One-Dimensional Nanoscale Si/Co Based on Layered Double Hydroxides towards Electrochemical Supercapacitor Electrodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Nanostructures
2.2. Physical Characterization
2.3. Electrochemical Measurements
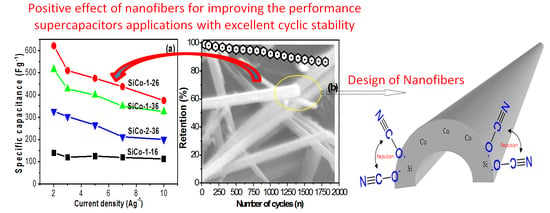
3. Results and Discussion
3.1. Scanning Electron Microscopy
3.2. Fourier Transform Infrared Spectroscopy
3.3. Powder X-ray Diffraction
3.4. Thermal Analyses
3.5. Formation Mechanism of One-Dimensional Nanofibers
3.6. Electrochemical Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, Y.; Zheng, S.; Xu, Y.; Xue, H.; Liu, C.; Pang, H. Ultrathin two-dimensional cobalt-organic frameworks nanosheets for electrochemical energy storage. Chem. Eng. J. 2019, 373, 1319–1328. [Google Scholar] [CrossRef]
- Yuan, M.; Guo, X.; Liu, Y.; Pang, H. Si-based materials derived from biomass: Synthesis and applications in electrochemical energy storage. J. Mater. Chem. A 2019, 7, 22123. [Google Scholar] [CrossRef]
- Chhetri, K.; Dahal, B.; Tiwari, A.P.; Mukhiya, T.; Muthurasu, A.; Ojha, G.P.; Lee, M.; Kim, T.; Chae, S.H.; Kim, H.Y. Controlled Selenium Infiltration of Cobalt Phosphide Nanostructure Arrays from a Two-Dimensional Cobalt Metal–Organic Framework: A Self-Supported Electrode for Flexible Quasi-Solid-State Asymmetric Supercapacitors. ACS Appl. Energy Mater. 2021, 4, 404–415. [Google Scholar] [CrossRef]
- Pan, Z.; Yang, J.; Zhang, Y.; Gao, X.; Wang, J. Quasi-solid-state fiber-shaped aqueous energy storage devices: Recent advances and prospects. J. Mater. Chem. A 2020, 8, 6406–6433. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Zheng, J.; Jiang, H.; Dong, X.; Liu, X.; Meng, C. Fabrication of vanadium sulfide (VS4) wrapped with carbonaceous materials as an enhanced electrode for symmetric supercapacitors. J. Colloid Interface Sci. 2020, 574, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Deka, B.K.; Hazarika, A.; Kim, J.; Kim, N.; Jeong, H.E.; Park, Y.-B.; Park, H.W. Bimetallic copper cobalt selenide nanowire-anchored woven carbon fiber-based structural supercapacitors. Chem. Eng. J. 2019, 355, 551–559. [Google Scholar] [CrossRef]
- Chhetri, K.; Tiwari, A.P.; Dahal, B.; PrasadOjha, G.; Mukhiya, T.; Lee, M.; Kim, T.; Chae, S.H.; Muthurasu, A.; Kim, H.Y. A ZIF-8-derived nanoporous carbon nanocomposite wrapped with Co3O4-polyaniline as an efficient electrode material for an asymmetric supercapacitor. J. Electroanaly. Chem. 2020, 856, 113670. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, J.; Baumann, D.; Peng, L.; Xu, Y.; Shakir, I.; Huang, Y.; Duan, X. Hierarchical 3D electrodes for electrochemical energy storage. Nat. Rev. Mater. 2019, 4, 45–60. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, L.; Li, C.; Yan, C.; Lee, P.S.; Ma, J. High–rate electrochemical capacitors from highly graphitic carbon–tipped manganese Oxide/Mesoporous Carbon/Manganese Oxide Hybrid Nanowires. Energy Environ. Sci. 2011, 4, 1813. [Google Scholar] [CrossRef]
- Miller, J.R.; Simon, P. Electrochemical capacitors for energy management. Science 2008, 321, 651. [Google Scholar] [CrossRef] [Green Version]
- Ansari, S.A.; Parveen, N.; Han, T.H.; Ansari, M.O.; Cho, M.H. Fibrous polyaniline@manganese oxide nanocomposites as supercapacitor electrode materials and cathode catalysts for improved power production in microbial fuel cells. Phys. Chem. Chem. Phys. 2016, 18, 9053. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, Y.; Xia, Y. Electrochemical capacitors: Mechanism, materials, systems, characterization, and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Zheng, J.; Liu, X.; Meng, C. Hydrothermal synthesis of VS4/CNTs composite with petal-shape structures performing a high specific capacity in a large potential range for high-performance symmetric supercapacitors. J. Colloid Interface Sci. 2019, 554, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Owusu, K.A.; Qu, L.; Li, J.; Wang, Z.; Zhao, K.; Yang, C.; Hercule, K.M.; Lin, C.; Shi, C.; Wei, Q.; et al. Low-crystalline iron oxide hydroxide nanoparticle anode for high-performance supercapacitors. Nat. Commun. 2017, 8, 14264. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhang, Y.; Meng, C.; Wang, X.; Liu, C.; Bo, M.; Pei, X.; Wei, Y.; Liv, T.; Cao, G. V2O3/C nanocomposites with interface defects for enhanced intercalation pseudo capacitance. Electrochim. Acta 2019, 318, 635–643. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, H.; Wang, Q.; Meng, C. In-situ hydrothermal growth of Zn4Si2O7(OH)2·H2O anchored on 3D N, S-enriched carbon derived from plant biomass for flexible solid-state asymmetrical supercapacitors. Chem. Eng. J. 2018, 352, 519–529. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, L.; Long, C.; Wei, T.; Fan, Z. Dual support system ensuring porous Co-Al hydroxide nanosheets with ultrahigh rate performance and high energy density for supercapacitors. Adv. Funct. Mater. 2015, 25, 1648–1655. [Google Scholar] [CrossRef]
- Wang, Q.; O’Hare, D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef]
- Feng, J.-T.; Lin, Y.-J.; Evans, D.G.; Duan, X.; Li, D.-Q. Enhanced metal dispersion and hydrodechlorination properties of a Ni/Al2O3 catalyst derived from layered double hydroxides. J. Catal. 2009, 266, 351–358. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, R.; Osada, M.; Iyi, N.; Ebina, Y.; Takada, K.; Sasaki, T. Synthesis, anion exchange, and delamination of Co-Al layered double hydroxide: Assembly of the exfoliated nanosheet/polyanion composite films and magneto-optical studies. J. Am. Chem. Soc. 2006, 128, 4872–4880. [Google Scholar] [CrossRef]
- Li, Q.; Yi, Z.; Cheng, Y.; Wang, X.; Yin, D.; Wang, L. Microwave-assisted synthesis of the sandwich-like porous Al2O3/RGO nanosheets anchoring NiO nanocomposite as anode materials for lithium-ion batteries. Appl. Surf. Sci. 2018, 427, 354–362. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, S.; Tschulik, K.; Compton, R.G.; Wei, M.; O’Hare, D.; Evans, D.G.; Duan, X. Molecular-scale hybridization of clay monolayers and conducting polymer for thin-film supercapacitors. Adv. Funct. Mater. 2015, 25, 2745–2753. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, J.; Xu, S.; Shao, M.; Zhang, Q.; Wei, F.; Ma, J.; Wei, M.; Evans, D.G.; Duan, X. Hierarchical NiMn layered double hydroxide/carbon nanotubes architecture with superb energy density for flexible supercapacitors. Adv. Funct. Mater. 2014, 24, 2938–2946. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Zhu, J.; Zhang, X.; San Hui, K.; Hui, K.N. 3D porous layered double hydroxides grown on graphene as advanced electrochemical pseudocapacitor materials. J. Mater. Chem. A 2013, 1, 9046. [Google Scholar] [CrossRef]
- Li, Z.; Shao, M.; Zhou, L.; Zhang, R.; Zhang, C.; Han, J.; Wei, M.; Evans, D.G.; Duan, X. A flexible all-solid-state micro-supercapacitor based on hierarchical CuO@ layered double hydroxide core shell nanoarrays. Nano Energy 2016, 20, 294–304. [Google Scholar] [CrossRef]
- Guan, X.; Huang, M.; Yang, L.; Wang, G.; Guan, X. Facial design and synthesis of CoSx/Ni-Co LDH nanocages with rhombic dodecahedral structure for high performance asymmetric supercapacitors. Chem. Eng. J. 2019, 372, 151–162. [Google Scholar] [CrossRef]
- Zheng, W.; Sun, S.; Xu, Y.; Yu, R.; Li, H. Sulfidation of hierarchical NiAl LDH/Ni-MOF composite for high-performance supercapacitor. Chem. Electro. Chem. 2019, 6, 3375–3382. [Google Scholar] [CrossRef]
- Gao, P.; Huang, X.; Zhao, Y.; Hu, X.; Cen, D.; Gao, G.; Bao, Z.; Mei, Y.; Di, Z.; Wu, G. formation of Si hollow structures as promising anode materials through reduction of silica in AlCl3-NaCl molten salt. ACS Nano 2018, 12, 11481–11490. [Google Scholar] [CrossRef]
- Zhang, H.; Zong, P.; Chen, M.; Jin, H.; Bai, Y.; Li, S.; Ma, F.; Xu, H.; Lian, K. Situ synthesis of multilayer carbon matrix decorated with copper particles: Enhancing the performance of Si as anode for Li-ion batteries. ACS Nano 2009, 13, 3054–3062. [Google Scholar] [CrossRef]
- Yi, Z.; Qian, Y.; Cao, C.; Lin, N.; Qian, Y. Porous Si/C microspheres decorated with stable outer carbon interphase and inner interpenetrated Si@C channels for enhanced lithium storage. Carbon 2019, 149, 664–671. [Google Scholar] [CrossRef]
- Prakash, S.; Zhang, C.; Park, J.D.; Razmjooei, F.; Yu, J.S. Silicon core-mesoporous shell carbon spheres as high stability lithium-ion battery anode. J. Colloid Interface Sci. 2019, 534, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Y.; Lu, B.; Yu, J.; Yuan, M.; Tan, Q.; Zhong, Z. Fabing Su. Hollow core-shell structured Si@NiAl-LDH composite as highperformance anode material in lithium-ion batteries. Electrochim. Acta 2020, 331, 135331. [Google Scholar] [CrossRef]
- Ramly, M.M.; Omar, F.S.; Rohaizad, A.; Aspanut, Z.; Rahman, S.A.; Goh, B.T. Solid-phase diffusion controlled growth of nickel silicide nanowires for supercapacitor electrode. Appl. Surf. Sci. 2018, 456, 515–525. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Z.; Li, B.; Zhang, J.; Niu, C. Ni3Si2 nanowires grown in situ on Ni foam for high-performance supercapacitors. J. Power Sources 2016, 320, 13–19. [Google Scholar] [CrossRef]
- Lee, J.; Yoo, C.Y.; Lee, Y.A.; Park, S.H.; Cho, Y.; Jun, J.H.; Kim, W.Y.; Kim, B.; Yoon, H. Single-crystalline Co2Si nanowires directly synthesized on silicon substrate for high-performance micro-supercapacitor. Chem. Engin. J. 2019, 370, 973–979. [Google Scholar] [CrossRef]
- Saber, O.; Kotb, H.M.; Osama, M.; Khater, H.A. An Effective Photocatalytic Degradation of Industrial Pollutants through Converting Titanium Oxide to Magnetic Nanotubes and Hollow Nanorods by Kirkendall Effect. Nanomaterials 2022, 12, 440. [Google Scholar] [CrossRef]
- Barrioni, B.R.; Norris, E.; Jones, J.R.; Pereira, M.M. The influence of cobalt incorporation and cobalt precursor selection on the structure and bioactivity of sol–gel-derived bioactive glass. J. Sol-Gel Sci. Technol. 2018, 88, 309–321. [Google Scholar] [CrossRef]
- Saber, O. Preparation and characterization of a new nano layered material, Co–Zr LDH. J. Mater. Sci. 2007, 42, 9905–9912. [Google Scholar] [CrossRef]
- Wakahara, A.; Fujiwara, T.; Okada, H.; Yoshida, A.; Ohshima1, T.; Itho, H. Energy-Back-Transfer Process in Rare-Earth Doped AlGaN. Mater. Res. Soc. Symp. Proc. 2005, 866, 391–396. [Google Scholar] [CrossRef]
- Gastuche, M.C.; Brown, G.; Mortland, M. Mixed magnesium-aluminum hydroxides. Clay Miner. 1967, 7, 177–201. [Google Scholar] [CrossRef]
- Saber, O.; Tagaya, H. Preparation of a new nano-layered materials and organic–inorganic nano-hybrid materials Zn–Si LDH. J. Porous Mater. 2009, 16, 81–89. [Google Scholar] [CrossRef]
- Salak, A.N.; Vieira, D.E.L.; Lukienko, I.M.; Shapovalov, Y.O.; Fedorchenko, A.V.; Fertman, E.L.; Pashkevich, Y.G.; Babkin, R.Y.; Shilin, A.D.; Rubanik, V.V.; et al. High-Power Ultrasonic Synthesis and Magnetic-Field-Assisted Arrangement of Nanosized Crystallites of Cobalt-Containing Layered Double Hydroxides. Chem. Eng. 2019, 3, 62. [Google Scholar] [CrossRef] [Green Version]
- Parveen, N.; Ansari, S.A.; Ansari, M.O.; Cho, M.H. Manganese dioxide nanorods intercalated reduced graphene oxide nanocomposite toward high performance electrochemical supercapacitive electrode materials. J. Colloid Interface Sci. 2017, 506, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Donga, X.; Yub, Y.; Zhang, Y.; Xu, Z.; Jiang, H.; Meng, C.; Huang, C. Synthesis of cobalt silicate nanosheets with mesoporous structure and high surface area as the promising electrode for high-performing hybrid supercapacitor. Electrochim. Acta 2021, 380, 138225. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Jiang, H.; Li, X.; Cheng, Y.; Meng, C. Designed mesoporous hollow sphere architecture metal (Mn, Co, Ni) silicate: A potential electrode material for flexible all solid-state asymmetric supercapacitor. Chem. Eng. J. 2019, 362, 818–829. [Google Scholar] [CrossRef]
- Rong, Q.; Long, L.-L.; Zhang, X.; Huang, Y.X.; Yu, H.-Q. Layered cobalt nickel silicate hollow spheres as a highly stable supercapacitor material. Appl. Energy 2015, 153, 63–69. [Google Scholar] [CrossRef]
- Parveen, N.; Hilal, M.; Han, J.I. Newly Design Porous/Sponge Red Phosphorus@ Graphene and Highly Conductive Ni2P Electrode for Asymmetric Solid State Supercapacitive Device with Excellent Performance. Nano-Micro Lett. 2020, 12, 25. [Google Scholar] [CrossRef] [Green Version]
- Parveen, N.; Ansari, S.A.; Al-Arjan, W.S.; Ansari, M.O. Manganese dioxide coupled with hollow carbon nanofiber toward high-performance electrochemical supercapacitive electrode materials. J. Sci. Adv. Mater. Devices 2021, 6, 472–482. [Google Scholar] [CrossRef]
- Dong, X.; Yu, Y.; Jing, X.; Jiang, H.; Hu, T.; Meng, C.; Huang, C.; Zhang, Y. Sandwich-like honeycomb Co2SiO4/rGO/honeycomb Co2SiO4 structures with enhanced electrochemical properties for high-performance hybrid supercapacitor. J. Power Source 2021, 492, 229643. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Dong, X.; Jiang, H.; Hu, T.; Meng, C.; Huang, C. Alkali etching metal silicates derived from bamboo leaves with enhanced electrochemical properties for solid-state hybrid supercapacitors. Chem. Eng. J. 2021, 417, 127964. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saber, O.; Ansari, S.A.; Osama, A.; Osama, M. One-Dimensional Nanoscale Si/Co Based on Layered Double Hydroxides towards Electrochemical Supercapacitor Electrodes. Nanomaterials 2022, 12, 1404. https://doi.org/10.3390/nano12091404
Saber O, Ansari SA, Osama A, Osama M. One-Dimensional Nanoscale Si/Co Based on Layered Double Hydroxides towards Electrochemical Supercapacitor Electrodes. Nanomaterials. 2022; 12(9):1404. https://doi.org/10.3390/nano12091404
Chicago/Turabian StyleSaber, Osama, Sajid Ali Ansari, Aya Osama, and Mostafa Osama. 2022. "One-Dimensional Nanoscale Si/Co Based on Layered Double Hydroxides towards Electrochemical Supercapacitor Electrodes" Nanomaterials 12, no. 9: 1404. https://doi.org/10.3390/nano12091404