Portable Nanocomposite System for Wound Healing in Space
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
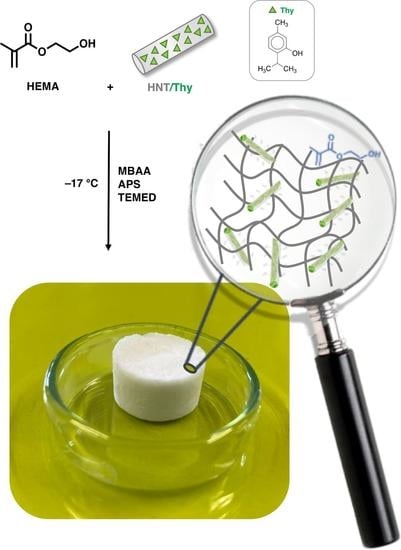
2.2. Synthesis of Cryogels
2.2.1. Preparation of HNTs/Thy Nanohybrid
2.2.2. Synthesis of HEMA and HEMA-HNT Cryogels
2.2.3. Synthesis of HEMA-HNT/Thy Cryogel
2.3. Cryogels Characterization
2.4. Thymol Release
2.5. Kinetic of Release
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wilkinson, H.N.; Hardman, M.J. Wound Healing: Cellular Mechanisms and Pathological Outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Cialdai, F.; Vignali, L.; Morbidelli, L.; Colciago, A.; Celotti, F.; Santi, A.; Caselli, A.; Cirri, P.; Monici, M. Modeled Microgravity Affects Fibroblast Functions Related to Wound Healing. Microgravity Sci. Technol. 2017, 29, 121–132. [Google Scholar] [CrossRef]
- Cancedda, R.; Liu, Y.; Ruggiu, A.; Tavella, S.; Biticchi, R.; Santucci, D.; Schwartz, S.; Ciparelli, P.; Falcetti, G.; Tenconi, C.; et al. The Mice Drawer System (MDS) Experiment and the Space Endurance Record-Breaking Mice. PLoS ONE 2012, 7, e32243. [Google Scholar] [CrossRef] [Green Version]
- Neutelings, T.; Nusgens, B.V.; Liu, Y.; Tavella, S.; Ruggiu, A.; Cancedda, R.; Gabriel, M.; Colige, A.; Lambert, C. Skin Physiology in Microgravity: A 3-Month Stay Aboard ISS Induces Dermal Atrophy and Affects Cutaneous Muscle and Hair Follicles Cycling in Mice. NPJ Microgravity 2015, 1, 15002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monici, M.; Cialdai, F.; Romano, G.; Fusi, F.; Egli, M.; Pezzatini, S.; Morbidelli, L. An in Vitro Study on Tissue Repair: Impact of Unloading on Cells Involved in the Remodelling Phase. Microgravity Sci. Technol. 2011, 23, 391–401. [Google Scholar] [CrossRef]
- Zagni, C.; Pistarà, V.; Oliveira, L.A.; Castilho, R.M.; Romeo, G.; Chiacchio, U.; Rescifina, A. Serendipitous Discovery of Potent Human Head and Neck Squamous Cell Carcinoma Anti-Cancer Molecules: A Fortunate Failure of a Rational Molecular Design. Eur. J. Med. Chem. 2017, 141, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Chiacchio, U.; Broggini, G.; Romeo, R.; Gazzola, S.; Chiacchio, M.A.; Giofrè, S.V.; Gabriele, B.; Mancuso, R.; Floresta, G.; Zagni, C. Intramolecular Oxidative Palladium-Catalyzed Diamination Reactions of Alkenyl Sulfamates: An Efficient Synthesis of [1,2,5]Thiadiazolo-Fused Piperazinones. RSC Adv. 2016, 6, 57521–57529. [Google Scholar] [CrossRef]
- Cardullo, N.; Catinella, G.; Floresta, G.; Muccilli, V.; Rosselli, S.; Rescifina, A.; Bruno, M.; Tringali, C. Synthesis of Rosmarinic Acid Amides as Antioxidative and Hypoglycemic Agents. J. Nat. Prod. 2019, 82, 573–582. [Google Scholar] [CrossRef]
- Langeveld, W.T.; Veldhuizen, E.J.A.; Burt, S.A. Synergy between Essential Oil Components and Antibiotics: A Review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef]
- Riella, K.R.; Marinho, R.R.; Santos, J.S.; Pereira-Filho, R.N.; Cardoso, J.C.; Albuquerque-Junior, R.L.C.; Thomazzi, S.M. Anti-Inflammatory and Cicatrizing Activities of Thymol, a Monoterpene of the Essential Oil from Lippia Gracilis, in Rodents. J. Ethnopharmacol. 2012, 143, 656–663. [Google Scholar] [CrossRef]
- Guimarães, A.G.; Quintans, J.S.S.; Quintans-Júnior, L.J. Monoterpenes with Analgesic Activity—A Systematic Review. Phytother. Res. 2013, 27, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.F.; Durço, A.O.; Rabelo, T.K.; Barreto, R.d.S.S.; Guimarães, A.G. Effects of Carvacrol, Thymol and Essential Oils Containing Such Monoterpenes on Wound Healing: A Systematic Review. J. Pharm. Pharm. 2019, 71, 141–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, V.; Grey, J.E.; Harding, K.G. Wound Dressings. BMJ 2006, 332, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Guo, B. Smart Wound Dressings for Wound Healing. Nano Today 2021, 41, 101290. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef] [PubMed]
- Pennetta, C.; Floresta, G.; Graziano, A.C.E.; Cardile, V.; Rubino, L.; Galimberti, M.; Rescifina, A.; Barbera, V. Functionalization of Single and Multi-Walled Carbon Nanotubes with Polypropylene Glycol Decorated Pyrrole for the Development of Doxorubicin Nano-Conveyors for Cancer Drug Delivery. Nanomaterials 2020, 10, 1073. [Google Scholar] [CrossRef]
- Patamia, V.; Fiorenza, R.; Brullo, I.; Marsala, M.Z.; Balsamo, S.A.; Distefano, A.; Furneri, P.M.; Barbera, V.; Scirè, S.; Rescifina, A. A Sustainable Porous Composite Material Based on Loofah-Halloysite for Gas Adsorption and Drug Delivery. Mater. Chem. Front. 2022, 6, 2233–2243. [Google Scholar] [CrossRef]
- Patamia, V.; Floresta, G.; Pistarà, V.; Rescifina, A. Green Efficient One-pot Synthesis and Separation of Nitrones in Water Assisted by a Self-assembled Nanoreactor. Int. J. Mol. Sci. 2022, 23, 236. [Google Scholar] [CrossRef]
- Patamia, V.; Tomarchio, R.; Fiorenza, R.; Zagni, C.; Scirè, S.; Floresta, G.; Rescifina, A. Carbamoyl-Decorated Cyclodextrins for Carbon Dioxide Adsorption. Catalysts 2023, 13, 41. [Google Scholar] [CrossRef]
- Patamia, V.; Gentile, D.; Fiorenza, R.; Muccilli, V.; Mineo, P.G.; Scirè, S.; Rescifina, A. Nanosponges Based on Self-Assembled Starfish-Shaped Cucurbit[6]Urils Functionalized with Imidazolium Arms. Chem. Commun. 2021, 57, 3664–3667. [Google Scholar] [CrossRef]
- Tomasella, P.; Sanfilippo, V.; Bonaccorso, C.; Cucci, L.M.; Consiglio, G.; Nicosia, A.; Mineo, P.G.; Forte, G.; Satriano, C. Theranostic Nanoplatforms of Thiolated Reduced Graphene Oxide Nanosheets and Gold Nanoparticles. Appl. Sci. 2020, 10, 5529. [Google Scholar] [CrossRef]
- Gentile, D.; Floresta, G.; Patamia, V.; Nicosia, A.; Mineo, P.G.; Rescifina, A. Cucurbit [7]Uril as a Catalytic Nanoreactor for One-Pot Synthesis of Isoxazolidines in Water. Org. Biomol. Chem. 2020, 18, 1194–1203. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lu, F.; Zhou, G.; Yu, K.; Lu, B.; Xiao, Y.; Dai, F.; Wu, D.; Lan, G. Silver Inlaid with Gold Nanoparticle/Chitosan Wound Dressing Enhances Antibacterial Activity and Porosity, and Promotes Wound Healing. Biomacromolecules 2017, 18, 3766–3775. [Google Scholar] [CrossRef]
- Yuan, H.; Chen, L.; Hong, F.F. A Biodegradable Antibacterial Nanocomposite Based on Oxidized Bacterial Nanocellulose for Rapid Hemostasis and Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 3382–3392. [Google Scholar] [CrossRef]
- Han, L.; Li, P.; Pengfei, T.; Wang, X.; Zhou, T.; Wang, K.; Ren, F.; Guo, T.; Lu, X. Mussel-Inspired Cryogels for Promoting Wound Regeneration through Photobiostimulation, Modulating Inflammatory Response and Suppressing Bacterial Invasion. Nanoscale 2019, 11, 15846–15861. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, N.; Sagbas, S.; Sahiner, M.; Silan, C. P(TA) Macro-, Micro-, Nanoparticle-Embedded Super Porous p(HEMA) Cryogels as Wound Dressing Material. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Diken Gür, S.; Bakhshpour, M.; Bereli, N.; Denizli, A. Antibacterial Effect against Both Gram-Positive and Gram-Negative Bacteria via Lysozyme Imprinted Cryogel Membranes. J. Biomater. Sci. Polym. Ed. 2021, 32, 1024–1039. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Y.; Yang, J.; Gao, Y.; Ma, C.; Che, L.; Wang, J.; Wu, J.; Zheng, J. De Novo Design of Allochroic Zwitterions. Mater. Today 2022, 60, 17–30. [Google Scholar] [CrossRef]
- Wan, Z.; He, J.; Yang, Y.; Chong, T.; Wang, J.; Guo, B.; Xue, L. Injectable Adhesive Self-Healing Biocompatible Hydrogel for Haemostasis, Wound Healing, and Postoperative Tissue Adhesion Prevention in Nephron-Sparing Surgery. Acta Biomater. 2022, 152, 157–170. [Google Scholar] [CrossRef]
- Wang, S.; Wang, F.; Shi, K.; Yuan, J.; Sun, W.; Yang, J.; Chen, Y.; Zhang, D.; Che, L. Osteichthyes Skin-Inspired Tough and Sticky Composite Hydrogels for Dynamic Adhesive Dressings. Compos. Part B Eng. 2022, 241, 110010. [Google Scholar] [CrossRef]
- Lvov, Y.M.; Shchukin, D.G.; Möhwald, H.; Price, R.R. Halloysite Clay Nanotubes for Controlled Release of Protective Agents. ACS Nano 2008, 2, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, G.; Milioto, S.; Lazzara, G. Halloysite Nanotubes: Interfacial Properties and Applications in Cultural Heritage. Langmuir 2020, 36, 3677–3689. [Google Scholar] [CrossRef] [PubMed]
- Abdullayev, E.; Lvov, Y. Halloysite Clay Nanotubes as a Ceramic “Skeleton” for Functional Biopolymer Composites with Sustained Drug Release. J. Mater. Chem. B 2013, 1, 2894–2903. [Google Scholar] [CrossRef] [PubMed]
- Okten Besli, N.S.; Orakdogen, N. One-Shot Preparation of Polybasic Ternary Hybrid Cryogels Consisting of Halloysite Nanotubes and Tertiary Amine Functional Groups: An Efficient and Convenient Way by Freezing-Induced Gelation. Gels 2021, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Lvov, Y.; Wang, W.; Zhang, L.; Fakhrullin, R. Halloysite Clay Nanotubes for Loading and Sustained Release of Functional Compounds. Adv. Mater. 2016, 28, 1227–1250. [Google Scholar] [CrossRef]
- Lvov, Y.; Abdullayev, E. Functional Polymer–Clay Nanotube Composites with Sustained Release of Chemical Agents. Prog. Polym. Sci. 2013, 38, 1690–1719. [Google Scholar] [CrossRef]
- Liu, M.; Jia, Z.; Jia, D.; Zhou, C. Recent Advance in Research on Halloysite Nanotubes-Polymer Nanocomposite. Prog. Polym. Sci. 2014, 39, 1498–1525. [Google Scholar] [CrossRef]
- Gorrasi, G.; Bugatti, V.; Ussia, M.; Mendichi, R.; Zampino, D.; Puglisi, C.; Carroccio, S.C. Halloysite Nanotubes and Thymol as Photo Protectors of Biobased Polyamide 11. Polym. Degrad. Stab. 2018, 152, 43–51. [Google Scholar] [CrossRef]
- Zagni, C.; Dattilo, S.; Mecca, T.; Gugliuzzo, C.; Scamporrino, A.A.; Privitera, V.; Puglisi, R.; Carola Carroccio, S. Single and Dual Polymeric Sponges for Emerging Pollutants Removal. Eur. Polym. J. 2022, 179, 111556. [Google Scholar] [CrossRef]
- Mecca, T.; Ussia, M.; Caretti, D.; Cunsolo, F.; Dattilo, S.; Scurti, S.; Privitera, V.; Carroccio, S.C. N-Methyl-D-Glucamine Based Cryogels as Reusable Sponges to Enhance Heavy Metals Removal from Water. Chem. Eng. J. 2020, 399, 125753. [Google Scholar] [CrossRef]
- Bruschi, M.L. Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Cambridge, UK, 2015; ISBN 978-0-08-100112-7. [Google Scholar]
- Fan, F.; Saha, S.; Hanjaya-Putra, D. Biomimetic Hydrogels to Promote Wound Healing. Front. Bioeng. Biotechnol. 2021, 9, 718377. [Google Scholar] [CrossRef] [PubMed]
- Nagoor Meeran, M.F.; Javed, H.; Al Taee, H.; Azimullah, S.; Ojha, S.K. Pharmacological Properties and Molecular Mechanisms of Thymol: Prospects for Its Therapeutic Potential and Pharmaceutical Development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veerabadran, N.G.; Mongayt, D.; Torchilin, V.; Price, R.R.; Lvov, Y.M. Organized Shells on Clay Nanotubes for Controlled Release of Macromolecules. Macromol. Rapid Commun. 2009, 30, 99–103. [Google Scholar] [CrossRef]
- Gorrasi, G. Dispersion of Halloysite Loaded with Natural Antimicrobials into Pectins: Characterization and Controlled Release Analysis. Carbohydr. Polym. 2015, 127, 47–53. [Google Scholar] [CrossRef]
- Koshy, S.T.; Zhang, D.K.Y.; Grolman, J.M.; Stafford, A.G.; Mooney, D.J. Injectable Nanocomposite Cryogels for Versatile Protein Drug Delivery. Acta Biomater. 2018, 65, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.; Mittapelly, N.; Pant, A.; Sharma, S.; Singh, P.; Banala, V.T.; Trivedi, R.; Shukla, P.K.; Mishra, P.R. Dual Functioning Microspheres Embedded Crosslinked Gelatin Cryogels for Therapeutic Intervention in Osteomyelitis and Associated Bone Loss. Eur. J. Pharm. Sci. 2016, 91, 105–113. [Google Scholar] [CrossRef]
- Barocas, V.; Drasler II, W.; Girton, T.; Guler, I.; Knapp, D.; Moeller, J.; Parsonage, E. A Dissolution-Diffusion Model for the TAXUSTM Drug-Eluting Stent with Surface Burst Estimated from Continuum Percolation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90B, 267–274. [Google Scholar] [CrossRef]
- Ghone, N.; PRICE, R.; Lvov, Y. Clay Nanotubes for Encapsulation and Sustained Release of Drugs. Nano 2007, 02. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Mathematical Modeling of Drug Dissolution. Int. J. Pharm. 2013, 453, 12–24. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Zhang, L.; Xu, Z.; Dai, H.; Wu, W. Nanocellulose/Gelatin Composite Cryogels for Controlled Drug Release. ACS Sustain. Chem. Eng. 2019, 7, 6381–6389. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A Simple Equation for Description of Solute Release II. Fickian and Anomalous Release from Swellable Devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]







| Thy Release | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Media | Zero Order | First Order | Higuchi | Korsmeyer–Peppas | Weibull | |||||||
| K | R2 | K | R2 | K | R2 | K | n | R2 | a | b | R2 | ||
| Hema-HNT/Thy | PBS | 0.487 | 0.795 | 0.008 | 0.850 | 5.375 | 0.918 | 16.523 | 0.245 | 0.973 | 0.161 | 0.324 | 0.976 |
| Hema-HNT/Thy | H2O | 0.545 | 0.808 | 0.010 | 0.873 | 6.014 | 0.929 | 18.877 | 0.240 | 0.986 | 0.184 | 0.333 | 0.988 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagni, C.; Scamporrino, A.A.; Riccobene, P.M.; Floresta, G.; Patamia, V.; Rescifina, A.; Carroccio, S.C. Portable Nanocomposite System for Wound Healing in Space. Nanomaterials 2023, 13, 741. https://doi.org/10.3390/nano13040741
Zagni C, Scamporrino AA, Riccobene PM, Floresta G, Patamia V, Rescifina A, Carroccio SC. Portable Nanocomposite System for Wound Healing in Space. Nanomaterials. 2023; 13(4):741. https://doi.org/10.3390/nano13040741
Chicago/Turabian StyleZagni, Chiara, Andrea Antonino Scamporrino, Paolo Maria Riccobene, Giuseppe Floresta, Vincenzo Patamia, Antonio Rescifina, and Sabrina Carola Carroccio. 2023. "Portable Nanocomposite System for Wound Healing in Space" Nanomaterials 13, no. 4: 741. https://doi.org/10.3390/nano13040741