Highly Efficient Energy Transfer from Silicon to Erbium in Erbium-Hyperdoped Silicon Quantum Dots
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Er-Hyperdoped Si QDs
2.3. Characterization
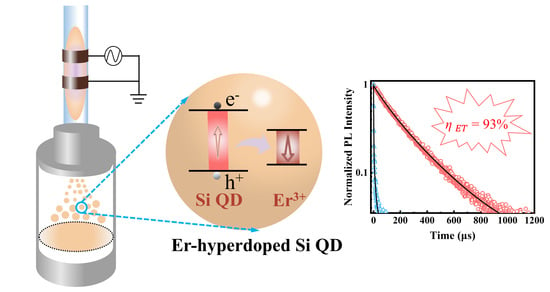
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, N.; Xin, M.; Su, Z.; Magden, E.S.; Singh, N.; Notaros, J.; Timurdogan, E.; Purnawirman, P.; Bradley, J.D.B.; Watts, M.R. A Silicon Photonic Data Link with a Monolithic Erbium-Doped Laser. Sci. Rep. 2020, 10, 1114. [Google Scholar] [CrossRef] [Green Version]
- Fu, P.; Yang, D.C.; Jia, R.; Yi, Z.J.; Liu, Z.F.; Li, X.; Eglitis, R.I.; Su, Z.M. Metallic Subnanometer Porous Silicon: A Theoretical Prediction. Phys. Rev. B 2021, 103, 014117. [Google Scholar] [CrossRef]
- Pavesi, L. Silicon-Based Light Sources for Silicon Integrated Circuits. Adv. Opt. Technol. 2008, 2008, 416926. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Chen, G.; Ng, D.K.T.; Lim, L.W.; Xue, J.; Ho, C.P.; Fu, Y.H.; Lee, L.Y.T. Integrated Lasers on Silicon at Communication Wavelength: A Progress Review. Adv. Opt. Mater. 2022, 10, 2201008. [Google Scholar] [CrossRef]
- Wojdak, M.; Klik, M.; Forcales, M.; Gusev, O.B.; Gregorkiewicz, T.; Pacifici, D.; Franzò, G.; Priolo, F.; Iacona, F. Sensitization of Er Luminescence by Si Nanoclusters. Phys. Rev. B 2004, 69, 233315. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.; Heitmann, J.; Scholz, R.; Zacharias, M. Bright Luminescence from Erbium Doped Nc-Si/SiO2 Superlattices. J. Non. Cryst. Solids 2002, 299–302, 678–682. [Google Scholar] [CrossRef] [Green Version]
- Wen, H.; He, J.; Hong, J.; Jin, S.; Xu, Z.; Zhu, H.; Liu, J.; Sha, G.; Yue, F.; Dan, Y. Efficient Er/O-Doped Silicon Light-Emitting Diodes at Communication Wavelength by Deep Cooling. Adv. Opt. Mater. 2020, 8, 2000720. [Google Scholar] [CrossRef]
- Fujii, M.; Yoshida, M.; Hayashi, S.; Yamamoto, K. Photoluminescence from SiO2 Films Containing Si Nanocrystals and Er: Effects of Nanocrystalline Size on the Photoluminescence Efficiency of Er3+. J. Appl. Phys. 1998, 84, 4525–4531. [Google Scholar] [CrossRef]
- Wang, K.; He, Q.; Yang, D.; Pi, X. Erbium-Hyperdoped Silicon Quantum Dots: A Platform of Ratiometric Near-Infrared Fluorescence. Adv. Opt. Mater. 2022, 10, 2201831. [Google Scholar] [CrossRef]
- Rinnert, H.; Adeola, G.W.; Vergnat, M. Influence of the Silicon Nanocrystal Size on the 1.54 μm Luminescence of Er-Doped SiO/SiO2 multilayers. J. Appl. Phys. 2009, 105, 2–5. [Google Scholar] [CrossRef]
- Timoshenko, V.Y.; Lisachenko, M.G.; Shalygina, O.A.; Kamenev, B.V.; Zhigunov, D.M.; Teterukov, S.A.; Kashkarov, P.K.; Heitmann, J.; Schmidt, M.; Zacharias, M. Comparative Study of Photoluminescence of Undoped and Erbium-Doped Size-Controlled Nanocrystalline Si/SiO2 Multilayered Structures. J. Appl. Phys. 2004, 96, 2254–2260. [Google Scholar] [CrossRef] [Green Version]
- Izeddin, I.; Timmerman, D.; Gregorkiewicz, T.; Moskalenko, A.S.; Prokofiev, A.A.; Yassievich, I.N.; Fujii, M. Energy Transfer in Er-Doped SiO2 Sensitized with Si Nanocrystals. Phys. Rev. B 2008, 78, 035327. [Google Scholar] [CrossRef] [Green Version]
- Dexter, D.L. A Theory of Sensitized Luminescence in Solids. J. Chem. Phys. 1953, 21, 836–850. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, S.; Gu, W.; Zhang, Y.; Qiao, X.; Ni, Z.; Pi, X.; Yang, D. Light-Emitting Diodes Based on Colloidal Silicon Quantum Dots with Octyl and Phenylpropyl Ligands. ACS Appl. Mater. Interfaces 2018, 10, 5959–5966. [Google Scholar] [CrossRef]
- Tessler, L.R.; Zanatta, A.R. Erbium Luminescence in A-Si:H. J. Non. Cryst. Solids 1998, 227–230, 399–402. [Google Scholar] [CrossRef]
- Kenyon, A.J.; Chryssou, C.E.; Pitt, C.W.; Shimizu-Iwayama, T.; Hole, D.E.; Sharma, N.; Humphreys, C.J. Luminescence from Erbium-Doped Silicon Nanocrystals in Silica: Excitation Mechanisms. J. Appl. Phys. 2002, 91, 367–374. [Google Scholar] [CrossRef]
- Mangolini, L.; Thimsen, E.; Kortshagen, U. High-Yield Plasma Synthesis of Luminescent Silicon Nanocrystals. Nano Lett. 2005, 5, 655–659. [Google Scholar] [CrossRef]
- Dasog, M.; De Los Reyes, G.B.; Titova, L.V.; Hegmann, F.A.; Veinot, J.G.C. Size vs Surface: Tuning the Photoluminescence of Freestanding Silicon Nanocrystals across the Visible Spectrum via Surface Groups. ACS Nano 2014, 8, 9636–9648. [Google Scholar] [CrossRef]
- Benton, J.L.; Michel, J.; Kimerling, L.C.; Jacobson, D.C.; Xie, Y.H.; Eaglesham, D.J.; Fitzgerald, E.A.; Poate, J.M. The Electrical and Defect Properties of Erbium-Implanted Silicon. J. Appl. Phys. 1991, 70, 2667–2671. [Google Scholar] [CrossRef]
- Raffa, A.G.; Ballone, P. Equilibrium Structure of Erbium-Oxygen Complexes in Crystalline Silicon. Phys. Rev. B 2002, 65, 121309. [Google Scholar] [CrossRef]
- Campbell, I.H.; Fauchet, P.M. The Effects of Microcrystal Size and Shape on the One Phonon Raman Spectra of Crystalline Semiconductors. Solid State Commun. 1986, 58, 739–741. [Google Scholar] [CrossRef]
- Ni, Z.; Pi, X.; Zhou, S.; Nozaki, T.; Grandidier, B.; Yang, D. Size-Dependent Structures and Optical Absorption of Boron-Hyperdoped Silicon Nanocrystals. Adv. Opt. Mater. 2016, 4, 700–707. [Google Scholar] [CrossRef]
- Zhou, S.; Pi, X.; Ni, Z.; Luan, Q.; Jiang, Y.; Jin, C.; Nozaki, T.; Yang, D. Boron- and Phosphorus-Hyperdoped Silicon Nanocrystals. Part. Part. Syst. Charact. 2015, 32, 213–221. [Google Scholar] [CrossRef]
- Tewell, C.R.; King, S.H. Observation of Metastable Erbium Trihydride. Appl. Surf. Sci. 2006, 253, 2597–2602. [Google Scholar] [CrossRef]
- Miritello, M.; Lo Savio, R.; Piro, A.M.; Franzò, G.; Priolo, F.; Iacona, F.; Bongiorno, C. Optical and Structural Properties of Er2O3 Films Grown by Magnetron Sputtering. J. Appl. Phys. 2006, 100, 013502. [Google Scholar] [CrossRef]
- Hannah, D.C.; Yang, J.; Podsiadlo, P.; Chan, M.K.Y.; Demortière, A.; Gosztola, D.J.; Prakapenka, V.B.; Schatz, G.C.; Kortshagen, U.; Schaller, R.D. On the Origin of Photoluminescence in Silicon Nanocrystals: Pressure-Dependent Structural and Optical Studies. Nano Lett. 2012, 12, 4200–4205. [Google Scholar] [CrossRef] [PubMed]
- Sangghaleh, F.; Sychugov, I.; Yang, Z.; Veinot, J.G.C.; Linnros, J. Near-Unity Internal Quantum Efficiency of Luminescent Silicon Nanocrystals with Ligand Passivation. ACS Nano 2015, 9, 7097–7104. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Zhou, S.; Zhao, S.; Peng, W.; Yang, D.; Pi, X. Silicon Nanocrystals: Unfading Silicon Materials for Optoelectronics. Mater. Sci. Eng. R Reports 2019, 138, 85–117. [Google Scholar] [CrossRef]
- Puzder, A.; Williamson, A.J.; Grossman, J.C.; Galli, G. Computational Studies of the Optical Emission of Silicon Nanocrystals. J. Am. Chem. Soc. 2003, 125, 2786–2791. [Google Scholar] [CrossRef]
- Fujii, M.; Yoshida, M.; Kanzawa, Y.; Hayashi, S.; Yamamoto, K. 1.54 μm Photoluminescence of Er3+ Doped into SiO2 Films Containing Si Nanocrystals: Evidence for Energy Transfer from Si Nanocrystals to Er3+. Appl. Phys. Lett. 1997, 71, 1198–1200. [Google Scholar] [CrossRef]
- Pradeep, J.A.; Agarwal, P. An Alternative Approach to Understand the Photoluminescence and the Photoluminescence Peak Shift with Excitation in Porous Silicon. J. Appl. Phys. 2008, 104, 123515. [Google Scholar] [CrossRef]
- Weissker, H.C.; Ning, N.; Bechstedt, F.; Vach, H. Luminescence and Absorption in Germanium and Silicon Nanocrystals: The Influence of Compression, Surface Reconstruction, Optical Excitation, and Spin-Orbit Splitting. Phys. Rev. B 2011, 83, 4–9. [Google Scholar] [CrossRef]
- Kik, P.G.; Brongersma, M.L.; Polman, A. Strong Exciton-Erbium Coupling in Si Nanocrystal-Doped SiO2. Appl. Phys. Lett. 2000, 76, 2325–2327. [Google Scholar] [CrossRef] [Green Version]
- Timmerman, D.; Izeddin, I.; Gregorkiewicz, T. Saturation of Luminescence from Si Nanocrystals Embedded in SiO2. Phys. Status Solidi Appl. Mater. Sci. 2010, 207, 183–187. [Google Scholar] [CrossRef]
- Gusev, O.B.; Bresler, M.S.; Pak, P.E.; Yassievich, I.N.; Forcales, M.; Vinh, N.Q.; Gregorkiewicz, T. Excitation Cross Section of Erbium in Semiconductor Matrices under Optical Pumping. Phys. Rev. B 2001, 64, 753021–753027. [Google Scholar] [CrossRef] [Green Version]
- Pacifici, D.; Franzò, G.; Priolo, F.; Iacona, F.; Dal Negro, L. Modeling and Perspectives of the Si Nanocrystals–Er Interaction for Optical Amplification. Phys. Rev. B 2003, 67, 245301. [Google Scholar] [CrossRef] [Green Version]
- Kenyon, A.J.; Wojdak, M.; Ahmad, I.; Loh, W.H.; Oton, C.J. Generalized Rate-Equation Analysis of Excitation Exchange between Silicon Nanoclusters and Erbium Ions. Phys. Rev. B 2008, 77, 035318. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, B.; Takeguchi, M.; Nakamura, J.; Nemoto, Y.; Hamaoka, T.; Chandra, S.; Shirahata, N. Origin of the Photoluminescence Quantum Yields Enhanced by Alkane-Termination of Freestanding Silicon Nanocrystals: Temperature-Dependence of Optical Properties. Sci. Rep. 2016, 6, 36951. [Google Scholar] [CrossRef] [Green Version]
- Navarro-Urrios, D.; Pitanti, A.; Daldosso, N.; Gourbilleau, F.; Rizk, R.; Garrido, B.; Pavesi, L. Energy Transfer between Amorphous Si Nanoclusters and Er3+ Ions in a SiO2 Matrix. Phys. Rev. B 2009, 79, 2–5. [Google Scholar] [CrossRef]
- Hijazi, K.; Rizk, R.; Cardin, J.; Khomenkova, L.; Gourbilleau, F. Towards an Optimum Coupling between Er Ions and Si-Based Sensitizers for Integrated Active Photonics. J. Appl. Phys. 2009, 106, 024311. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; He, Q.; Yang, D.; Pi, X. Highly Efficient Energy Transfer from Silicon to Erbium in Erbium-Hyperdoped Silicon Quantum Dots. Nanomaterials 2023, 13, 277. https://doi.org/10.3390/nano13020277
Wang K, He Q, Yang D, Pi X. Highly Efficient Energy Transfer from Silicon to Erbium in Erbium-Hyperdoped Silicon Quantum Dots. Nanomaterials. 2023; 13(2):277. https://doi.org/10.3390/nano13020277
Chicago/Turabian StyleWang, Kun, Qiang He, Deren Yang, and Xiaodong Pi. 2023. "Highly Efficient Energy Transfer from Silicon to Erbium in Erbium-Hyperdoped Silicon Quantum Dots" Nanomaterials 13, no. 2: 277. https://doi.org/10.3390/nano13020277