Bioinspired Hierarchical Carbon Structures as Potential Scaffolds for Wound Healing and Tissue Regeneration Applications
Abstract
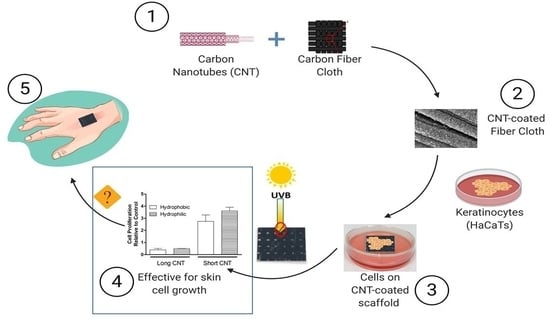
:1. Introduction
2. Materials and Methods
2.1. Preparation and Surface Treatment of CNT-Coated Scaffolds
2.2. Structure Analysis of Scaffolds Using Scanning Electron Microscopy (SEM)
2.3. Analyses of Surface Chemical States Using X-ray Photoelectron Spectroscopy (XPS)
2.4. Cell Culture and Cell Seeding on Scaffolds
2.5. Cell Seeding with CNT-Coated Scaffolds
2.6. Elution Test
2.7. Cell Labeling
2.8. Ultraviolet-B (UVB) Treatment
2.9. Cell Proliferation
2.10. Cytotoxicity Analysis
2.11. Cell Migration from CNT-Coated Scaffolds
2.12. Sandwich Enzyme-Linked Immunosorbent Assay (ELISA)
2.13. Cytokine Analysis
2.14. Data Analysis
3. Results
3.1. Surface Morphology of the CNT-Coated Carbon Fiber Cloth
3.2. Influence of Surface Oxidation Treatment on Surface Chemical States of CNT Carpet
3.3. Influence of Surface Oxidation Treatment on Surface Wettability and Water Contact Angle
3.4. CNT-Coated Scaffolds Do Not Induce Cytotoxicity in Keratinocytes
3.5. Short CNTs Can Effectively Support Keratinocyte Cell Proliferation
3.6. CNT-Coated Scaffolds Support Cell Migration
3.7. Scaffolds Provide Cytoprotection against UVB Exposure
3.8. CNT-Coated Scaffolds May Modulate Immune Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, J.F.; Yannas, I.V.; Quinby, W.C.; Bondoc, C.C.; Jung, W.K. Successful Use of a Physiologically Acceptable Artificial Skin in the Treatment of Extensive Burn Injury. Ann. Surg. 1981, 194, 413–428. [Google Scholar] [CrossRef] [PubMed]
- Hassanshahi, A.; Hassanshahi, M.; Khabbazi, S.; Hosseini-Khah, Z.; Peymanfar, Y.; Ghalamkari, S.; Su, Y.-W.; Xian, C.J. Adipose-Derived Stem Cells for Wound Healing. J. Cell. Physiol. 2019, 234, 7903–7914. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Midha, S.; Giri, S.; Mohanty, S. Functional Skin Grafts: Where Biomaterials Meet Stem Cells. Stem Cells Int. 2019, 2019, 1286054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braza, M.E.; Fahrenkopf, M.P. Split-Thickness Skin Grafts; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Horch, R.E.; Jeschke, M.G.; Spilker, G.; Herndon, D.N.; Kopp, J. Treatment of Second Degree Facial Burns with Allografts—Preliminary Results. Burns 2005, 31, 597–602. [Google Scholar] [CrossRef]
- Lee, K.H. Tissue-Engineered Human Living Skin Substitutes: Development and Clinical Application. Yonsei Med. J. 2000, 41, 774–779. [Google Scholar] [CrossRef] [Green Version]
- Negut, I.; Dorcioman, G.; Grumezescu, V. Scaffolds for Wound Healing Applications. Polymer 2020, 12, 2010. [Google Scholar] [CrossRef]
- Turner, N.J.; Badylak, S.F. The Use of Biologic Scaffolds in the Treatment of Chronic Nonhealing Wounds. Adv. Wound Care 2015, 4, 490–500. [Google Scholar] [CrossRef] [Green Version]
- Har-el, Y.; Gerstenhaber, J.A.; Brodsky, R.; Huneke, R.B.; Lelkes, P.I. Electrospun Soy Protein Scaffolds as Wound Dressings: Enhanced Reepithelialization in a Porcine Model of Wound Healing. Wound Med. 2014, 5, 9–15. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, F.J. Biomaterials & Scaffolds for Tissue Engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Lu, L.; Peter, S.J.; Lyman, M.D.; Lai, H.L.; Leite, S.M.; Tamada, J.A.; Uyama, S.; Vacanti, J.P.; Langer, R.; Mikos, A.G. In Vitro and in Vivo Degradation of Porous Poly(DL-Lactic-Co-Glycolic Acid) Foams. Biomaterials 2000, 21, 1837–1845. [Google Scholar] [CrossRef]
- Smith, I.O.; Liu, X.H.; Smith, L.A.; Ma, P.X. Nano-Structured Polymer Scaffolds for Tissue Engineering and Regenerative Medicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 226–236. [Google Scholar] [CrossRef] [Green Version]
- Lobo, A.; Antunes, E.; Palma, M.; Pacheco-Soares, C.; Corat, M.; Trava-Airoldi, V.; Corat, E. Biocompatibility Differences Between Dispersed And Vertically-Aligned Carbon Nanotubes: An In Vitro Assays Review. In Carbon Nanotubes: New Research; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2009. [Google Scholar]
- Yi, H.; Ur Rehman, F.; Zhao, C.; Liu, B.; He, N. Recent Advances in Nano Scaffolds for Bone Repair. Bone Res. 2016, 4, 16050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balasundaram, G.; Storey, D.M.; Webster, T.J. Novel Nano-Rough Polymers for Cartilage Tissue Engineering. Int. J. Nanomed. 2014, 9, 1845–1853. [Google Scholar] [CrossRef] [Green Version]
- Veetil, J.V.; Ye, K. Tailored Carbon Nanotubes for Tissue Engineering Applications. Biotechnol. Prog. 2009, 25, 709–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianco, A.; Kostarelos, K.; Partidos, C.D.; Prato, M. Biomedical Applications of Functionalised Carbon Nanotubes. Chem. Commun. 2005, 5, 571–577. [Google Scholar] [CrossRef]
- Bellucci, S.; Chiaretti, M.; Onorato, P.; Rossella, F.; Grandi, M.S.; Galinetto, P.; Sacco, I.; Micciulla, F. Micro-Raman Study of the Role of Sterilization on Carbon Nanotubes for Biomedical Applications. Available online: https://www.futuremedicine.com/doi/abs/10.2217/nnm.09.100 (accessed on 24 January 2021).
- Wang, X.; Li, Q.; Xie, J.; Jin, Z.; Wang, J.; Li, Y.; Jiang, K.; Fan, S. Fabrication of Ultralong and Electrically Uniform Single-Walled Carbon Nanotubes on Clean Substrates. Nano Lett. 2009, 9, 3137–3141. [Google Scholar] [CrossRef]
- Mao, H.; Kawazoe, N.; Chen, G. Uptake and Intracellular Distribution of Collagen-Functionalized Single-Walled Carbon Nanotubes. Biomaterials 2013, 34, 2472–2479. [Google Scholar] [CrossRef]
- Villa, C.H.; Dao, T.; Ahearn, I.; Fehrenbacher, N.; Casey, E.; Rey, D.A.; Korontsvit, T.; Zakhaleva, V.; Batt, C.A.; Philips, M.R.; et al. Single-Walled Carbon Nanotubes Deliver Peptide Antigen into Dendritic Cells and Enhance IgG Responses to Tumor-Associated Antigens. ACS Nano 2011, 5, 5300–5311. [Google Scholar] [CrossRef]
- Mutlu, G.M.; Budinger, G.R.S.; Green, A.A.; Urich, D.; Soberanes, S.; Chiarella, S.E.; Alheid, G.F.; McCrimmon, D.R.; Szleifer, I.; Hersam, M.C. Biocompatible Nanoscale Dispersion of Single Walled Carbon Nanotubes Minimizes in Vivo Pulmonary Toxicity. Nano Lett. 2010, 10, 1664–1670. [Google Scholar] [CrossRef] [Green Version]
- Kostarelos, K.; Bianco, A.; Prato, M. Promises, Facts and Challenges for Carbon Nanotubes in Imaging and Therapeutics. Nat. Nanotechnol. 2009, 4, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Kostarelos, K.; Lacerda, L.; Pastorin, G.; Wu, W.; Wieckowski, S.; Luangsivilay, J.; Godefroy, S.; Pantarotto, D.; Briand, J.-P.; Muller, S.; et al. Cellular Uptake of Functionalized Carbon Nanotubes Is Independent of Functional Group and Cell Type. Nat. Nanotech. 2007, 2, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Pantarotto, D.; Singh, R.; McCarthy, D.; Erhardt, M.; Briand, J.; Prato, M.; Kostarelos, K.; Bianco, A. Functionalized Carbon Nanotubes for Plasmid DNA Gene Delivery. Angew. Chem. Int. Ed. 2004, 43, 5242–5246. [Google Scholar] [CrossRef]
- Zhang, M.; Deng, Y.; Yang, M.; Nakajima, H.; Yudasaka, M.; Iijima, S.; Okazaki, T. A Simple Method for Removal of Carbon Nanotubes from Wastewater Using Hypochlorite. Sci. Rep. 2019, 9, 1284. [Google Scholar] [CrossRef] [Green Version]
- Saito, N.; Usui, Y.; Aoki, K.; Narita, N.; Shimizu, M.; Hara, K.; Ogiwara, N.; Nakamura, K.; Ishigaki, N.; Kato, H.; et al. Carbon Nanotubes: Biomaterial Applications. Chem. Soc. Rev. 2009, 38, 1897. [Google Scholar] [CrossRef]
- Dvir, T.; Timko, B.P.; Kohane, D.S.; Langer, R. Nanotechnological Strategies for Engineering Complex Tissues. Nat. Nanotechnol. 2011, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Hirata, E.; Uo, M.; Nodasaka, Y.; Takita, H.; Ushijima, N.; Akasaka, T.; Watari, F.; Yokoyama, A. 3D Collagen Scaffolds Coated with Multiwalled Carbon Nanotubes: Initial Cell Attachment to Internal Surface. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 93, 544–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karumuri, A.K.; He, L.; Mukhopadhyay, S.M. Tuning the Surface Wettability of Carbon Nanotube Carpets in Multiscale Hierarchical Solids. Appl. Surf. Sci. 2015, 327, 122–130. [Google Scholar] [CrossRef]
- He, L.; Karumuri, A.; Mukhopadhyay, S.M. Wettability Tailoring of Nanotube Carpets: Morphology-Chemistry Synergy for Hydrophobic–Hydrophilic Cycling. RSC Adv. 2017, 7, 25265–25275. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.; Mukundan, S.; Wang, W.; Karumuri, A.; Sant, V.; Mukhopadhyay, S.M.; Sant, S. Carbon-Based Hierarchical Scaffolds for Myoblast Differentiation: Synergy between Nano-Functionalization and Alignment. Acta Biomater. 2016, 32, 77–88. [Google Scholar] [CrossRef] [Green Version]
- Ezzati Nazhad Dolatabadi, J.; Omidi, Y.; Losic, D. Carbon Nanotubes as an Advanced Drug and Gene Delivery Nanosystem. Curr. Nanosci. 2011, 7, 297–314. [Google Scholar] [CrossRef]
- Pei, B.; Wang, W.; Dunne, N.; Li, X. Applications of Carbon Nanotubes in Bone Tissue Regeneration and Engineering: Superiority, Concerns, Current Advancements, and Prospects. Nanomaterials 2019, 9, 1501. [Google Scholar] [CrossRef] [Green Version]
- Shetti, N.P.; Malode, S.J.; Nayak, D.S.; Reddy, C.V.; Reddy, K.R. Novel Biosensor for Efficient Electrochemical Detection of Methdilazine Using Carbon Nanotubes-Modified Electrodes. Mater. Res. Express 2019, 6, 116308. [Google Scholar] [CrossRef]
- Simon-Deckers, A.; Gouget, B.; Mayne-L’Hermite, M.; Herlin-Boime, N.; Reynaud, C.; Carriere, M. In Vitro Investigation of Oxide Nanoparticle and Carbon Nanotube Toxicity and Intracellular Accumulation in A549 Human Pneumocytes. Toxicology 2008, 253, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.; Huaux, F.; Moreau, N.; Misson, P.; Heilier, J.-F.; Delos, M.; Arras, M.; Fonseca, A.; Nagy, J.B.; Lison, D. Respiratory Toxicity of Multi-Wall Carbon Nanotubes. Toxicol. Appl. Pharmacol. 2005, 207, 221–231. [Google Scholar] [CrossRef]
- Sachar, S.; Saxena, R.K. Cytotoxic Effect of Poly-Dispersed Single Walled Carbon Nanotubes on Erythrocytes in Vitro and in Vivo. PLoS ONE 2011, 6, e22032. [Google Scholar] [CrossRef]
- Lin, S.H.; Kleinberg, L.R. Carmustine Wafers: Localized Delivery of Chemotherapeutic Agents in CNS Malignancies. Expert Rev. Anticancer Ther. 2008, 8, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.R.; Kisin, E.; Leonard, S.S.; Young, S.H.; Kommineni, C.; Kagan, V.E.; Castranova, V.; Shvedova, A.A. Oxidative Stress and Inflammatory Response in Dermal Toxicity of Single-Walled Carbon Nanotubes. Toxicology 2009, 257, 161–171. [Google Scholar] [CrossRef]
- Vitkina, T.I.; Yankova, V.I.; Gvozdenko, T.A.; Kuznetsov, V.L.; Krasnikov, D.V.; Nazarenko, A.V.; Chaika, V.V.; Smagin, S.V.; Tsatsakis, A.Μ.; Engin, A.B.; et al. The Impact of Multi-Walled Carbon Nanotubes with Different Amount of Metallic Impurities on Immunometabolic Parameters in Healthy Volunteers. Food Chem. Toxicol. 2016, 87, 138–147. [Google Scholar] [CrossRef]
- Palmer, B.C.; Phelan-Dickenson, S.J.; DeLouise, L.A. Multi-Walled Carbon Nanotube Oxidation Dependent Keratinocyte Cytotoxicity and Skin Inflammation. Part Fibre. Toxicol. 2019, 16, 3. [Google Scholar] [CrossRef] [Green Version]
- Mukhopadhyay, S.M.; Karumuri, A.K. Nanotube Attachment for Prevention of Interfacial Delamination. J. Phys. D Appl. Phys. 2010, 43, 365301. [Google Scholar] [CrossRef] [Green Version]
- Karumuri, A.K.; Maleszewski, A.A.; Oswal, D.P.; Hostetler, H.A.; Mukhopadhyay, S.M. Fabrication and Characterization of Antibacterial Nanoparticles Supported on Hierarchical Hybrid Substrates. J. Nanoparticle Res. 2014, 16, 2346. [Google Scholar] [CrossRef]
- Vijwani, H.; Agrawal, A.; Mukhopadhyay, S.M. Dechlorination of Environmental Contaminants Using a Hybrid Nanocatalyst: Palladium Nanoparticles Supported on Hierarchical Carbon Nanostructures. J. Nanotechnol. 2012, 2012, 478381. [Google Scholar] [CrossRef] [Green Version]
- Mukhopadhyay, S.M.; Karumuri, A.; Barney, I.T. Hierarchical Nanostructures by Nanotube Grafting on Porous Cellular Surfaces. J. Phys. D Appl. Phys. 2009, 42, 195503. [Google Scholar] [CrossRef]
- Vijwani, H.; Nadagouda, M.N.; Namboodiri, V.; Mukhopadhyay, S.M. Hierarchical Hybrid Carbon Nano-Structures as Robust and Reusable Adsorbents: Kinetic Studies with Model Dye Compound. Chem. Eng. J. 2015, 268, 197–207. [Google Scholar] [CrossRef]
- Jones, G.; Cartmell, S.H. Optimization of Cell Seeding Efficiencies on a Three-Dimensional Gelatin Scaffold for Bone Tissue Engineering. J. Appl. Biomater. Biomech. 2006, 4, 172–180. [Google Scholar]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal Keratinization in a Spontaneously Immortalized Aneuploid Human Keratinocyte Cell Line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [Green Version]
- Cerutti, A.; Zan, H.; Schaffer, A.; Bergsagel, L.; Harindranath, N.; Max, E.E.; Casali, P. CD40 Ligand and Appropriate Cytokines Induce Switching to IgG, IgA, and IgE and Coordinated Germinal Center and Plasmacytoid Phenotypic Differentiation in a Human Monoclonal IgM+IgD+ B Cell Line. J. Immunol. 1998, 160, 2145–2157. [Google Scholar] [CrossRef]
- Bernstein, R.M.; Mills, F.C.; Mitchell, M.; Max, E.E. Complex Mechanisms for Inhibition of Immunoglobulin Gene Expression in a Germinal Center B Cell Line. Mol. Immunol. 2004, 41, 63–72. [Google Scholar] [CrossRef]
- Saiki, O.; Ralph, P. Clonal Differences in Response to T Cell Replacing Factor (TRF) for IgM Secretion and TRF Receptors in a Human B Lymphoblast Cell Line. Eur. J. Immunol. 1983, 13, 31–34. [Google Scholar] [CrossRef]
- Bradford, C.; Freeman, R.; Percival, S.L. In Vitro Study of Sustained Antimicrobial Activity of a New Silver Alginate Dressing. J. Am. Coll. Certif. Wound Spec. 2009, 1, 117–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, L.; Tang, J.; Liu, H.; Shen, C.; Rong, M.; Zhang, Z.; Lai, R. A Potential Wound-Healing-Promoting Peptide from Salamander Skin. FASEB J. 2014, 28, 3919–3929. [Google Scholar] [CrossRef] [Green Version]
- Boissel, J.-P.; Ohly, D.; Bros, M.; Gödtel-Armbrust, U.; Förstermann, U.; Frank, S. The Neuronal Nitric Oxide Synthase Is Upregulated in Mouse Skin Repair and in Response to Epidermal Growth Factor in Human HaCaT Keratinocytes. J. Investig. Dermatol. 2004, 123, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.R.; Kang, S.Y.; Kim, H.O.; Park, C.W.; Chung, B.Y. Role of Aryl Hydrocarbon Receptor Activation and Autophagy in Psoriasis-Related Inflammation. Int. J. Mol. Sci. 2020, 21, 2195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, L.; Wu, W.; Fu, W.; Hu, Y. The Effects of Phototherapy and Melanocytes on Keratinocytes. Exp. Ther. Med. 2018, 15, 3459–3466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulentic, C.E.W.; Holsapple, M.P.; Kaminski, N.E. Aryl Hydrocarbon Receptor-Dependent Suppression by 2,3,7,8-Tetrachlorodibenzo-p-Dioxin of IgM Secretion in Activated B Cells. Mol Pharm. 1998, 53, 623–629. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Mukhopadhyay, S.M. Hierarchical Nanostructured Surface Design for Robust and Flexible Multifunctional Devices. Carbon Trends 2021, 5, 100096. [Google Scholar] [CrossRef]
- Quinton, B.T. Aligned Carbon Nanotube Carpets on Carbon Substrates for High Power Electronic Applications; Mechanical and Thermal Systems Branch; Power and Control Division Wright-Patterson Air Force Base United States: Dayton, OH, USA, 2016. [Google Scholar]
- Kumar, C.V.; Pattammattel, A. Chapter 3—Characterization Techniques for Graphene. In Introduction to Graphene; Kumar, C.V., Pattammattel, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 45–74. ISBN 978-0-12-813182-4. [Google Scholar]
- Barney, I.T. Fabrication and Testing of Hierarchical Carbon Nanostructures for Multifunctional Applications. Ph.D. Thesis, Wright State University, Dayton, OH, USA, 2012. [Google Scholar]
- Wang, W. Hierarchical Hybrid Materials from Flexible Fabric Substrates. Ph.D. Thesis, Wright State University, Dayton, OH, USA, 2020. [Google Scholar]
- He, L.A. Surface Treatments To Tailor The Wettability Of Carbon Nanotube Arrays. Master’s Thesis, Wright State University, Dayton, OH, USA, 2015. [Google Scholar]
- Parikh, S.D.; Dave, S.; Huang, L.; Wang, W.; Mukhopadhyay, S.M.; Mayes, D.A. Multi-Walled Carbon Nanotube Carpets as Scaffolds for U87MG Glioblastoma Multiforme Cell Growth. Mater. Sci. Eng. C 2020, 108, 110345. [Google Scholar] [CrossRef]
- Lin, C.; Fugetsu, B.; Su, Y.; Watari, F. Studies on Toxicity of Multi-Walled Carbon Nanotubes on Arabidopsis T87 Suspension Cells. J. Hazard. Mater. 2009, 170, 578–583. [Google Scholar] [CrossRef] [Green Version]
- Pharmacopeia, U. 87>Biological Reactivity Tests. Vitro. USP Rockv. MD USA 2015. [Google Scholar]
- Lutz, J.B.; Zehrer, C.L.; Solfest, S.E.; Walters, S.-A. A New in Vivo Test Method to Compare Wound Dressing Fluid Handling Characteristics and Wear Time. Ostomy Wound Manag. 2011, 57, 28–36. [Google Scholar]
- Naderi, J.; Hung, M.; Pandey, S. Oxidative Stress-Induced Apoptosis in Dividing Fibroblasts Involves Activation of P38 MAP Kinase and over-Expression of Bax: Resistance of Quiescent Cells to Oxidative Stress. Apoptosis 2003, 8, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Mitchison, T.J. The Proliferation Rate Paradox in Antimitotic Chemotherapy. Mol Biol Cell 2012, 23, 1–6. [Google Scholar] [CrossRef]
- Use of International Standard ISO 10993-1, “Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing within a Risk Management Process”—Guidance for Industry and Food and Drug Administration Staff. 68. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/use-international-standard-iso-10993-1-biological-evaluation-medical-devices-part-1-evaluation-and (accessed on 1 March 2023).
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte-Fibroblast Interactions in Wound Healing. J. Investig. Derm. 2007, 127, 998–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallé, A.; Zuber, C.E.; Defrance, T.; Djossou, O.; De Rie, M.; Banchereau, J. Activation of Human B Lymphocytes through CD40 and Interleukin 4. Eur. J. Immunol. 1989, 19, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Maurer, E.; Hussain, S.; Mukhopadhyay, S.M. Cell Growth in a Porous Microcellular Structure: Influence of Surface Modification and Nanostructures. Nanosci. Nanotechnol. Lett. 2011, 3, 110–113. [Google Scholar] [CrossRef]
- Kostarelos, K. The Long and Short of Carbon Nanotube Toxicity. Nat Biotechnol. 2008, 26, 774–776. [Google Scholar] [CrossRef]
- Cendrowski, K.; Jedrzejczak-Silicka, M. Carbon Nanotubes with Controlled Length—Preparation, Characterization and Their Cytocompatibility Effects. Pol. J. Chem. Technol. 2018, 20, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Murphy, F.A.; Poland, C.A.; Duffin, R.; Donaldson, K. Length-Dependent Pleural Inflammation and Parietal Pleural Responses after Deposition of Carbon Nanotubes in the Pulmonary Airspaces of Mice. Nanotoxicology 2012, 7, 1157–1167. [Google Scholar] [CrossRef]
- Glatkowski, P.J.; Piché, J.W.; Conroy, J.L.; Bolduc, R.; LaBlanc, P. (54) Nanotube Based Sunscreen. 13. Available online: https://patents.google.com/patent/US7195754B1/en (accessed on 1 March 2023).
- MacNeil, S. Progress and Opportunities for Tissue-Engineered Skin. Nature 2007, 445, 874–880. [Google Scholar] [CrossRef]
- Yannas, I.V.; Burke, J.F.; Orgill, D.P.; Skrabut, E.M. Wound Tissue Can Utilize a Polymeric Template to Synthesize a Functional Extension of Skin. Science 1982, 215, 174–176. [Google Scholar] [CrossRef]
- Tanaka, M.; Aoki, K.; Haniu, H.; Kamanaka, T.; Takizawa, T.; Sobajima, A.; Yoshida, K.; Okamoto, M.; Kato, H.; Saito, N. Applications of Carbon Nanotubes in Bone Regenerative Medicine. Nanomaterials 2020, 10, 659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; He, D.; Xiao, X.; Yu, G.; Hu, G.; Zhang, W.; Wen, X.; Lin, Y.; Li, X.; Lin, H.; et al. Nitrogen-Doped Multiwalled Carbon Nanotubes Enhance Bone Remodeling through Immunomodulatory Functions. ACS Appl. Mater. Interfaces 2021, 13, 25290–25305. [Google Scholar] [CrossRef] [PubMed]
- Arnott, C.H.; Scott, K.A.; Moore, R.J.; Robinson, S.C.; Thompson, R.G.; Balkwill, F.R. Expression of Both TNF-Alpha Receptor Subtypes Is Essential for Optimal Skin Tumour Development. Oncogene 2004, 23, 1902–1910. [Google Scholar] [CrossRef] [Green Version]
- Bachelez, H. Immunopathogenesis of Psoriasis: Recent Insights on the Role of Adaptive and Innate Immunity. J. Autoimmun. 2005, 25, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Bahar-Shany, K.; Ravid, A.; Koren, R. Upregulation of MMP-9 Production by TNFalpha in Keratinocytes and Its Attenuation by Vitamin D. J. Cell Physiol. 2010, 222, 729–737. [Google Scholar] [CrossRef]
- Pasparakis, M.; Haase, I.; Nestle, F.O. Mechanisms Regulating Skin Immunity and Inflammation. Nat. Rev. Immunol. 2014, 14, 289–301. [Google Scholar] [CrossRef]
- Fusco, L.; Pelin, M.; Mukherjee, S.; Keshavan, S.; Sosa, S.; Martín, C.; González, V.; Vázquez, E.; Prato, M.; Fadeel, B.; et al. Keratinocytes Are Capable of Selectively Sensing Low Amounts of Graphene-Based Materials: Implications for Cutaneous Applications. Carbon 2020, 159, 598–610. [Google Scholar] [CrossRef]
- Giannakou, C.; Park, M.V.D.Z.; Bosselaers, I.E.M.; de Jong, W.H.; van der Laan, J.W.; van Loveren, H.; Vandebriel, R.J.; Geertsma, R.E. Nonclinical Regulatory Immunotoxicity Testing of Nanomedicinal Products: Proposed Strategy and Possible Pitfalls. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1633. [Google Scholar] [CrossRef] [Green Version]
- Orecchioni, M.; Bedognetti, D.; Sgarrella, F.; Marincola, F.M.; Bianco, A.; Delogu, L. Impact of Carbon Nanotubes and Graphene on Immune Cells. J. Transl. Med. 2014, 12, 138. [Google Scholar] [CrossRef] [Green Version]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parikh, S.D.; Wang, W.; Nelson, M.T.; Sulentic, C.E.W.; Mukhopadhyay, S.M. Bioinspired Hierarchical Carbon Structures as Potential Scaffolds for Wound Healing and Tissue Regeneration Applications. Nanomaterials 2023, 13, 1791. https://doi.org/10.3390/nano13111791
Parikh SD, Wang W, Nelson MT, Sulentic CEW, Mukhopadhyay SM. Bioinspired Hierarchical Carbon Structures as Potential Scaffolds for Wound Healing and Tissue Regeneration Applications. Nanomaterials. 2023; 13(11):1791. https://doi.org/10.3390/nano13111791
Chicago/Turabian StyleParikh, Soham D., Wenhu Wang, M. Tyler Nelson, Courtney E. W. Sulentic, and Sharmila M. Mukhopadhyay. 2023. "Bioinspired Hierarchical Carbon Structures as Potential Scaffolds for Wound Healing and Tissue Regeneration Applications" Nanomaterials 13, no. 11: 1791. https://doi.org/10.3390/nano13111791