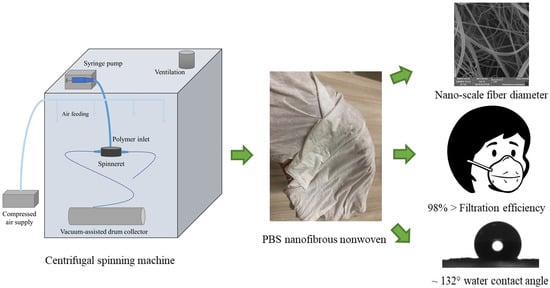
Optimization of the Centrifugal Spinning Parameters to Prepare Poly(butylene succinate) Nanofibers Mats for Aerosol Filter Applications
Abstract
:1. Introduction
2. Experimental Study
2.1. Production of PBS Fiber
2.2. Characterizations and Measurements
2.3. Statistical Analysis
3. Results and Discussion
3.1. Model Development
3.2. The Effect of the CS Procedure on PBS Nanofiber
3.3. Investigation of PBS Nanofiber as a Filter
3.4. Further Analysis of PBS Filter
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonfim, D.P.F.; Cruz, F.G.S.; Bretas, R.E.S.; Guerra, V.G.; Aguiar, M.L. A Sustainable Recycling Alternative: Electrospun PET-Membranes for Air Nanofiltration. Polymers 2021, 13, 1166. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, A.; Sarngan, P.P.; Sarkar, D. Inorganic-organic nanofiber networks with antibacteria porperties enhaced particulate filtration: The critical role of amorphous titania. Chemosphere 2022, 286, 131671. [Google Scholar] [CrossRef] [PubMed]
- Selvaranjan, K.; Navaratnam, S.; Rajeev, P.; Ravintherakumaran, N. Environmental challenges induced by extensive use of face masks during COVID-19: A review and potential solutions. Environ. Chall. 2021, 3, 100039. [Google Scholar] [CrossRef]
- Fadare, O.O.; Okoffo, E.D. COVID-19 face masks: A potential source of microplastic fibers in the environment. Sci. Total Environ. 2020, 737, 140279. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Pashalidis, I. Single-use surgical face masks, as a potential source of microplastics: Do they act as pollutant carriers? J. Mol. Liq. 2021, 326, 115247. [Google Scholar] [CrossRef]
- Kutralam-Muniasamy, G.; Pérez-Guevara, F.; Shruti, V.C. A critical synthesis of current peer-reviewed literature on the environmental and human health impacts of COVID-19 PPE litter: New findings and next steps. J. Hazard. Mater. 2022, 422, 126945. [Google Scholar] [CrossRef]
- de Matos Costa, A.R.; Crocitti, A.; Hecker de Carvalho, L.; Carroccio, S.C.; Cerruti, P.; Santagata, G. Properties of Biodegradable Films Based on Poly(butylene Succinate) (PBS) and Poly(butylene Adipate-co-Terephthalate) (PBAT) Blends. Polymers 2020, 12, 2317. [Google Scholar] [CrossRef]
- Kim, S.H.; Cho, J.Y.; Cho, D.H.; Jung, H.J.; Kim, B.C.; Bhatia, S.K.; Park, S.H.; Park, K.; Yang, Y.H. Acceleration of Polybutylene Succinate Biodegradation by Terribacillus sp. JY49 Isolated from a Marine Environment. Polymers 2022, 14, 3978. [Google Scholar] [CrossRef]
- Platnieks, O.; Gaidukovs, S.; Barkane, A.; Sereda, A.; Gaidukova, G.; Grase, L.; Thakur, V.K.; Filipova, I.; Fridrihsone, V.; Skute, M.; et al. Bio-based poly(butylene succinate)/microcrystalline cellulose/nanofibrillated cellulose-based sustainable polymer composites: Thermo-mechanical and biodegradation studies. Polymers 2020, 12, 1472. [Google Scholar] [CrossRef]
- Lv, D.; Zhu, M.; Jiang, Z.; Jiang, S.; Zhang, Q.; Xiong, R.; Huang, C. Green Electrospun Nanofibers and Their Application in Air Filtration. Macromol. Mater. Eng. 2018, 303, 1800336. [Google Scholar] [CrossRef]
- Borojeni, I.A.; Gajewski, G.; Riahi, R.A. Application of Electrospun Nonwoven Fibers in Air Filters. Fibers 2022, 10, 15. [Google Scholar] [CrossRef]
- Jeong, E.H.; Im, S.S.; Youk, J.H. Electrospinning and structural characterization of ultrafine poly(butylene succinate) fibers. Polymer 2005, 46, 9538–9543. [Google Scholar] [CrossRef]
- Liu, Y.; He, J.H.; Yu, J.Y. Preparation and morphology of poly (butylene succinate) nanofibers via electrospinning. Fibres Text. East. Eur. 2007, 15, 30–33. [Google Scholar]
- Zhang, D.; Chang, J.; Zeng, Y. Fabrication of fibrous poly (butylene succinate)/wollastonite/apatite composite scaffolds by electrospinning and biomimetic process. J. Mater. Sci. Mater. Med. 2008, 19, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Wang, P.; Zhao, Z.; Ji, J. Antimicrobial Activity of Electrospun Poly (butylenes Succinate) Fiber Mats Containing PVPCapped Silver Nanoparticles. Appl. Biochem. Biotechnol. 2013, 171, 1890–1899. [Google Scholar] [CrossRef] [PubMed]
- Klairutsamee, W.; Supaphol, P.; Jangchud, I. Electrospinnability of Poly (butylene Succinate): Effects of Solvents and Organic Salt on the Fiber Size and Morphology. J. Appl. Polym. Sci. 2015, 132, 42716. [Google Scholar] [CrossRef]
- Llorens, E.; Ibañez, H.; Del Valle, L.J.; Puiggalí, J. Biocompatibility and drug release behavior of scaffolds prepared by coaxial electrospinning of poly(butylene succinate) and polyethylene glycol. Mater. Sci. Eng. C 2015, 49, 472–484. [Google Scholar] [CrossRef]
- Cooper, C.J.; Mohanty, A.K.; Misra, M. Electrospinning Process and Structure Relationship of Biobased Poly(butylene succinate) for Nanoporous Fibers. ACS Omega 2018, 3, 5547–5557. [Google Scholar] [CrossRef]
- Kurokawa, N.; Kimura, S.; Hotta, A. Mechanical properties of poly(butylene succinate) composites with aligned cellulose-acetate nanofibers. J. Appl. Polym. Sci. 2018, 135, 45429. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; Posati, T.; Sotgiu, G.; Tiboni, M.; Barbalinardo, M.; Valle, F.; Casettari, L.; Zamboni, R.; Lotti, N.; et al. Regenerated wool keratin-polybutylene succinate nanofibrous mats for drug delivery and cells culture. Polym. Degrad. Stab. 2020, 179, 109272. [Google Scholar] [CrossRef]
- Gavande, V.; Im, D.; Jin, Y.; Lim, K.T.; Lee, W.K. 3D bio polybutylene succinate electrospun nanofiber scaffolds for biomimetic structure. Mol. Cryst. Liq. Cryst. 2020, 706, 55–61. [Google Scholar] [CrossRef]
- Atıcı, B.; Ünlü, C.H.; Yanilmaz, M. A Review on Centrifugally Spun Fibers and Their Applications. Polym. Rev. 2022, 62, 1–64. [Google Scholar] [CrossRef]
- Xu, H.; Yagi, S.; Ashour, S.; Du, L.; Hoque, M.E.; Tan, L. A Review on Current Nanofiber Technologies: Electrospinning, Centrifugal Spinning, and Electro-Centrifugal Spinning. Macromol. Mater. Eng. 2023, 308, 2200502. [Google Scholar] [CrossRef]
- Vanheusden, C.; Vanminsel, J.; Reddy, N.; Samyn, P.; D’Haen, J.; Peeters, R.; Ethirajan, A.; Buntinx, M. Fabrication of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) Fibers Using Centrifugal Fiber Spinning: Structure, Properties and Application Potential. Polymers 2023, 15, 1181. [Google Scholar] [CrossRef] [PubMed]
- Atıcı, B.; Ünlü, C.H.; Yanilmaz, M. A statistical analysis on the influence of process and solution properties on centrifugally spun nanofiber morphology. J. Ind. Text. 2022, 51, 613S–639S. [Google Scholar] [CrossRef]
- Doan, H.N.; Nguyen, D.K.; Vo, P.P.; Hayashi, K.; Kinashi, K.; Sakai, W.; Tsutsumi, N.; Huynh, D.P. Facile and Scalable Fabrication of Porous Polystyrene Fibers for OilRemoval by Centrifugal Spinning. ACS Omega 2019, 4, 15992–16000. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zou, C.; Shao, J.; Zhang, X.; Li, Y. Preparation of SiO2/PS Superhydrophobic Fibers with Bionic Controllable Micro–Nano Structure via Centrifugal Spinning. RSC Adv. 2017, 7, 11041–11048. [Google Scholar] [CrossRef]
- Weitz, R.T.; Harnau, L.; Rauschenbach, S.; Burghard, M.; Kern, K. Polymer Nanofibersvia Nozzle-Free Centrifugal Spinning. Nano Lett. 2008, 8, 1187–1191. [Google Scholar] [CrossRef]
- Patlan, R.; Mejias, J.; McEachin, Z.; Salinas, A.; Lozano, K. Fabrication and Characterization of Poly(L-Lactic Acid) Fiber Mats Using Centrifugal Spinning. Fibers Polym. 2018, 19, 1271–1277. [Google Scholar] [CrossRef]
- Venkataraman, D.; Shabani, E.; Joshi, K.; Widjaja, O.; Park, J.H. Comparative Investigation of Electrospun and Centrifugal Spun Polylactic Acid for Filtration Performance and Reusability. ACS Appl. Eng. Mater. 2023, 1, 2315–2323. [Google Scholar] [CrossRef]
- Fang, Y.; Dulaney, A.R.; Gadley, J.; Maia, J.; Ellison, C.J. A Comparative Parameter Study: Controlling Fiber Diameter and Diameter Distribution in Centrifugal Spinning ofPhotocurable Monomers. Polymer 2016, 88, 102–111. [Google Scholar] [CrossRef]
- McEachin, Z.; Lozano, K. Production and Characterization of Polycaprolactone Nanofibers via ForcespinningTM Technology. J. Appl. Polym. Sci. 2012, 126, 473–479. [Google Scholar] [CrossRef]
- Yang, S.B.; Yeum, J.H. Morphological Comparison of Aligned Poly (Vinyl Alcohol) Nanofibers Fabricated by Modified Electrospinning and Centrifugal Jet Spinning Techniques. J. Nanosci. Nanotechnol. 2017, 17, 9056–9062. [Google Scholar] [CrossRef]
- Andjani, D.; Sriyanti, I.; Fauzi, A.; Edikresnha, D.; Munir, M.M.; Khairurrijal, A.D. Fabrication of polyvinylpyrrolidone fibers by Means of Rotary Forcespinning Method. IOP Conf. Ser. Mater. Sci. Eng. 2018, 367, 012044. [Google Scholar] [CrossRef]
- Vazquez, B.; Vasquez, H.; Lozano, K. Preparation and Characterization of Polyvinylidene Fluoride Nanofibrous Membranes by ForcespinningTM. Polym. Eng. Sci. 2012, 52, 2260–2265. [Google Scholar] [CrossRef]
- Vo, P.; Doan, H.; Kinashi, K.; Sakai, W.; Tsutsumi, N.; Huynh, D. Centrifugally Spun Recycled PET: Processing and Characterization. Polymers 2018, 10, 680. [Google Scholar] [CrossRef] [PubMed]
- Hammami, M.A.; Krifa, M.; Harzallah, O. Centrifugal Force Spinning of PA6 Nanofibers—Processability and Morphology of Solution-Spun Fibers. J. Ind. Text. 2014, 105, 637–647. [Google Scholar] [CrossRef]
- Pereira Rodrigues, I.C.; Tamborlin, L.; Rodrigues, A.A.; Jardini, A.L.; Ducati Luchessi, A.; Maciel Filho, R.; Najar Lopes, É.S.; Pellizzer Gabriel, L. Polyurethane Fibrous Membranes Tailored by Rotary Jet Spinning for Tissue Engineering Applications. J. Appl. Polym. Sci. 2020, 137, 48455. [Google Scholar] [CrossRef]
- De la Garza, D.; De Santiago, F.; Materon, L.; Chipara, M.; Alcoutlabi, M. Fabrication and Characterization of Centrifugally Spun Poly(Acrylic Acid) Nanofibers. J. Appl. Polym. Sci. 2019, 136, 47480. [Google Scholar] [CrossRef]
- Chantre, C.O.; Gonzalez, G.M.; Ahn, S.; Cera, L.; Campbell, P.H.; Hoerstrup, S.P.; Parker, K.K. Porous Biomimetic Hyaluronic Acid and Extracellular Matrix Protein Nanofiber Scaffolds for Accelerated Cutaneous Tissue Repair. ACS Appl. Mater. Interfaces 2019, 11, 45498–45510. [Google Scholar] [CrossRef]
- Li, Z.; Mei, S.; Dong, Y.; She, F.; Kong, L. High Efficiency Fabrication of Chitosan Composite Nanofibers with Uniform Morphology via Centrifugal Spinning. Polymers 2019, 11, 1550. [Google Scholar] [CrossRef] [PubMed]
- Stojanovska, E.; Kurtulus, M.; Abdelgawad, A.; Candan, Z.; Kilic, A. Developing Lignin-Based Bio-Nanofibers by Centrifugal Spinning Technique. Int. J. Biol. Macromol. 2018, 113, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sun, J.; Shao, M.; Yang, B. A Comparison of Centrifugally-Spun and Electrospun Regenerated Silk Fibroin Nanofiber Structures and Properties. RSC Adv. 2015, 5, 98553–98558. [Google Scholar] [CrossRef]
- Arican, F.; Uzuner-Demir, A.; Polat, O.; Sancakli, A.; Ismar, E. Fabrication of gelatin nanofiber webs via centrifugal spinning for N95 respiratory filters. Bull. Mater. Sci. 2022, 45, 93. [Google Scholar] [CrossRef]
- NIOSH 42 CFR Part 84 Standard; Approval of Respiratory Protective Devices. eCFR: London, UK, 2004.
- Aslan, N.; Cebeci, Y. Application of Box–Behnken design and Response Surface Methodology for modeling of some Turkish coals. Fuel 2007, 86, 90–97. [Google Scholar] [CrossRef]
- Salimbeigi, G.; Oliveira, R.N.; McGuinness, G.B. Electrospun poly(e-caprolactone)/propolis fiber morphology: A process optimisation study. J. Appl. Polym. Sci. 2022, 139, e52131. [Google Scholar] [CrossRef]
- Merchiers, J.; Meurs, W.; Deferme, W.; Peeters, R.; Buntinx, M.; Reddy, N.K. Influence of polymer concentration and nozzle material on centrifugal fiber spinning. Polymers 2020, 12, 575. [Google Scholar] [CrossRef]
- Xia, L.; Lu, L.; Liang, Y. Preparation and Characterization of Poly(lactic acid) Micro- and Nanofibers Fabricated by Centrifugal Spinning. Fibers Polym. 2020, 21, 1422–1429. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Y.; Zhang, S.; Xu, G.; Fu, K.; Lee, H.; Zhang, X. Parameter study and characterization for polyacrylonitrile nanofibers fabricated via centrifugal spinning process. Eur. Polym. J. 2013, 49, 3834–3845. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Zhang, X.; Huang, C.; Jin, X. Design of electret polypropylene melt blown air filtration material containing nucleating agent for effective PM2. 5 capture. RSC Adv. 2018, 8, 7932–7941. [Google Scholar] [CrossRef]
- Matulevicius, J.; Kliucininkas, L.; Martuzevicius, D.; Krugly, E.; Tichonovas, M.; Baltrusaitis, J. Design and characterization of electrospun polyamide nanofiber media for air filtration applications. J. Nanomater. 2014, 2014, 859656. [Google Scholar] [CrossRef]
- Qian, Y.; Willeke, K.; Grinshpun, S.A.; Donnelly, J.; Coffey, C.C. Performance of N95 Respirators: Filtration Efficiency for Airborne Microbial and Inert Particles. Am. Ind. Hyg. Assoc. J. 1998, 59, 128–132. [Google Scholar] [CrossRef] [PubMed]
- EN 149:2001; Respiratory Protective Devices—Filtering Half Masks to Protect Against Particles—Requirements, Testing, Marking. BSI: London, UK, 2009.
- Wang, Z.; Zhao, C.C.; Pan, Z.J. Porous bead-on-string poly(lactic acid) fibrous membranes for air filtration. J. Colloid Interface Sci. 2015, 441, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Wang, Z.; Li, X. Improving Filtration Performance of Electrospun Nanofiber Mats by a Bimodal Method. J. Appl. Polym. Sci. 2013, 128, 1089–1094. [Google Scholar] [CrossRef]
- Leung, W.W.F.; Hung, C.H.; Yuen, P.T. Effect of face velocity, nanofiber packing density and thickness on filtration performance of filters with nanofibers coated on a substrate. Sep. Purif. Technol. 2010, 71, 30–37. [Google Scholar] [CrossRef]
- Li, H.; Wang, Z.; Zhang, H.Y.; Pan, Z.J. Nanoporous PLA/(Chitosan Nanoparticle) Composite Fibrous Membranes with Excellent Air Filtration and Antibacterial Performance. Polymers 2018, 10, 1085. [Google Scholar] [CrossRef]
- Hu, J.; Xiong, Z.J.; Liu, Y.Q.; Lin, J.Y. A biodegradable composite filter made from electrospun zein fibers underlaid on the cellulose paper towel. Int. J. Biol. Macromol. 2022, 204, 419–428. [Google Scholar] [CrossRef]
- Akduman, C. Cellulose acetate and polyvinylidene fluoride nanofiber mats for N95 respirators. J. Ind. Text. 2021, 50, 1239–1261. [Google Scholar] [CrossRef]
- Širc, J.; Hobzová, R.; Kostina, N.; Munzarová, M.; Juklíčková, M.; Lhotka, M.; Kubinová, Š.; Zajícová, A.; Michálek, J. Morphological Characterization of Nanofibers: Methods and Application in Practice. J. Nanomater. 2012, 2012, 327369. [Google Scholar] [CrossRef]
- Bang, J.; Park, S.; Hwang, S.W.; Oh, J.K.; Yeo, H.; Jin, H.J.; Kwak, H.W. Biodegradable and hydrophobic nanofibrous membranes produced by solution blow spinning for efficient oil/water separation. Chemosphere 2023, 312, 137240. [Google Scholar] [CrossRef]








| Run | Concentration (%) | Rotational Speed (rpm) | Needle Size (mm) | Average Fiber Diameter (nm) | |
|---|---|---|---|---|---|
| Experimental | Predicted | ||||
| R1 | 7 | 6000 | 0.7 | 172 | 166 |
| R2 | 13 | 6000 | 0.7 | 286 | 275 |
| R3 | 7 | 10,000 | 0.7 | 177 | 188 |
| R4 | 13 | 10,000 | 0.7 | 315 | 321 |
| R5 | 7 | 8000 | 0.6 | 206 | 201 |
| R6 | 13 | 8000 | 0.6 | 302 | 303 |
| R7 | 7 | 8000 | 0.8 | 214 | 214 |
| R8 | 13 | 8000 | 0.8 | 349 | 354 |
| R9 | 10 | 6000 | 0.6 | 253 | 264 |
| R10 | 10 | 10,000 | 0.6 | 339 | 333 |
| R11 | 10 | 6000 | 0.8 | 324 | 330 |
| R12 | 10 | 10,000 | 0.8 | 340 | 329 |
| R13 | 10 | 8000 | 0.7 | 277 | 275 |
| R14 | 10 | 8000 | 0.7 | 264 | 275 |
| R15 | 10 | 8000 | 0.7 | 283 | 275 |
| Factors | Variable Levels and Range | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Concentration (%) | 7 | 10 | 13 |
| Rotational speed (rpm) | 6000 | 8000 | 10,000 |
| Needle size (mm) | 0.6 | 0.7 | 0.8 |
| Source | SS | df | MS | F-Value | ρ-Value | |
|---|---|---|---|---|---|---|
| Mean vs. Total | 1.121 × 106 | 1 | 1.121 × 106 | |||
| Linear vs. Mean | 33,489.25 | 3 | 11,163.08 | 8.48 | 0.0034 | |
| 2FI vs. Linear | 1749.25 | 3 | 583.08 | 0.3662 | 0.7795 | |
| Quadratic vs. 2FI | 11,881.18 | 3 | 3960.39 | 23.08 | 0.0023 | Suggested |
| Cubic vs. Quadratic | 669.25 | 3 | 223.08 | 2.36 | 0.3110 | Aliased |
| Residual | 188.67 | 2 | 94.33 | |||
| Total | 1.169 × 106 | 15 | 77,946.07 |
| Linear | 0.0034 | 0.0573 | 0.6157 | 0.3573 | |
| 2FI | 0.7795 | 0.0438 | 0.5353 | −0.3972 | |
| Quadratic | 0.0023 | 0.3110 | 0.9499 | 0.7680 | Suggested |
| Cubic | 0.3110 | 0.9725 | Aliased |
| Source | SS | df | MS | F-Value | ρ-Value | |
|---|---|---|---|---|---|---|
| Model | 47,119.68 | 9 | 5235.52 | 30.51 | 0.0008 | significant |
| A-Concentration | 29,161.13 | 1 | 29,161.13 | 169.95 | <0.0001 | |
| B-Rotational speed | 2312.00 | 1 | 2312.00 | 13.47 | 0.0144 | |
| C-Needle size | 2016.13 | 1 | 2016.13 | 11.75 | 0.0187 | |
| AB | 144.00 | 1 | 144.00 | 0.8392 | 0.4016 | |
| AC | 380.25 | 1 | 380.25 | 2.22 | 0.1967 | |
| BC | 1225.00 | 1 | 1225.00 | 7.14 | 0.0442 | |
| A2 | 6423.08 | 1 | 6423.08 | 37.43 | 0.0017 | |
| B2 | 76.16 | 1 | 76.16 | 0.4439 | 0.5348 | |
| C2 | 4469.39 | 1 | 4469.39 | 26.05 | 0.0038 | |
| Residual | 857.92 | 5 | 171.58 | |||
| Lack of Fit | 669.25 | 3 | 223.08 | 2.36 | 0.3110 | not significant |
| Pure Error | 188.67 | 2 | 94.33 | |||
| Cor Total | 47,977.60 | 14 |
| Materials | Production Method | Air Velocity/Flow Rate | Filtration Efficiency (%) | Pressure Drop (Pa) | QF (Pa−1) | References |
|---|---|---|---|---|---|---|
| PBS | Centrifugal Spinning | 5.33 cm/s | 98.61 | 95 | 0.045 | This study |
| 14.17 cm/s | 98.35 | 238 | 0.017 | This study | ||
| 15.83 cm/s | 98.14 | 248 | 0.016 | This study | ||
| PLA | Centrifugal Spinning | 14.17 cm/s | 99.8 | 140 | 0.044 | [30] |
| Gelatin | Centrifugal Spinning | 15.83 cm/s | - | - | 0.011 | [44] |
| PLA | Electrospinning | 5.8 cm/s | 99.997 | 165.3 | 0.064 | [55] |
| Chitosan/PLA | Electrospinning | 14 cm/s | 98.99 | 147.63 | 0.031 | [58] |
| Zein | Electrospinning | 5.3 cm/s | 99 | 109 | 0.042 | [59] |
| Cellulose acetate | Electrospinning | 85 L/min | 95.33 | 298 | 0.010 | [60] |
| Surface Area (m2/g) | Cumulative Pore Volume (cc/g) | Pore Diameter (nm) | Tensile Strength (MPa) | Young Module (MPa) | Elongation (%) | Contact Angle (°) |
|---|---|---|---|---|---|---|
| 8.93 | 0.016 | 3.4 | 0.10 ± 0.01 | 0.16 ± 0.12 | 58.14 ± 2.13 | 131.98 ± 3.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pakolpakçıl, A.; Kılıç, A.; Draczynski, Z. Optimization of the Centrifugal Spinning Parameters to Prepare Poly(butylene succinate) Nanofibers Mats for Aerosol Filter Applications. Nanomaterials 2023, 13, 3150. https://doi.org/10.3390/nano13243150
Pakolpakçıl A, Kılıç A, Draczynski Z. Optimization of the Centrifugal Spinning Parameters to Prepare Poly(butylene succinate) Nanofibers Mats for Aerosol Filter Applications. Nanomaterials. 2023; 13(24):3150. https://doi.org/10.3390/nano13243150
Chicago/Turabian StylePakolpakçıl, Ayben, Ali Kılıç, and Zbigniew Draczynski. 2023. "Optimization of the Centrifugal Spinning Parameters to Prepare Poly(butylene succinate) Nanofibers Mats for Aerosol Filter Applications" Nanomaterials 13, no. 24: 3150. https://doi.org/10.3390/nano13243150