Angew. Chem. Int. Ed. 2006, 45, 5974–5978. [Google Scholar] [CrossRef]
- Horcajada, P.; Serre, C.; Maurin, G.; Ramsahye, N.A.; Balas, F.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Flexible Porous Metal-Organic Frameworks for a Controlled Drug Delivery. J. Am. Chem. Soc. 2008, 130, 6774–6780. [Google Scholar] [CrossRef]
- Rojas, S.; Colinet, I.; Cunha, D.; Hidalgo, T.; Salles, F.; Serre, C.; Guillou, N.; Horcajada, P. Toward Understanding Drug Incorporation and Delivery from Biocompatible Metal–Organic Frameworks in View of Cutaneous Administration. ACS Omega 2018, 3, 2994–3003. [Google Scholar] [CrossRef]
- Zheng, C.; Wang, Y.; Phua, S.Z.F.; Lim, W.Q.; Zhao, Y. ZnO–DOX@ZIF-8 Core–Shell Nanoparticles for pH-Responsive Drug Delivery. ACS Biomater. Sci. Eng. 2017, 3, 2223–2229. [Google Scholar] [CrossRef]
- Wankar, J.; Kotla, N.G.; Gera, S.; Rasala, S.; Pandit, A.; Rochev, Y.A. Recent Advances in Host–Guest Self-Assembled Cyclodextrin Carriers: Implications for Responsive Drug Delivery and Biomedical Engineering. Adv. Funct. Mater. 2020, 30, 1909049. [Google Scholar] [CrossRef]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef]
- Ke, F.; Zhang, M.; Qin, N.; Zhao, G.; Chu, J.; Wan, X. Synergistic antioxidant activity and anticancer effect of green tea catechin stabilized on nanoscale cyclodextrin-based metal–organic frameworks. J. Mater. Sci. 2019, 54, 10420–10429. [Google Scholar] [CrossRef]
- Souza, B.E.; Donà, L.; Titov, K.; Bruzzese, P.; Zeng, Z.; Zhang, Y.; Babal, A.S.; Möslein, A.F.; Frogley, M.D.; Wolna, M.; et al. Elucidating the Drug Release from Metal–Organic Framework Nanocomposites via In Situ Synchrotron Microspectroscopy and Theoretical Modeling. ACS Appl. Mater. Interfaces 2020, 12, 5147–5156. [Google Scholar] [CrossRef]
- National Institute of Biomedical Imaging and Bioengineering. Magnetic Resonance Imaging (MRI). Available online: https://www.nibib.nih.gov/science-education/science-topics/magnetic-resonance-imaging-mri (accessed on 12 December 2022).
- Chowdhury, M.A. Metal-Organic-Frameworks as Contrast Agents in Magnetic Resonance Imaging. ChemBioEng Rev. 2017, 4, 225–239. [Google Scholar] [CrossRef]
- Rieter, W.J.; Taylor, K.M.L.; An, H.; Lin, W.; Lin, W. Nanoscale Metal−Organic Frameworks as Potential Multimodal Contrast Enhancing Agents. J. Am. Chem. Soc. 2006, 128, 9024–9025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinna, A.; Ricco, R.; Migheli, R.; Rocchitta, G.; Serra, P.A.; Falcaro, P.; Malfatti, L.; Innocenzi, P. A MOF-based carrier for in situ dopamine delivery. RSC Adv. 2018, 8, 25664–25672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, R.; Wang, T.; Zhang, L.; Li, L.; Wang, C. A combination of tri-modal cancer imaging and in vivo drug delivery by metal–organic framework based composite nanoparticles. Biomater. Sci. 2015, 3, 1270–1278. [Google Scholar] [CrossRef]
- Cai, W.; Wang, J.; Chu, C.; Chen, W.; Wu, C.; Liu, G. Metal–Organic Framework-Based Stimuli-Responsive Systems for Drug Delivery. Adv. Sci. 2019, 6, 1801526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Vázquez-González, M.; Willner, I. Stimuli-responsive metal–organic framework nanoparticles for controlled drug delivery and medical applications. Chem. Soc. Rev. 2021, 50, 4541–4563. [Google Scholar] [CrossRef]
- Angelos, S.; Khashab, N.M.; Yang, Y.-W.; Trabolsi, A.; Khatib, H.A.; Stoddart, J.F.; Zink, J.I. pH Clock-Operated Mechanized Nanoparticles. J. Am. Chem. Soc. 2009, 131, 12912–12914. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, S.; Wang, L.; Liao, Z.; Zhang, H.; Wei, T.; Dai, Z. Functionalized biological metal–organic framework with nanosized coronal structure and hierarchical wrapping pattern for enhanced targeting therapy. Chem. Eng. J. 2023, 456, 140963. [Google Scholar] [CrossRef]
- Yang, J.; Chen, A.; He, X.; Lu, S. Fabrication of baicalein-encapsulated zeolitic imidazole framework as a novel nanocomposited wound closure material to persuade pH-responsive healing efficacy in post-caesarean section wound care. Int. Wound J. 2022. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gong, Y.; Li, X.; Li, M.; Wei, Q.; Zhou, B.; Zhang, J. Alkaline-Stable Peroxidase Mimics Based on Biological Metal–Organic Frameworks for Recyclable Scavenging of Hydrogen Peroxide and Detecting Glucose in Apple Fruits. ACS Sustain. Chem. Eng. 2022, 10, 10685–10698. [Google Scholar] [CrossRef]
- Liang, Z.; Yang, Z.; Yuan, H.; Wang, C.; Qi, J.; Liu, K.; Cao, R.; Zheng, H. A protein@metal–organic framework nanocomposite for pH-triggered anticancer drug delivery. Dalton Trans. 2018, 47, 10223–10228. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Zhang, L.; An, J.; Wang, T.; Li, L.; Si, X.; He, L.; Wu, X.; Wang, C.; Su, Z. Polyacrylic acid@zeolitic imidazolate framework-8 nanoparticles with ultrahigh drug loading capability for pH-sensitive drug release. Chem. Commun. 2014, 50, 1000–1002. [Google Scholar] [CrossRef]
- Zhuang, J.; Kuo, C.-H.; Chou, L.-Y.; Liu, D.-Y.; Weerapana, E.; Tsung, C.-K. Optimized Metal–Organic-Framework Nanospheres for Drug Delivery: Evaluation of Small-Molecule Encapsulation. ACS Nano 2014, 8, 2812–2819. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, Y.; Liu, L.; Wan, W.; Guo, P.; Nyström, A.M.; Zou, X. One-pot Synthesis of Metal–Organic Frameworks with Encapsulated Target Molecules and Their Applications for Controlled Drug Delivery. J. Am. Chem. Soc. 2016, 138, 962–968. [Google Scholar] [CrossRef]
- Abazari, R.; Ataei, F.; Morsali, A.; Slawin, A.M.Z.; Carpenter-Warren, C.L. A Luminescent Amine-Functionalized Metal–Organic Framework Conjugated with Folic Acid as a Targeted Biocompatible pH-Responsive Nanocarrier for Apoptosis Induction in Breast Cancer Cells. ACS Appl. Mater. Interfaces 2019, 11, 45442–45454. [Google Scholar] [CrossRef]
- Liu, X.; Yan, C.-H.; Capobianco, J.A. Photon upconversion nanomaterials. Chem. Soc. Rev. 2015, 44, 1299–1301. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Shao, Q.; Deng, R.; Wang, C.; Teng, X.; Cheng, K.; Cheng, Z.; Huang, L.; Liu, Z.; Liu, X.; et al. In Vitro and In Vivo Uncaging and Bioluminescence Imaging by Using Photocaged Upconversion Nanoparticles. Angew. Chem. Int. Ed. 2012, 51, 3125–3129. [Google Scholar] [CrossRef]
- Zagami, R.; Rapozzi, V.; Piperno, A.; Scala, A.; Triolo, C.; Trapani, M.; Xodo, L.E.; Monsù Scolaro, L.; Mazzaglia, A. Folate-Decorated Amphiphilic Cyclodextrins as Cell-Targeted Nanophototherapeutics. Biomacromolecules 2019, 20, 2530–2544. [Google Scholar] [CrossRef]
- Menon, D.; Bhatia, D. Biofunctionalized metal–organic frameworks and host–guest interactions for advanced biomedical applications. J. Mater. Chem. B 2022, 10, 7194–7205. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, W.; Wu, Z.-H.; Xu, H. Nanoscale Metal−Organic Frameworks and Their Nanomedicine Applications. Front. Chem. 2022, 9, 834171. [Google Scholar] [CrossRef]
- An, J.; Geib, S.J.; Rosi, N.L. Cation-Triggered Drug Release from a Porous Zinc−Adeninate Metal−Organic Framework. J. Am. Chem. Soc. 2009, 131, 8376–8377. [Google Scholar] [CrossRef]
- Hu, Q.; Yu, J.; Liu, M.; Liu, A.; Dou, Z.; Yang, Y. A Low Cytotoxic Cationic Metal–Organic Framework Carrier for Controllable Drug Release. J. Med. Chem. 2014, 57, 5679–5685. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hu, Q.; Zhang, Q.; Jiang, K.; Lin, W.; Yang, Y.; Cui, Y.; Qian, G. A Large Capacity Cationic Metal–Organic Framework Nanocarrier for Physiological pH Responsive Drug Delivery. Mol. Pharm. 2016, 13, 2782–2786. [Google Scholar] [CrossRef] [PubMed]
- Hergt, R.; Dutz, S.; Müller, R.; Zeisberger, M. Magnetic particle hyperthermia: Nanoparticle magnetism and materials development for cancer therapy. J. Phys. Condens. Matter 2006, 18, S2919–S2934. [Google Scholar] [CrossRef]
- Yusuf, V.F.; Malek, N.I.; Kailasa, S.K. Review on Metal–Organic Framework Classification, Synthetic Approaches, and Influencing Factors: Applications in Energy, Drug Delivery, and Wastewater Treatment. ACS Omega 2022, 7, 44507–44531. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Hu, Y.; Tian, Z.; Zhu, Y. Metal-organic framework-coated magnetite nanoparticles for synergistic magnetic hyperthermia and chemotherapy with pH-triggered drug release. Sci. Technol. Adv. Mater. 2019, 20, 1043–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, P.; Kumar, A.; Bhamidipati, K.; Puvvada, N.; Sahu, S.K. Facile Strategy to Synthesize Magnetic Upconversion Nanoscale Metal–Organic Framework Composites for Theranostics Application. ACS Appl. Bio Mater. 2020, 3, 869–880. [Google Scholar] [CrossRef]
- Jeyaseelan, C.; Jain, P.; Soin, D.; Gupta, D. Metal organic frameworks: An effective application in drug delivery systems. Inorg. Nano-Met. Chem. 2022, 52, 1463–1475. [Google Scholar] [CrossRef]
- Nagata, S.; Kokado, K.; Sada, K. Metal–organic framework tethering pH- and thermo-responsive polymer for ON–OFF controlled release of guest molecules. CrystEngComm 2020, 22, 1106–1111. [Google Scholar] [CrossRef]
- Roy, D.; Brooks, W.L.A.; Sumerlin, B.S. New directions in thermoresponsive polymers. Chem. Soc. Rev. 2013, 42, 7214–7243. [Google Scholar] [CrossRef]
- Lin, W.; Cui, Y.; Yang, Y.; Hu, Q.; Qian, G. A biocompatible metal–organic framework as a pH and temperature dual-responsive drug carrier. Dalton Trans. 2018, 47, 15882–15887. [Google Scholar] [CrossRef]
- Meng, F.; Hennink, W.E.; Zhong, Z. Reduction-sensitive polymers and bioconjugates for biomedical applications. Biomaterials 2009, 30, 2180–2198. [Google Scholar] [CrossRef]
- Li, J.; Huo, M.; Wang, J.; Zhou, J.; Mohammad, J.M.; Zhang, Y.; Zhu, Q.; Waddad, A.Y.; Zhang, Q. Redox-sensitive micelles self-assembled from amphiphilic hyaluronic acid-deoxycholic acid conjugates for targeted intracellular delivery of paclitaxel. Biomaterials 2012, 33, 2310–2320. [Google Scholar] [CrossRef] [PubMed]
- Pourmadadi, M.; Ostovar, S.; Eshaghi, M.M.; Rajabzadeh-Khosroshahi, M.; Safakhah, S.; Ghotekar, S.; Rahdar, A.; Díez-Pascual, A.M. Nanoscale metallic-organic frameworks as an advanced tool for medical applications: Challenges and recent progress. Appl. Organomet. Chem. 2023, 37, e6982. [Google Scholar] [CrossRef]
- Elhassan, E.; Devnarain, N.; Mohammed, M.; Govender, T.; Omolo, C.A. Engineering hybrid nanosystems for efficient and targeted delivery against bacterial infections. J. Control. Release 2022, 351, 598–622. [Google Scholar] [CrossRef] [PubMed]
- Lei, B.; Wang, M.; Jiang, Z.; Qi, W.; Su, R.; He, Z. Constructing Redox-Responsive Metal–Organic Framework Nanocarriers for Anticancer Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 16698–16706. [Google Scholar] [CrossRef]
- Gao, M.; Yi, J.; Zhu, J.; Minikes, A.M.; Monian, P.; Thompson, C.B.; Jiang, X. Role of Mitochondria in Ferroptosis. Mol. Cell 2019, 73, 354–363.e3. [Google Scholar] [CrossRef] [Green Version]
- Gaschler, M.M.; Andia, A.A.; Liu, H.; Csuka, J.M.; Hurlocker, B.; Vaiana, C.A.; Heindel, D.W.; Zuckerman, D.S.; Bos, P.H.; Reznik, E.; et al. FINO2 initiates ferroptosis through GPX4 inactivation and iron oxidation. Nat. Chem. Biol. 2018, 14, 507–515. [Google Scholar] [CrossRef]
- Liu, M.; Liu, B.; Liu, Q.; Du, K.; Wang, Z.; He, N. Nanomaterial-induced ferroptosis for cancer specific therapy. Coord. Chem. Rev. 2019, 382, 160–180. [Google Scholar] [CrossRef]
- Seibt, T.M.; Proneth, B.; Conrad, M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Rad. Biol. Med. 2019, 133, 144–152. [Google Scholar] [CrossRef]
- Hangauer, M.J.; Viswanathan, V.S.; Ryan, M.J.; Bole, D.; Eaton, J.K.; Matov, A.; Galeas, J.; Dhruv, H.D.; Berens, M.E.; Schreiber, S.L.; et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 2017, 551, 247–250. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Du, L.; Guo, H.; An, Y.; Lu, L.; Chen, Y.; Wang, Y.; Zhong, H.; Shen, J.; Wu, J.; et al. Redox Responsive Metal Organic Framework Nanoparticles Induces Ferroptosis for Cancer Therapy. Small 2020, 16, 2001251. [Google Scholar] [CrossRef]
- Moharramnejad, M.; Ehsani, A.; Salmani, S.; Shahi, M.; Malekshah, R.E.; Robatjazi, Z.S.; Parsimehr, H. Zinc-based metal-organic frameworks: Synthesis and recent progress in biomedical application. J. Inorg. Organomet. Polym. Mater. 2022, 32, 3339–3354. [Google Scholar] [CrossRef]
- Cai, X.; Bao, X.; Wu, Y. Metal–Organic Frameworks as Intelligent Drug Nanocarriers for Cancer Therapy. Pharmaceutics 2022, 14, 2641. [Google Scholar] [CrossRef]
- Yang, X.; Tang, Q.; Jiang, Y.; Zhang, M.; Wang, M.; Mao, L. Nanoscale ATP-Responsive Zeolitic Imidazole Framework-90 as a General Platform for Cytosolic Protein Delivery and Genome Editing. J. Am. Chem. Soc. 2019, 141, 3782–3786. [Google Scholar] [CrossRef]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in Photodynamic Therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef]
- Ding, L.; Lin, X.; Lin, Z.; Wu, Y.; Liu, X.; Liu, J.; Wu, M.; Zhang, X.; Zeng, Y. Cancer Cell-Targeted Photosensitizer and Therapeutic Protein Co-Delivery Nanoplatform Based on a Metal–Organic Framework for Enhanced Synergistic Photodynamic and Protein Therapy. ACS Appl. Mater. Interfaces 2020, 12, 36906–36916. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, Z.; Xie, Y.; Lin, J.; Zhang, B.; Fan, J. Engineering Bio-MOF/polydopamine as a biocompatible targeted theranostic system for synergistic multi-drug chemo-photothermal therapy. Int. J. Pharm. 2022, 623, 121912. [Google Scholar] [CrossRef]
- Nguyen, L.H.T.; Thi Dang, Y.; Nguyen, T.T.T.; Le, B.Q.G.; Mai, N.X.D.; Nguyen, H.V.; Le, M.-T.; Phan, T.B.; Doan, T.L.H. Pore engineering of biomolecule-based metal–organic framework nanocarriers for improving loading and release of paclitaxel. New J. Chem. 2022, 46, 6630–6635. [Google Scholar] [CrossRef]
- World Cancer Research Fund International. Breast Cancer Statistics. Available online: https://www.wcrf.org/dietandcancer/breast-cancer-statistics/ (accessed on 3 December 2022).
- Dhull, A.K.; Kaushal, V.; Singh, S.; Sen, R. Tamoxifen-induced endometrial carcinoma after a lag of 14 years. South Asian J. Cancer 2013, 2, 6. [Google Scholar] [CrossRef]
- Alves, R.C.; Perosa Fernandes, R.; Lira de Farias, R.; da Silva, P.B.; Santos Faria, R.; Quijia, C.R.; Galvão Frem, R.C.; Azevedo, R.B.; Chorilli, M. Fabrication of Functional BioMOF-100 Prototype as Drug Delivery System for Breast Cancer Therapy. Pharmaceutics 2022, 14, 2458. [Google Scholar] [CrossRef]
- Javad Farhangi, M.; Es-haghi, A.; Taghavizadeh Yazdi, M.E.; Rahdar, A.; Baino, F. MOF-Mediated Synthesis of CuO/CeO2 Composite Nanoparticles: Characterization and Estimation of the Cellular Toxicity against Breast Cancer Cell Line (MCF-7). J. Funct. Biomater. 2021, 12, 53. [Google Scholar] [CrossRef]
- Kodaira, H.; Kusuhara, H.; Ushiki, J.; Fuse, E.; Sugiyama, Y. Kinetic Analysis of the Cooperation of P-Glycoprotein (P-gp/Abcb1) and Breast Cancer Resistance Protein (Bcrp/Abcg2) in Limiting the Brain and Testis Penetration of Erlotinib, Flavopiridol, and Mitoxantrone. J. Pharmacol. Exp. Ther. 2010, 333, 788. [Google Scholar] [CrossRef] [Green Version]
- Mozzetti, S.; Ferlini, C.; Concolino, P.; Filippetti, F.; Raspaglio, G.; Prislei, S.; Gallo, D.; Martinelli, E.; Ranelletti, F.O.; Ferrandina, G.; et al. Class III beta-tubulin overexpression is a prominent mechanism of paclitaxel resistance in ovarian cancer patients. Clin. Cancer Res. 2005, 11, 298–305. [Google Scholar] [CrossRef]
- Yeh, J.J.; Hsu, W.H.; Wang, J.J.; Ho, S.T.; Kao, A. Predicting Chemotherapy Response to Paclitaxel-Based Therapy in Advanced Non-Small-Cell Lung Cancer with P-Glycoprotein Expression. Respiration 2003, 70, 32–35. [Google Scholar] [CrossRef]
- Lebok, P.; Öztürk, M.; Heilenkötter, U.; Jaenicke, F.; Müller, V.; Paluchowski, P.; Geist, S.; Wilke, C.; Burandt, E.; Lebeau, A.; et al. High levels of class III β-tubulin expression are associated with aggressive tumor features in breast cancer. Oncol. Lett. 2016, 11, 1987–1994. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Yu, X.; Wang, Y.; Yuan, Y.; Xiao, H.; Cheng, D.; Shuai, X. A Reduction and pH Dual-Sensitive Polymeric Vector for Long-Circulating and Tumor-Targeted siRNA Delivery. Adv. Mater. 2014, 26, 8217–8224. [Google Scholar] [CrossRef]
- Zheng, W.; Cao, C.; Liu, Y.; Yu, Q.; Zheng, C.; Sun, D.; Ren, X.; Liu, J. Multifunctional polyamidoamine-modified selenium nanoparticles dual-delivering siRNA and cisplatin to A549/DDP cells for reversal multidrug resistance. Acta Biomater. 2015, 11, 368–380. [Google Scholar] [CrossRef]
- Sinha, R.; El-Bayoumy, K. Apoptosis is a Critical Cellular Event in Cancer Chemoprevention and Chemotherapy by Selenium Compounds. Curr. Cancer Drug Targets 2004, 4, 13–28. [Google Scholar] [CrossRef]
- Abdulah, R.; Miyazaki, K.; Nakazawa, M.; Koyama, H. Chemical forms of selenium for cancer prevention. J. Trace Elem. Med. Biol. 2005, 19, 141–150. [Google Scholar] [CrossRef]
- Hatfield, D.L.; Tsuji, P.A.; Carlson, B.A.; Gladyshev, V.N. Selenium and selenocysteine: Roles in cancer, health, and development. Trends Biochem. Sci. 2014, 39, 112–120. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhang, J.; Yu, H. Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: Comparison with selenomethionine in mice. Free Rad. Biol. Med. 2007, 42, 1524–1533. [Google Scholar] [CrossRef]
- Levina, A.; Mitra, A.; Lay, P.A. Recent developments in ruthenium anticancer drugs. Metallomics 2009, 1, 458–470. [Google Scholar] [CrossRef]
- Bergamo, A.; Gaiddon, C.; Schellens, J.H.M.; Beijnen, J.H.; Sava, G. Approaching tumour therapy beyond platinum drugs: Status of the art and perspectives of ruthenium drug candidates. J. Inorg. Biochem. 2012, 106, 90–99. [Google Scholar] [CrossRef]
- Bonnet, S.; Archer, S.L.; Allalunis-Turner, J.; Haromy, A.; Beaulieu, C.; Thompson, R.; Lee, C.T.; Lopaschuk, G.D.; Puttagunta, L.; Bonnet, S.; et al. A Mitochondria-K+ Channel Axis Is Suppressed in Cancer and Its Normalization Promotes Apoptosis and Inhibits Cancer Growth. Cancer Cell 2007, 11, 37–51. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.; Lin, J.; Wang, S.; Zhou, Z.; Li, Z.; Wang, Z.; Zhang, C.; Yue, X.; Niu, G.; Yang, M.; et al. Photosensitizer-conjugated silica-coated gold nanoclusters for fluorescence imaging-guided photodynamic therapy. Biomaterials 2013, 34, 4643–4654. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.; Li, Z.; Lin, J.; Yang, D.; Gao, G.; Xu, C.; Bao, L.; Zhang, C.; Wang, K.; Song, H.; et al. Photosensitizer-conjugated magnetic nanoparticles for in vivo simultaneous magnetofluorescent imaging and targeting therapy. Biomaterials 2011, 32, 3447–3458. [Google Scholar] [CrossRef]
- Huang, P.; Lin, J.; Wang, X.; Wang, Z.; Zhang, C.; He, M.; Wang, K.; Chen, F.; Li, Z.; Shen, G.; et al. Light-Triggered Theranostics Based on Photosensitizer-Conjugated Carbon Dots for Simultaneous Enhanced-Fluorescence Imaging and Photodynamic Therapy. Adv. Mater. 2012, 24, 5104–5110. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.; Wang, S.; Zheng, R.; Zhu, X.; Jiang, X.; Fu, D.; Yang, W. Magnetic nanoparticle clusters for photothermal therapy with near-infrared irradiation. Biomaterials 2015, 39, 67–74. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Zhao, J.; Liu, X.; Bu, J.; Yan, X.; Huang, R. Multifunctional Mesoporous Silica-Coated Graphene Nanosheet Used for Chemo-Photothermal Synergistic Targeted Therapy of Glioma. J. Am. Chem. Soc. 2013, 135, 4799–4804. [Google Scholar] [CrossRef]
- Biswal, B.P.; Shinde, D.B.; Pillai, V.K.; Banerjee, R. Stabilization of graphene quantum dots (GQDs) by encapsulation inside zeolitic imidazolate framework nanocrystals for photoluminescence tuning. Nanoscale 2013, 5, 10556–10561. [Google Scholar] [CrossRef]
- Yang, K.; Feng, L.; Liu, Z. The advancing uses of nano-graphene in drug delivery. Expert Opin. Drug Deliv. 2015, 12, 601–612. [Google Scholar] [CrossRef]
- World Health Organization. Cancer; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global Epidemiology of Lung Cancer. Ann. Glob. Health 2019, 85, 8. [Google Scholar] [CrossRef] [Green Version]
- Barash, O.; Peled, N.; Hirsch, F.R.; Haick, H. Sniffing the Unique “Odor Print” of Non-Small-Cell Lung Cancer with Gold Nanoparticles. Small 2009, 5, 2618–2624. [Google Scholar] [CrossRef]
- Raju, P.; Balakrishnan, K.; Mishra, M.; Ramasamy, T.; Natarajan, S. Fabrication of pH responsive FU@Eu-MOF nanoscale metal organic frameworks for lung cancer therapy. J. Drug Deliv. Sci. Technol. 2022, 70, 103223. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, H.; Yan, B. Determination of urinary N-acetylneuraminic acid for early diagnosis of lung cancer by a boric acid covalently functionalized lanthanide MOFs and its intelligent visual molecular robot application. Sens. Actuators B Chem. 2021, 349, 130736. [Google Scholar] [CrossRef]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin. 2012, 62, 10–29. [Google Scholar] [CrossRef] [Green Version]
- Rawla, P.; Sunkara, T.; Muralidharan, P.; Raj, J.P. Update in global trends and aetiology of hepatocellular carcinoma. Contemp. Oncol. 2018, 22, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Juaid, N.; Amin, A.; Abdalla, A.; Reese, K.; Alamri, Z.; Moulay, M.; Abdu, S.; Miled, N. Anti-Hepatocellular Carcinoma Biomolecules: Molecular Targets Insights. Int. J. Mol. Sci. 2021, 22, 10774. [Google Scholar] [CrossRef]
- Chong, D.Q.; Tan, I.B.; Choo, S.-P.; Toh, H.C. The evolving landscape of therapeutic drug development for hepatocellular carcinoma. Contemp. Clin. Trials 2013, 36, 605–615. [Google Scholar] [CrossRef]
- Warsame, R.; Grothey, A. Treatment options for advanced pancreatic cancer: A review. Expert Rev. Anticancer Ther. 2012, 12, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Keating, G.M.; Santoro, A. Sorafenib. Drugs 2009, 69, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.M.; Adnane, L.; Newell, P.; Villanueva, A.; Llovet, J.M.; Lynch, M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol. Cancer Ther. 2008, 7, 3129. [Google Scholar] [CrossRef] [Green Version]
- Urtasun, N.; Vidal-Pla, A.; Pérez-Torras, S.; Mazo, A. Human pancreatic cancer stem cells are sensitive to dual inhibition of IGF-IR and ErbB receptors. BMC Cancer 2015, 15, 223. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Zhang, W.; Wu, Q.; Fu, C.; Ren, X.; Lv, K.; Ma, T.; Chen, X.; Tan, L.; Meng, X. Lanthanide europium MOF nanocomposite as the theranostic nanoplatform for microwave thermo-chemotherapy and fluorescence imaging. J. Nanobiotechnol. 2022, 20, 133. [Google Scholar] [CrossRef]
- Li, J.; Lee, S.H.; Yoo, D.K.; Woo, H.C.; Jhung, S.H.; Jović, M.; Girault, H.; Lee, H.J. A spatially multiplexed voltammetric magneto-sandwich assay involving Fe3O4/Fe-based metal-organic framework for dual liver cancer biomarkers. Sens. Actuators B Chem. 2023, 380, 133313. [Google Scholar] [CrossRef]
- Speciale, A.; Muscarà, C.; Molonia, M.S.; Cristani, M.; Cimino, F.; Saija, A. Recent Advances in Glycyrrhetinic Acid-Functionalized Biomaterials for Liver Cancer-Targeting Therapy. Molecules 2022, 27, 1775. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, X.; Liu, G.; Guo, H.; Li, C.; Zhang, Y.; Zhang, F.; Zhao, Z.; Cheng, H. Novel galactosylated biodegradable nanoparticles for hepatocyte-delivery of oridonin. Int. J. Pharm. 2016, 502, 47–60. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Hu, B. The anti-tumor effect of folate-targeted liposome microbubbles loaded with oridonin as ultrasound-triggered tumor-targeted therapeutic carrier system. J. Drug Target 2017, 25, 83–91. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, T.; Sheng, R.; Li, H.; Sun, J.; Cao, A. Amphiphilic Diblock Terpolymer PMAgala-b-P(MAA-co-MAChol)s with Attached Galactose and Cholesterol Grafts and Their Intracellular pH-Responsive Doxorubicin Delivery. Biomacromolecules 2016, 17, 98–110. [Google Scholar] [CrossRef]
- Li, S.; Chen, Z.; Tan, L.; Wu, Q.; Ren, X.; Fu, C.; Niu, M.; Li, H.; Meng, X. MOF@COF nanocapsule for the enhanced microwave thermal-dynamic therapy and anti-angiogenesis of colorectal cancer. Biomaterials 2022, 283, 121472. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Lan, Q.; Li, C.; Xia, X.-H. Morphologically Flex Sm-MOF Based Electrochemical Immunosensor for Ultrasensitive Detection of a Colon Cancer Biomarker. Anal. Chem. 2022, 94, 3013–3019. [Google Scholar] [CrossRef]
- Khatibi, Z.; Kazemi, N.M.; Khaleghi, S. Targeted and biocompatible NMOF as efficient nanocomposite for delivery of methotrexate to colon cancer cells. J. Drug Deliv. Sci. Technol. 2022, 73, 103441. [Google Scholar] [CrossRef]
- Al-Sagur, H.; Sundaram, K.S.; Kaya, E.N.; Durmuş, M.; Basova, T.V.; Hassan, A. Amperometric glucose biosensing performance of a novel graphene nanoplatelets-iron phthalocyanine incorporated conducting hydrogel. Biosens. Bioelectron. 2019, 139, 111323. [Google Scholar] [CrossRef]
- Liang, J.; Xie, Y.-Q.; Wang, X.-S.; Wang, Q.; Liu, T.-T.; Huang, Y.-B.; Cao, R. An imidazolium-functionalized mesoporous cationic metal–organic framework for cooperative CO2 fixation into cyclic carbonate. Chem. Commun. 2018, 54, 342–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, S.J.; Karapetis, C.S.; Gibbs, P.; Pavlakis, N.; Desai, J.; Michael, M.; Tebbutt, N.C.; Price, T.J.; Tabernero, J. Overview of biomarkers in metastatic colorectal cancer: Tumour, blood and patient-related factors. Crit. Rev. Oncol. Hematol. 2013, 85, 121–135. [Google Scholar] [CrossRef]
- Szabo, C.; Coletta, C.; Chao, C.; Módis, K.; Szczesny, B.; Papapetropoulos, A.; Hellmich, M.R. Tumor-derived hydrogen sulfide, produced by cystathionine-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 12474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, H.; Zhou, Q.; Zhang, L.; Tian, Y. Rational Design of Specific Recognition Molecules for Simultaneously Monitoring of Endogenous Polysulfide and Hydrogen Sulfide in the Mouse Brain. Angew. Chem. Int. Ed. 2019, 58, 13948–13953. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, Q.; Zhai, X.; Mao, F.; Jiang, A.; Zhou, J. Rationally designed pure-inorganic upconversion nanoprobes for ultra-highly selective hydrogen sulfide imaging and elimination in vivo. Chem. Sci. 2019, 10, 1193–1200. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Sun, Y.; Sugimoto, K.; Luo, Z.; Ishigaki, Y.; Pu, K.; Suzuki, T.; Chen, H.-Y.; Ye, D. Engineering of Electrochromic Materials as Activatable Probes for Molecular Imaging and Photodynamic Therapy. J. Am. Chem. Soc. 2018, 140, 16340–16352. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ni, D.; Rosenkrans, Z.T.; Cao, T.; Cai, W. Smart H2S-Triggered/Therapeutic System (SHTS)-Based Nanomedicine. Adv. Sci. 2019, 6, 1901724. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Yu, G.; Wang, Z.; Jacobson, O.; Lin, L.-S.; Yang, W.; Deng, H.; He, Z.; Liu, Y.; Chen, Z.-Y.; et al. Enhanced Antitumor Efficacy by a Cascade of Reactive Oxygen Species Generation and Drug Release. Angew. Chem. Int. Ed. 2019, 58, 14758–14763. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, X.; Lei, H.; Geng, Y.; Zhao, Q.; Gong, F.; Yang, Z.; Dong, Z.; Liu, Z.; Cheng, L. Hollow Cu2Se Nanozymes for Tumor Photothermal-Catalytic Therapy. Chem. Mater. 2019, 31, 6174–6186. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef]
- Liu, J.; Yang, L.; Cao, X.; Chen, M.; Li, J.; Wang, X.; Wu, S.; Zhang, Z. PEGylated Mn containing MOF nanoparticles for potential immunotherapy of pancreatic cancer via manganese induced activation of anti-tumor immunity. Colloid Interface Sci. Commun. 2021, 42, 100409. [Google Scholar] [CrossRef]
- Sakamaki, Y.; Ozdemir, J.; Heidrick, Z.; Azzun, A.; Watson, O.; Tsuji, M.; Salmon, C.; Sinha, A.; Batta-Mpouma, J.; McConnell, Z.; et al. A Bioconjugated Chlorin-Based Metal–Organic Framework for Targeted Photodynamic Therapy of Triple Negative Breast and Pancreatic Cancers. ACS Appl. Bio Mater. 2021, 4, 1432–1440. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Ferrone, C.R.; Ferrone, S.; Nasr, M.L. Engineered nanomedicines to overcome resistance of pancreatic cancer to immunotherapy. Drug Discov. Today 2023, 28, 103434. [Google Scholar] [CrossRef]
- Kawamori, T.; Nakatsugi, S.; Ohta, T.; Sugimura, T.; Wakabayashi, K. Chemopreventive effects of nimesulide, a selective cyclooxygenase-2 inhibitor, against PhIP-induced mammary carcinogenesis. Adv. Exp. Med. Biol. 2002, 507, 371–376. [Google Scholar] [PubMed]
- Nakatsugi, S.; Ohta, T.; Kawamori, T.; Mutoh, M.; Tanigawa, T.; Watanabe, K.; Sugie, S.; Sugimura, T.; Wakabayashi, K. Chemoprevention by nimesulide, a selective cyclooxygenase-2 inhibitor, of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-induced mammary gland carcinogenesis in rats. Jpn. J. Cancer Res. 2000, 91, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, F.; Nishikawa, A.; Lee, I.S.; Kanki, K.; Umemura, T.; Okazaki, K.; Kawamori, T.; Wakabayashi, K.; Hirose, M. A cyclooxygenase-2 inhibitor, nimesulide, inhibits postinitiation phase of N-nitrosobis(2-oxopropyl)amine-induced pancreatic carcinogenesis in hamsters. Int. J. Cancer 2003, 104, 269–273. [Google Scholar] [CrossRef]
- Clinic, M. Bladder Cancer. Available online: https://www.mayoclinic.org/diseases-conditions/bladder-cancer/symptoms-causes/syc-20356104 (accessed on 14 December 2022).
- Massoumi, R. CYLD: A deubiquitination enzyme with multiple roles in cancer. Future Oncol. 2011, 7, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sun, Y.; Zhang, Y.; Han, S. Downregulation of miR-181b inhibits human colon cancer cell proliferation by targeting CYLD and inhibiting the NF-κB signaling pathway. Int. J. Mol. Med. 2020, 46, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, Y.; Wei, T.; Zhao, X.; Li, F.; Li, Y.; Wang, F.; Cai, Y.; Jin, J. KAT8/MOF-Mediated Anti-Cancer Mechanism of Gemcitabine in Human Bladder Cancer Cells. Biomol. Ther. 2021, 29, 184–194. [Google Scholar] [CrossRef]
- Li, F.; Xu, M.; Zhuang, J. Dual biomineralized metal−organic frameworks-mediated conversion of chemical energy to electricity enabling portable PEC sensing of telomerase activity in bladder cancer tissues. Biosens. Bioelectron. 2022, 204, 114070. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Monk, B.J.; Sood, A.K.; Herzog, T.J. Latest research and treatment of advanced-stage epithelial ovarian cancer. Nat. Rev. Clin. Oncol. 2013, 10, 211–224. [Google Scholar] [CrossRef] [Green Version]
- World Cancer Research Fund International. Ovarian Cancer. Available online: https://www.wcrf.org/dietandcancer/ovarian-cancer/ (accessed on 14 December 2022).
- Vaughan, S.; Coward, J.I.; Bast, R.C.; Berchuck, A.; Berek, J.S.; Brenton, J.D.; Coukos, G.; Crum, C.C.; Drapkin, R.; Etemadmoghadam, D.; et al. Rethinking ovarian cancer: Recommendations for improving outcomes. Nat. Rev. Cancer 2011, 11, 719–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koshiyama, M.; Matsumura, N.; Konishi, I. Subtypes of Ovarian Cancer and Ovarian Cancer Screening. Diagnostics 2017, 7, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathieu, K.B.; Bedi, D.G.; Thrower, S.L.; Qayyum, A.; Bast, R.C., Jr. Screening for ovarian cancer: Imaging challenges and opportunities for improvement. Ultrasound Obstet. Gynecol. 2018, 51, 293–303. [Google Scholar] [CrossRef] [Green Version]
- Wright, A.A.; Bohlke, K.; Armstrong, D.K.; Bookman, M.A.; Cliby, W.A.; Coleman, R.L.; Dizon, D.S.; Kash, J.J.; Meyer, L.A.; Moore, K.N.; et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. Gynecol. Oncol. 2016, 143, 3–15. [Google Scholar] [CrossRef] [Green Version]
- van Dam, G.M.; Themelis, G.; Crane, L.M.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W.; Sarantopoulos, A.; de Jong, J.S.; Arts, H.J.; van der Zee, A.G.; et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: First in-human results. Nat. Med. 2011, 17, 1315–1319. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Yellepeddi, V.K.; Vangara, K.K.; Kumar, A.; Palakurthi, S. Comparative Evaluation of Small-molecule Chemosensitizers in Reversal of Cisplatin Resistance in Ovarian Cancer Cells. Anticancer Res. 2012, 32, 3651–3658. [Google Scholar] [PubMed]
- Xiong, X.-B.; Lavasanifar, A. Traceable Multifunctional Micellar Nanocarriers for Cancer-Targeted Co-delivery of MDR-1 siRNA and Doxorubicin. ACS Nano 2011, 5, 5202–5213. [Google Scholar] [CrossRef]
- Meng, H.; Liong, M.; Xia, T.; Li, Z.; Ji, Z.; Zink, J.I.; Nel, A.E. Engineered Design of Mesoporous Silica Nanoparticles to Deliver Doxorubicin and P-Glycoprotein siRNA to Overcome Drug Resistance in a Cancer Cell Line. ACS Nano 2010, 4, 4539–4550. [Google Scholar] [CrossRef]
- Li, S.; Hu, C.; Chen, C.; Zhang, J.; Bai, Y.; Tan, C.S.; Ni, G.; He, F.; Li, W.; Ming, D. Molybdenum Disulfide Supported on Metal–Organic Frameworks as an Ultrasensitive Layer for the Electrochemical Detection of the Ovarian Cancer Biomarker CA125. ACS Appl. Bio Mater. 2021, 4, 5494–5502. [Google Scholar] [CrossRef]
- Vandghanooni, S.; Sanaat, Z.; Barar, J.; Adibkia, K.; Eskandani, M.; Omidi, Y. Recent advances in aptamer-based nanosystems and microfluidics devices for the detection of ovarian cancer biomarkers. TrAC Trends Anal. Chem. 2021, 143, 116343. [Google Scholar] [CrossRef]
- Pfeilschifter, W.; Czech, B.; Hoffmann, B.P.; Sujak, M.; Kahles, T.; Steinmetz, H.; Neumann-Haefelin, T.; Pfeilschifter, J. Pyrrolidine Dithiocarbamate Activates p38 MAPK and Protects Brain Endothelial Cells From Apoptosis: A Mechanism for the Protective Effect in Stroke? Neurochem. Res. 2010, 35, 1391–1401. [Google Scholar] [CrossRef]
- Keter, F.K.; Guzei, I.A.; Nell, M.; van Zyl, W.E.; Darkwa, J. Phosphinogold(I) Dithiocarbamate Complexes: Effect of the Nature of Phosphine Ligand on Anticancer Properties. Inorg. Chem. 2014, 53, 2058–2067. [Google Scholar] [CrossRef]
- Mills, G.B.; Moolenaar, W.H. The emerging role of lysophosphatidic acid in cancer. Nat. Rev. Cancer 2003, 3, 582–591. [Google Scholar] [CrossRef]
- Xu, Y.; Shen, Z.; Wiper, D.W.; Wu, M.; Morton, R.E.; Elson, P.; Kennedy, A.W.; Belinson, J.; Markman, M.; Casey, G. Lysophosphatidic Acid as a Potential Biomarker for Ovarian and Other Gynecologic Cancers. JAMA 1998, 280, 719–723. [Google Scholar] [CrossRef] [Green Version]
- Umezu-Goto, M.; Tanyi, J.; Lahad, J.; Liu, S.; Yu, S.; Lapushin, R.; Hasegawa, Y.; Lu, Y.; Trost, R.; Bevers, T.; et al. Lysophosphatidic acid production and action: Validated targets in cancer? J. Cell. Biochem. 2004, 92, 1115–1140. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Xiao, Y.-j.; Singh, L.S.; Zhao, X.; Zhao, Z.; Feng, L.; Rose, T.M.; Prestwich, G.D.; Xu, Y. Lysophosphatidic Acid Is Constitutively Produced by Human Peritoneal Mesothelial Cells and Enhances Adhesion, Migration, and Invasion of Ovarian Cancer Cells. Cancer Res. 2006, 66, 3006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutphen, R.; Xu, Y.; Wilbanks, G.D.; Fiorica, J.; Grendys, E.C.; LaPolla, J.P.; Arango, H.; Hoffman, M.S.; Martino, M.; Wakeley, K.; et al. Lysophospholipids Are Potential Biomarkers of Ovarian Cancer. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1185. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, B.; Qian, G. Lanthanide metal-organic frameworks for luminescent sensing and light-emitting applications. Coord. Chem. Rev. 2014, 273–274, 76–86. [Google Scholar] [CrossRef]
- Dahiya, N.; Sherman-Baust, C.A.; Wang, T.-L.; Davidson, B.; Shih, I.-M.; Zhang, Y.; Wood, W., III; Becker, K.G.; Morin, P.J. MicroRNA Expression and Identification of Putative miRNA Targets in Ovarian Cancer. PLoS ONE 2008, 3, e2436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Cervical Cancer. Available online: https://www.who.int/health-topics/cervical-cancer#tab=tab_1 (accessed on 10 October 2022).
- Cai, X.; Zhao, Y.; Wang, L.; Hu, M.; Wu, Z.; Liu, L.; Zhu, W.; Pei, R. Synthesis of Au@MOF core–shell hybrids for enhanced photodynamic/photothermal therapy. J. Mater. Chem. B 2021, 9, 6646–6657. [Google Scholar] [CrossRef]
- Rao, D.; Wang, B.; Zhong, H.; Yan, Y.; Ding, C.-F. Construction of boric acid-functionalized metal–organic frameworks for glycopeptide recognition in the serum of cervical cancer patients. Rapid Commun. Mass Spectrom. 2022, 36, e9314. [Google Scholar] [CrossRef]
- Darroudi, M.; Nazari, S.E.; Asgharzadeh, F.; Khalili-Tanha, N.; Khalili-Tanha, G.; Dehghani, T.; Karimzadeh, M.; Maftooh, M.; Fern, G.A.; Avan, A.; et al. Fabrication and application of cisplatin-loaded mesoporous magnetic nanobiocomposite: A novel approach to smart cervical cancer chemotherapy. Cancer Nanotechnol. 2022, 13, 36. [Google Scholar] [CrossRef]
- Fan, W.; Yung, B.; Huang, P.; Chen, X. Nanotechnology for Multimodal Synergistic Cancer Therapy. Chem. Rev. 2017, 117, 13566–13638. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Yan, H.; Dong, F.; Zhang, L.; Du, N.; Sun, L.; Li, N.; Yu, G.; Yang, Z.; Wang, Y.; et al. Tumor-targeted biomimetic nanoplatform precisely integrates photodynamic therapy and autophagy inhibition for collaborative treatment of oral cancer. Biomater. Sci. 2022, 10, 1456–1469. [Google Scholar] [CrossRef]
- Bai, Y.-T.; Zhang, X.-Q.; Chen, X.-J.; Zhou, G. Nanomedicines in oral cancer: Inspiration comes from extracellular vesicles and biomimetic nanoparticles. Nanomedicine 2022, 17, 1761–1778. [Google Scholar] [CrossRef] [PubMed]
- Meyers, J.D.; Cheng, Y.; Broome, A.-M.; Agnes, R.S.; Schluchter, M.D.; Margevicius, S.; Wang, X.; Kenney, M.E.; Burda, C.; Basilion, J.P. Peptide-Targeted Gold Nanoparticles for Photodynamic Therapy of Brain Cancer. Part. Part. Syst. Charact. 2015, 32, 448–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, A.; Shukla, A.; Maiti, P. Biomaterials for Interfacing Cell Imaging and Drug Delivery: An Overview. Langmuir 2019, 35, 12285–12305. [Google Scholar] [CrossRef]
- Bazi Alahri, M.; Arshadizadeh, R.; Raeisi, M.; Khatami, M.; Sadat Sajadi, M.; Kamal Abdelbasset, W.; Akhmadeev, R.; Iravani, S. Theranostic applications of metal–organic frameworks (MOFs)-based materials in brain disorders: Recent advances and challenges. Inorg. Chem. Commun. 2021, 134, 108997. [Google Scholar] [CrossRef]
- Ebrahimpour, A.; Riahi Alam, N.; Abdolmaleki, P.; Hajipour-Verdom, B.; Tirgar, F.; Ebrahimi, T.; Khoobi, M. Magnetic Metal–Organic Framework Based on Zinc and 5-Aminolevulinic Acid: MR Imaging and Brain Tumor Therapy. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1208–1216. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, A.; Li, C.; Panwar Hazari, P.; Mahajan, S.D.; Aalinkeel, R.; Sharma, R.K.; Swihart, M.T. A cannabidiol-loaded Mg-gallate metal–organic framework-based potential therapeutic for glioblastomas. J. Mater. Chem. B 2021, 9, 2505–2514. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Zhu, C.; Su, S.; Li, D.; He, Y.; Huang, Q.; Fan, C. Self-Catalyzed, Self-Limiting Growth of Glucose Oxidase-Mimicking Gold Nanoparticles. ACS Nano 2010, 4, 7451–7458. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Fei, J.; Li, G.; Yuan, T.; Xu, X.; Li, J. Nanozyme-Catalyzed Cascade Reactions for Mitochondria-Mimicking Oxidative Phosphorylation. Angew. Chem. Int. Ed. 2019, 58, 5572–5576. [Google Scholar] [CrossRef]
- Stuehr, D.J. Enzymes of the L-Arginine to Nitric Oxide Pathway. J. Nutr. 2004, 134, 2748S–2751S. [Google Scholar] [CrossRef] [Green Version]
- Fan, W.; Lu, N.; Huang, P.; Liu, Y.; Yang, Z.; Wang, S.; Yu, G.; Liu, Y.; Hu, J.; He, Q.; et al. Glucose-Responsive Sequential Generation of Hydrogen Peroxide and Nitric Oxide for Synergistic Cancer Starving-Like/Gas Therapy. Angew. Chem. Int. Ed. 2017, 56, 1229–1233. [Google Scholar] [CrossRef]
- Keshet, R.; Erez, A. Arginine and the metabolic regulation of nitric oxide synthesis in cancer. Dis. Model. Mech. 2018, 11, dmm033332. [Google Scholar] [CrossRef] [Green Version]
- Fan, W.; Yung, B.C.; Chen, X. Stimuli-Responsive NO Release for On-Demand Gas-Sensitized Synergistic Cancer Therapy. Angew. Chem. Int. Ed. 2018, 57, 8383–8394. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, C.; Zhao, H.; Ren, X.; Peng, S.; Zuo, Z. Autoregulation of inducible nitric oxide synthase expression by RNA interference provides neuroprotection in neonatal rats. Theranostics 2015, 5, 504–514. [Google Scholar] [CrossRef] [Green Version]
- Chandrawati, R.; Chang, J.Y.H.; Reina-Torres, E.; Jumeaux, C.; Sherwood, J.M.; Stamer, W.D.; Zelikin, A.N.; Overby, D.R.; Stevens, M.M. Localized and Controlled Delivery of Nitric Oxide to the Conventional Outflow Pathway via Enzyme Biocatalysis: Toward Therapy for Glaucoma. Adv. Mater. 2017, 29, 1604932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clinic, M. Leukemia. Available online: https://www.mayoclinic.org/diseases-conditions/leukemia/symptoms-causes/syc-20374373 (accessed on 14 December 2022).
- Reynolds, J.G.; Geretti, E.; Hendriks, B.S.; Lee, H.; Leonard, S.C.; Klinz, S.G.; Noble, C.O.; Lücker, P.B.; Zandstra, P.W.; Drummond, D.C.; et al. HER2-targeted liposomal doxorubicin displays enhanced anti-tumorigenic effects without associated cardiotoxicity. Toxicol. Appl. Pharmacol. 2012, 262, 1–10. [Google Scholar] [CrossRef]
- Elsabahy, M.; Wooley, K.L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012, 41, 2545–2561. [Google Scholar] [CrossRef] [Green Version]
- Draz, M.S.; Fang, B.A.; Zhang, P.; Hu, Z.; Gu, S.; Weng, K.C.; Gray, J.W.; Chen, F.F. Nanoparticle-mediated systemic delivery of siRNA for treatment of cancers and viral infections. Theranostics 2014, 4, 872–892. [Google Scholar] [CrossRef] [PubMed]
- Williford, J.-M.; Wu, J.; Ren, Y.; Archang, M.M.; Leong, K.W.; Mao, H.-Q. Recent Advances in Nanoparticle-Mediated siRNA Delivery. Annu. Rev. Biomed. Eng. 2014, 16, 347–370. [Google Scholar] [CrossRef] [PubMed]














| MOF | Cancer | Targeting Material/Drug | Cell Lines | Activity | Ref |
|---|---|---|---|---|---|
| MIL-101-NH2(Fe) | Breast | single gold nanostar (AuNS), targeted peptide (ZD2) | MDA-MB-231 | MRI, photothermal therapy | [111] (Zhang) |
| IRMOF-3 | Breast | curcumin (CCM), folic acid (FA) | MDA-MB-468, 4T1 | ROS-mediated DNA, mitochondrial DNA damage | [112] (Laha) |
| MIL-101(Fe) | Breast | selenium/ruthenium nanoparticles, siRNAs | MCF-7/T | instability of MTs and disruption of mitotic spindle formation | [113] (Chen) |
| UiO-66 | Breast | triphenylphosphonium (TPP), dichloroacetate (DCA) | MCF-7 | mitochondria-targeted | [126] (Haddad) |
| Fe3O4@UiO-66-NH2 | Breast | Fe3O4, DOX, highly fluorescent carbon dots (CDs), nucleolin-binding aptamer, AS1411 | MDA-MB-231 | cellular bioimaging and chemotherapy | [200] (Alijani) |
| ZIF-8 | Breast | AuNCs, DOX | - | photothermal therapy, chemotherapy | [131] (Zhang) |
| ZIF-8 | Breast | graphene quantum dots (GQDs) | 4T1 | Photothermal therapy, chemotherapy | [133] (Tian) |
| CS/Zn-MOF@GO | Breast | chitosan (CS), graphene oxide (GO), 5-Fu | MDA-MB 231 | chemotherapy, pH sensitive | [193] (pooresmail) |
| Zr-Fc MOF Nanosheet | Breast | ferrocene-based MOF (Zr-Fc MOF) nanosheet | 4T1 | Photothermal therapy and chemodynamic therapy | [210] (Deng) |
| MD@Lip | Breast | dichloroacetic acid, (DCA), Fe(II) MOF | MDA-MB-231 | ROS chemotherapy | |
| Zr-based porphyrinic MOF | Breast, Lung | porphyrin, DOX, indocyanine green (ICG) | 4T1, A549, U87MG | photothermal therapy, chemotherapy | [191] (Sun) |
| ZIF-67 | Breast | phosphorus nanosheets (BPNSs), ferrocene (Fc), indium tin oxide (ITO) slice, methylene blue (MB)-labeled single- strand DNA aptamer | MCF-7 | aptasensor for detecting specific cancer cell-derived exosomes | [192] (Sun) |
| porphyrin Pd-MOF | Breast | Pd, porphyrin | 4T1, HeLa, 4T1 tumor-bearing mice | hydrogenothermal chemotherapy, photoacoustic imaging | [190] (Zhou) |
| porphyrin MOF | Breast | porphyrin-like single atom Fe(III) centers, DOX | MCF-7 | photodynamic therapy, photothermal therapy, photoacoustic imaging | [188] (Wang) |
| HKUST-1 | Breast | Cu2+, Vk3 | 4T1 | chemodynamic therapy | [189] (Tian) |
| Cu-MOF/Ce6 | Breast | Cu2+, chlorin e6 (Ce6) | MCF-7 | chemodynamic therapy, sonodynamic therapy | [207] (Zhang) |
| N3-bio-MOF-100 | Breast | CCM, FA | 4T1 | chemotherapy, pH sensitive | [202] (Alves) |
| Tb-MOF-on-Fe-MOF | Breast | Bimetallic FeTb-MOFs | MCF-7 | aptasensor for detecting CA125 | [184] (Wang) |
| TA-Fe/ART@ZIF-8 | Breast | artemisinin (ART), tannic acid (TA), Fe(II) | MDA-MB-231, MDA-MB-231 xenograft tumors | chemotherapy | [206] (Li) |
| HA-PCN | Breast | PCN-224, HA, DOX | MCF-7/MDR | photodynamic therapy, chemotherapy | [208] |
| La(III)-MOF | Breast, Lung | La(III)-MOF (fluorophore) Ag NPs (quencher), 5′-amino-labeled ssDNA strands (aptamers) | miRNA-155 (biomarker) | photoluminescence Quenching-Based Detection of miRNA-155 | [195] (Afzalinia) |
| Fe-MOF@PEM | Breast, Lung | polyelectrolyte multilayer (PEM), DOX | MCF-7, A549 | ROS, chemotherapy | [197] (Wang) |
| Gd-MOF | Lung | 5-Fu | A549 | chemotherapy | [198] (Wei) |
| IRMOF-3 | Oral | thermosensitive hydrogel, DOX, celecoxib (Cel) | KB, SCC-9 | Chemotherapy (pH responsive | [185] (Tan) |
| Fe3O4@C nanocomposite | Oral | Fe3O4, DOX | CAL27 | MRI-guided magnetic-triggered hyperthermia and chemotherapy | [196] (Xiang) |
| MIL-53(Fe) | Hepatic | oridonin (Ori) | HepG2 | chemotherapy | [144] (Leng) |
| DHA@ZIF-8 | Hepatic | Dihydroartemisinin (DHA) | HepG2, SMMC-7721, BEL-7404 | chemotherapy | [194] (Li) |
| Fe2+ doped ZIF-8 | Hepatic | Ferrous ion, DHA | HepG2 | chemotherapy | [203] (Xiao) |
| porphyrin MOFs | Hepatic | FA, gadolinium (Gd) | HepG2, L02 | photodynamic therapy, MRI | [147] (chen) |
| MIL-100(Fe) | Hepatic | - | HepG2 | chemotherapy | [148] (chen) |
| Gd(BCB)(DMF)](H2O)2 | Hepatic | 5-Fu | Hep-G2 | chemotherapy | [149] (Sun) |
| PCN-224 | Hepatic | Galactose, DOX | HepG2, Huh7 | Interventional photodynamic therapy, chemotherapy | [150] (Hu) |
| HKUST-1 | Pancreatic | Fe3O4 nanorods, Nimesulide | - | chemotherapy | [81] (ke) |
| Cu-GA NMOF | Pancreatic | methylene blue | Panc-1 cells | chemotherapy, photodynamic therapy | [156] (Sharma) |
| UiO-Cis | Ovarian | siRNA, cisplatin | SKOV3 cells | chemotherapy | [168] (He) |
| Ln-ZMOFs | Ovarian | Terbium (Tb), Europium (Eu) | LPA (biomarker) | Biochemical sensing | [174] (Zhang) |
| Zn-MOF | Ovarian | dinuclear gold(I) pyrrolidinedithiocarbamato | A2780, A2870cis, HepG2, U-87 MG, MDCK | chemotherapy | [176] (Sun) |
| HKUST-1 | Ovarian | Cu-MOF | SKOV3 | chemotherapy | [199] (Chen) |
| MIL-88A | Ovarian | minicircle DNA (MC), MC encoding anti-CD3/anti-EpCAM bispecific T cell engager (MC.BiTE) | SKOV3 | Bispecific T-cell engager (BiTE) immunotherapy | [201] (Zhao) |
| FeN200@GOx@M | Ovarian | FeN, glucose oxidase (GOx) | A2780 | chemotherapy | [204] |
| UiO-68 | Ovarian, Breast | complementary sequence to (miRNA)-21 or miRNA-221, DOX | OVCAR-3, MCF-7 | miRNA responsive, chemotherapy | [179] (Chen) |
| Cu-MOF | Ovarian | - | Hey ovarian cancer cells | chemotherapy by ROS accumulation | [181] (Li) |
| NZIF-8 | Cervical | CCM | HeLa, xenograft tumors of U14 | chemotherapy | [183] (Zheng) |
| NH2-MIL-88B (Fe) | Cervical | Chloroquine (CQ) | cervical carcinoma cell line HeLa, A375 | nanocatalytic therapy, ROS-induced oxidative damage | [186] (Yang) |
| HKUST-1 | Colon, Cervical | Cu2+ | CT26, CT26 tumor-bearing mice, human cervix cancer cells HeLa | H2S-activated photothermal therapy, chemodynamic therapy | [187] (Li) |
| [Zn2(L)(H2O)2](DMA)2 | Colon | SW60 | chemotherapy | ||
| InIII-MOF | Colon | 5-Fu | SW60 | ROS, chemotherapy | [205] (Li) |
| Cr-MOF@CoPc | Colorectal | cobalt phthalocyanine (CoPc) NPs | CT26 | biosensing | [209] (Duan) |
| Ni(II) MOF | Leukemia | HL-60 | ROS, chemotherapy | [207] (Xi) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmehrath, S.; Nguyen, H.L.; Karam, S.M.; Amin, A.; Greish, Y.E. BioMOF-Based Anti-Cancer Drug Delivery Systems. Nanomaterials 2023, 13, 953. https://doi.org/10.3390/nano13050953
Elmehrath S, Nguyen HL, Karam SM, Amin A, Greish YE. BioMOF-Based Anti-Cancer Drug Delivery Systems. Nanomaterials. 2023; 13(5):953. https://doi.org/10.3390/nano13050953
Chicago/Turabian StyleElmehrath, Sandy, Ha L. Nguyen, Sherif M. Karam, Amr Amin, and Yaser E. Greish. 2023. "BioMOF-Based Anti-Cancer Drug Delivery Systems" Nanomaterials 13, no. 5: 953. https://doi.org/10.3390/nano13050953